Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Noboru Imai | -- | 2312 | 2023-06-30 10:38:48 | | | |
| 2 | Sirius Huang | Meta information modification | 2312 | 2023-07-03 03:01:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Imai, N. Circadian and Circannual Rhythms in Migraine. Encyclopedia. Available online: https://encyclopedia.pub/entry/46263 (accessed on 07 February 2026).
Imai N. Circadian and Circannual Rhythms in Migraine. Encyclopedia. Available at: https://encyclopedia.pub/entry/46263. Accessed February 07, 2026.
Imai, Noboru. "Circadian and Circannual Rhythms in Migraine" Encyclopedia, https://encyclopedia.pub/entry/46263 (accessed February 07, 2026).
Imai, N. (2023, June 30). Circadian and Circannual Rhythms in Migraine. In Encyclopedia. https://encyclopedia.pub/entry/46263
Imai, Noboru. "Circadian and Circannual Rhythms in Migraine." Encyclopedia. Web. 30 June, 2023.
Copy Citation
Migraine—a primary headache—has circadian and circannual rhythms in the onset of attacks. The circadian and circannual rhythms involve the hypothalamus, which is strongly associated with pain processing in migraines. Moreover, the role of melatonin in circadian rhythms has been implied in the pathophysiology of migraines.
migraine
circadian rhythms
circannual rhythms
suprachiasmatic nucleus
PACAP
1. Introduction
Migraine is a neurological disorder that causes recurrent headaches with intensity ranging from moderate to severe. It is characterized by pulsating pain that is often on one side of the head and aggravated by routine physical activity, and is typically accompanied by nausea, vomiting, and sensitivity to light and sound [1]. Migraine headaches are caused by a combination of genetic and environmental factors [2]. Although the exact cause of migraines is yet to be determined, it is associated with changes in the brainstem and its interactions with the trigeminal nerve—a major pain pathway [2].
The hypothalamus is a small region at the base of the brain that plays a critical role in regulating various bodily functions, including sleep, appetite, and hormone release. It also plays a role in the development of migraines [3][4]. The hypothalamus is involved in regulating the body’s circadian rhythms, the internal biological processes that control sleep and wake cycles [5][6]. Migraineurs often report that their headaches are triggered by changes in sleep patterns or irregular sleep schedules, suggesting that disruptions in the circadian rhythms may play a role in the development of migraines [7][8][9].
Circadian and circannual rhythms regulate various physiological functions, including sleep–wake cycles, hormone secretion, metabolism, and immune function [5][6][8][9][10]. These rhythms are driven by endogenous biological clocks that are located in various tissues and organs throughout the body. The central pacemaker of these rhythms is the suprachiasmatic nucleus (SCN) of the hypothalamus, which generates and synchronizes circadian rhythms in mammals.
2. The SCN of the Hypothalamus
The SCN controls the body’s circadian rhythms [10][11]. These rhythms are biological processes occurring on a 24 h cycle and include sleep–wake cycles, hormone production, and other bodily functions. The SCN is located in the hypothalamus, a region of the brain that regulates many bodily functions, including hunger, thirst, and temperature regulation. It is a small region of approximately 20,000 neurons located in the anterior hypothalamus, just above the optic chiasm (Figure 1) [5]. Additionally, it can be divided into two subregions—“core” and “shell”—based on their connection to the retina and output pathways, as well as the expression of specific neuropeptides. The core region contains cells that express vasoactive intestinal (VIP) and gastrin-releasing (GRP) peptides, whereas the shell region consists of cells that express arginine vasopressin (AVP) [10]. The optic chiasm is the point where the optic nerves (from the eyes) cross. This location is important for the function of the SCN because it allows it to receive information about light and darkness.
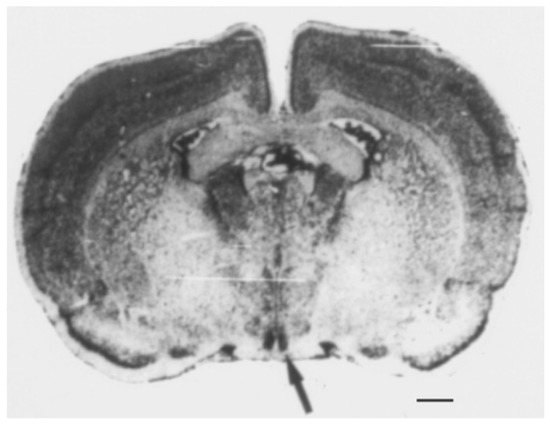
Figure 1. The suprachiasmatic nucleus (SCN) of the hypothalamus. The area symmetrically stained black through Nissl staining in the coronal section of the rat brain is the SCN, as indicated by the arrow. Scale bar = 1 mm. Data from Schwartz W.J. [5].
One of the most important functions of the SCN is to regulate the sleep–wake cycle [5][6][7][8][9][10][11][12][13][14][15][16], also known as the circadian rhythm. This cycle controls when humans feel sleepy and wakefulness. The SCN receives information about light and darkness through the optic nerves and uses this information to adjust the body clock. When the eyes detect light, the SCN is activated and sends signals to the pineal gland to stop producing melatonin, a hormone that induces drowsiness. The SCN is less active when it is dark. Thus, the pineal gland begins to produce melatonin, leading to drowsiness and eventual sleep.
The SCN can maintain its own circadian rhythm, even in the absence of external cues [5][10][11][12][13][14][15][16]. This ability is known as “endogenous rhythmicity” and is due to the molecular clock within the region. This clock consists of a group of proteins that interact with each other in a rhythmic manner, with the levels of each protein peaking and declining at specific times of the day. These proteins include the circadian locomotor output cycles protein kaput (CLOCK) and brain and muscle ARNT-like 1 (BMAL1; also, ARNTL), and the period (PER) and cryptochrome (CRY) proteins (Figure 1) [11]. The molecular clock regulates the expression of genes that control circadian rhythms in the body. Moreover, the regulation of circadian rhythms depends on intracellular transcription–translation negative feedback loops (TTFLs) [11][12], which have been identified in neurons and astrocytes of the SCN. The astrocytic TTFL can independently drive molecular oscillations and circadian behavior in mice, even in the absence of other cellular clocks.
The molecular clock is not unique to the SCN and is present in many cells and tissues throughout the body [5][7][11][12]. However, the molecular clock in the SCN is particularly important because it acts as a “master clock” that synchronizes rhythms in the rest of the body. The SCN sends signals to other regions of the brain and the rest of the body to regulate the timing of physiological processes such as hormone production and metabolism.
3. Circadian Rhythm
The circadian rhythm is a 24 h cycle of physiological and behavioral processes controlled by a molecular clock composed of clock genes and their protein products [5][6][8][9][10][11]. Its regulation is complex and involves the interaction of several neurotransmitters, hormones, and signaling pathways [5][6][7][8][9][10][11][12][13][14][15][16]. For example, the neurotransmitter serotonin plays a critical role in regulating the sleep–wake cycle and the timing of SCN activity. Serotonin is synthesized from tryptophan by the enzyme tryptophan hydroxylase and metabolized by the enzyme monoamine oxidase. Dysregulation of the serotonin system has been implicated in several sleep disorders, such as insomnia and hypersomnia, and mood disorders, such as depression and anxiety.
The molecular clock controlling the circadian rhythm is present in nearly all cells in the body. Furthermore, it is regulated by a network of transcriptional and post-transcriptional feedback loops involving the expression and degradation of clock genes and their protein products (Figure 2).
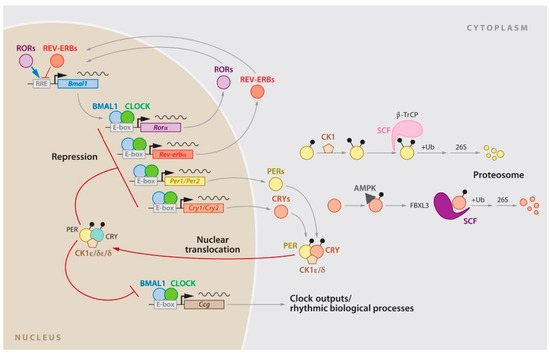
Figure 2. The circadian gene network in mammals. The clock network is driven by the transcriptional activators CLOCK and BMAL1, which stimulate Per1, Per2, Cry1, and Cry2 expression. The resulting proteins interact to repress their own transcription, with the stability of PER and CRY tightly regulated by parallel E3 ubiquitin ligase pathways. CLOCK and BMAL1 also regulate the nuclear receptors Rev-erbα/β, which rhythmically repress the transcription of Bmal1 and Nfil3, a process driven by the activators RORa/b. In turn, NFIL3 represses the PAR-bZip factor DBP, which regulates a rhythm in ROR nuclear receptors. These three interconnected transcriptional feedback loops are master regulators of most cycling genes. Data from Mohawk J.A, et al. [11].
Central components of the clock network include the transcriptional activators—CLOCK (along with its paralog NPAS2) and BMAL1—which work together to stimulate the expression of the period (Per1 and Per2) and cryptochrome (Cry1 and Cry2) genes at the beginning of each cycle. As the products of Per and Cry genes accumulate, they form dimers. Additionally, they form a complex that translocates to the nucleus where it interacts with CLOCK and BMAL1 to ultimately repress its own transcription. The feedback loop occurs over approximately 24 h, and the degradation of the PER and CRY proteins is strictly controlled by E3 ubiquitin ligase complexes. In addition to the core CLOCK-BMAL1/PER-CRY feedback loop, there are other interlocking loops. One of the most notable interlocking loops involves Rev-Erbα (Nr1d1) and Rora. These two components are direct targets of CLOCK-BMAL1 and exert feedback effects on the transcription of Bmal1 (and to a lesser extent CLOCK), resulting in the antiphase oscillation of BMAL1. Other feedback loops include members of the PAR-bZip family, such as DBP, HLF, and TEF, as well as the bZip protein E4BP4 (Nfil3) and the bHLH proteins DEC1 and DEC2 (Bhlhb2 and Bhlhb3). All these components are transcriptional targets of CLOCK-BMAL1.
4. Circadian and Circannual Rhythms in Migraine
The potential role of circadian rhythms in the pathogenesis of migraines has been investigated, linking mutations in a component of the molecular circadian clock to migraines in animals [17]. Similarly, circadian phase delays and lower plasma melatonin levels have been reported in migraineurs [18]. Research on the prophylactic effect of melatonin in migraines has shown contrasting results. A randomized, placebo-controlled trial of 2 mg extended-release melatonin showed no prophylactic effect [19]. Contrastingly, melatonin had a considerable effect compared to a placebo and amitriptyline in a randomized trial using a placebo, 25 mg of amitriptyline, and 3 mg of melatonin (Figure 3) [20], which was better tolerated than amitriptyline. The dim light melatonin onset study showed that circadian misalignment and delayed sleep timing were associated with higher migraine frequency and severity, independent of sleep duration [21]. These findings suggest that circadian factors may play a role in the development and maintenance of chronic migraines, particularly in combination with biological or environmental factors, and that melatonin may be a target for migraine prophylaxis.
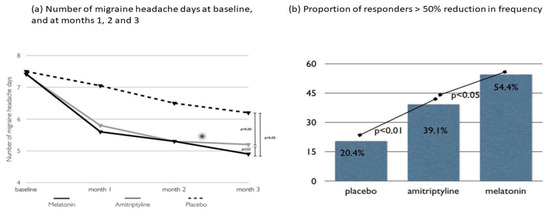
Figure 3. Randomized clinical trial comparing 3 mg of melatonin, 25 mg of amitriptyline, and placebo for migraine prevention. The primary efficacy endpoint of this study was the frequency of migraine headache days per month. Both melatonin and amitriptyline were superior to the placebo (p < 0.05) when comparing the baseline to the last month of observation. For the secondary endpoints, melatonin and amitriptyline were more effective than the placebo in reducing the number of analgesics taken as well as the duration and intensity of migraine headache attacks. Although melatonin and amitriptyline were equally effective for the primary endpoint, melatonin was superior to amitriptyline (p < 0.05) and the placebo (p < 0.01) for the secondary endpoint of the number of responders (migraineurs with a > 50% improvement in headache frequency). NS: Non-significant. Data from Gonçalves AL, et al. [20].
Research on the possible role of circannual rhythms in the pathogenesis of migraine is limited. In an Arctic region where light conditions are extreme, migraineurs with aura were more likely to have attacks during the light season, with light exposure being a key factor [22]. These migraineurs also reported interictal light hypersensitivity, light exposure as a precipitating factor, and frequent use of sunglasses to prevent attacks [22]. Another study showed that female migraineurs with aura had a marked seasonal variation, with more attacks during the light season [23]. A recent study using Google Trends found that interest in headaches and migraine peaked in February, October, and November and was lowest in July [24]. These studies suggest that many migraineurs may experience seasonal variations in their headaches and that further study is required in clinical practice.
5. PACAP and Circadian Rhythm
The circadian rhythm is regulated by the SCN through cell-autonomous transcription–translation feedback loops. Light-sensitive projections from the retinohypothalamic tract to the SCN signal via the neurotransmitters glutamate, aspartate, and PACAP [25][26][27]. Glutamate is the major neurotransmitter used for signaling [28][29]. Furthermore, PACAP is involved in the daytime regulation of the circadian cycle and contributes to the gain control mechanism for glutamate-induced phase shifts [30]. Knock-out mice deficient in PACAP or PAC1 show the stable expression of clock genes but have impaired photic entrainment and disrupted circadian food anticipation behavior, suggesting the importance of PACAP in regulating the circadian rhythm [31][32][33][34]. PACAP and glutamate induce phase changes via the light-sensitive clock genes per1 and per2 [30]. Glutamate induces per1 and per2 expression, whereas PACAP modulates their expression, inducing per1 and per2 at nanomolar concentrations and blocking glutamate-induced expression at micromolar concentrations [30].
Casein kinase 1δ (CKIδ) has been identified as an issue in migraines and circadian rhythms related to PER2 regulation. In a previous study, two families with familial migraines with aura and familial advanced sleep phase syndrome had a distinct missense mutation in the gene encoding casein kinase Iδ [35]. CK1δ regulates the rate of the mammalian circadian clock [35][36][37]. The resulting mutations (T44A and H46R) occurred in the conserved catalytic domain of CKIδ and caused reduced enzyme activity. Mice engineered to carry the CKIδ T44A allele were more sensitive to pain after treatment with nitroglycerin, a drug that triggers a migraine-like attack. In addition, they had a reduced threshold for cortical spreading depression and greater arterial dilation during cortical spreading depression. The mutation in the gene encoding CKIδ co-segregated with both the presence of migraine and advanced sleep phase [17].
6. PACAP and Migraine
The role of neuropeptides, particularly calcitonin gene-related peptide (CGRP) and PACAP, in the pathogenesis and treatment of migraines has received increasing attention over the past decade [38][39][40]. Monoclonal antibodies (mAbs) that block CGRP have been effective in many migraineurs. However, approximately 40% of migraineurs do not respond to this treatment.
PACAP is a member of the vasoactive intestinal peptide (VIP)/secretin/glucagon family of peptides and is widely distributed throughout the central and peripheral nervous systems. It has two isoforms—PACAP-27 and PACAP-38—which differ by six amino acids at the C-terminal (Figure 4) [40].

Figure 4. Sequence comparison of amino acids making up the isoforms of PACAP and VIP. This image shows a sequence comparison of the amino acids that make up the isoforms of PACAP (PACAP-38 and PACAP-27) and VIP. PACAP-27 is produced via post-translational truncation at the C-terminus of PACAP-38, and the amino acid sequence at the N-terminus is retained. PACAP-38 has a high degree of homology with VIP (approximately 68%). Data from Guo S, et al. [40].
PACAP binds to three G protein-coupled receptors—PAC1, VPAC1, and VPAC2—which are expressed in different tissues throughout the body. PACAP-27 and PACAP-38 induce migraine attacks when administered to migraineurs [39][41][42]. An animal study showed that the peptide PACAP-38 can cause considerable hypersensitivity and dilation of the carotid arteries independent of CGRP. Unlike glyceryl trinitrate, which requires CGRP to induce hypersensitivity, PACAP-38-induced hypersensitivity is only partially inhibited by ATP-sensitive potassium channels. These findings establish the PACAP pathway as distinct from other migraine-provoking pathways, such as CGRP and glyceryl trinitrate, based on several migraine-relevant models. This understanding of PACAP signaling may lead to the identification of novel therapeutic targets, particularly for migraineurs who do not respond to anti-CGRP therapy. PACAP may be involved in non-responsiveness to CGRP mAbs, and a neutralizing mAb against PACAP is in phase II clinical development [40].
References
- Headache Classification Committee of the International Headache Society (Healthcare Infection Society). The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2018, 38, 1–211.
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876.
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of migraine: A disorder of sensory processing. Physiol. Rev. 2017, 97, 553–622.
- Schulte, L.H.; May, A. The migraine generator revisited: Continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016, 139, 1987–1993.
- Schwartz, W.J. Suprachiasmatic nucleus. Curr. Biol. 2002, 12, R644.
- Porcu, A.; Nilsson, A.; Booreddy, S.; Barnes, S.A.; Welsh, D.K.; Dulcis, D. Seasonal changes in day length induce multisynaptic neurotransmitter switching to regulate hypothalamic network activity and behavior. Sci. Adv. 2022, 8, eabn9867.
- Vgontzas, A.; Pavlović, J.M. Sleep disorders and migraine: Review of literature and potential pathophysiology mechanisms. Headache 2018, 58, 1030–1039.
- de Tommaso, M.; Delussi, M. Circadian rhythms of migraine attacks in episodic and chronic patients: A cross sectional study in a headache center population. BMC Neurol. 2018, 18, 94.
- Tiseo, C.; Vacca, A.; Felbush, A.; Filimonova, T.; Gai, A.; Glazyrina, T.; Hubalek, I.A.; Marchenko, Y.; Overeem, L.H.; Piroso, S.; et al. Migraine and sleep disorders: A systematic review. J. Headache Pain 2020, 21, 126.
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu. Rev. Physiol. 2010, 72, 551–577.
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462.
- Brancaccio, M.; Edwards, M.D.; Patton, A.P.; Smyllie, N.J.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 2019, 363, 187–192.
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179.
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102.
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84.
- Naito, E.; Watanabe, T.; Tei, H.; Yoshimura, T.; Ebihara, S. Reorganization of the suprachiasmatic nucleus coding for day length. J. Biol. Rhythms 2008, 23, 140–149.
- Brennan, K.C.; Bates, E.A.; Shapiro, R.E.; Zyuzin, J.; Hallows, W.C.; Huang, Y.; Lee, H.Y.; Jones, C.R.; Fu, Y.H.; Charles, A.C.; et al. Casein kinase idelta mutations in familial migraine and advanced sleep phase. Sci. Transl. Med. 2013, 5, 183ra56.
- Peres, M.F.; Valença, M.M.; Amaral, F.G.; Cipolla-Neto, J. Current understanding of pineal gland structure and function in headache. Cephalalgia 2019, 39, 1700–1709.
- Alstadhaug, K.B.; Odeh, F.; Salvesen, R.; Bekkelund, S.I. Prophylaxis of migraine with melatonin: A randomized controlled trial. Neurology 2010, 75, 1527–1532.
- Gonçalves, A.L.; Ferreira, A.M.; Ribeiro, R.T.; Zukerman, E.; Cipolla-Neto, J.; Peres, M.F.P. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1127–1132.
- Ong, J.C.; Taylor, H.L.; Park, M.; Burgess, H.J.; Fox, R.S.; Snyder, S.; Rains, J.C.; Espie, C.A.; Wyatt, J.K. Can circadian dysregulation exacerbate migraines? Headache 2018, 58, 1040–1051.
- Alstadhaug, K.B.; Salvesen, R.; Bekkelund, S.I. Seasonal variation in migraine. Cephalalgia 2005, 25, 811–816.
- Alstadhaug, K.B.; Bekkelund, S.; Salvesen, R. Circannual periodicity of migraine? Eur. J. Neurol. 2007, 14, 983–988.
- Radziwon, J.; Waszak, P. Seasonal changes of internet searching suggest circannual rhythmicity of primary headache disorders. Headache 2022, 62, 811–817.
- Hannibal, J.; Møller, M.; Ottersen, O.P.; Fahrenkrug, J. PACAP and glutamate are co-stored in the retinohypothalamic tract. J. Comp. Neurol. 2000, 418, 147–155.
- Hannibal, J.; Fahrenkrug, J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res. 2004, 316, 99–113.
- Hannibal, J.; Kankipati, L.; Strang, C.E.; Peterson, B.B.; Dacey, D.; Gamlin, P.D. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J. Comp. Neurol. 2014, 522, 2231–2248.
- Moldavan, M.G.; Irwin, R.P.; Allen, C.N. Presynaptic GABA(B) receptors regulate retinohypothalamic tract synaptic transmission by inhibiting voltage-gated Ca2+ channels. J. Neurophysiol. 2006, 95, 3727–3741.
- Colwell, C.S.; Menaker, M. NMDA as well as non-NMDA receptor antagonists can prevent the phase-shifting effects of light on the circadian system of the golden hamster. J. Biol. Rhythms 1992, 7, 125–136.
- Nielsen, H.S.; Hannibal, J.; Knudsen, S.M.; Fahrenkrug, J. Pituitary adenylate cyclase-activating polypeptide induces period1 and period2 gene expression in the rat suprachiasmatic nucleus during late night. Neuroscience 2001, 103, 433–441.
- Okamura, H. Suprachiasmatic nucleus clock time in the mammalian circadian system. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 551–556.
- Hannibal, J.; Brabet, P.; Fahrenkrug, J. Mice lacking the PACAP type I receptor have impaired photic entrainment and negative masking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R2050–R2058.
- Hannibal, J.; Jamen, F.; Nielsen, H.S.; Journot, L.; Brabet, P.; Fahrenkrug, J. Dissociation between light-induced phase shift of the circadian rhythm and clock gene expression in mice lacking the pituitary adenylate cyclase activating polypeptide type 1 receptor. J. Neurosci. 2001, 21, 4883–4890.
- Hannibal, J.; Georg, B.; Fahrenkrug, J. Altered circadian food anticipatory activity rhythms in PACAP receptor 1 (PAC1) deficient mice. PLoS ONE 2016, 11, e0146981.
- Etchegaray, J.P.; Machida, K.K.; Noton, E.; Constance, C.M.; Dallmann, R.; Di Napoli, M.N.; DeBruyne, J.P.; Lambert, C.M.; Yu, E.A.; Reppert, S.M.; et al. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol. Cell. Biol. 2009, 29, 3853–3866.
- Eng, G.W.L.; Virshup, D.M. Site-specific phosphorylation of casein kinase 1 δ (CK1δ) regulates its activity towards the circadian regulator PER2. PLoS ONE 2017, 12, e0177834.
- Keesler, G.A.; Camacho, F.; Guo, Y.; Virshup, D.; Mondadori, C.; Yao, Z. Phosphorylation and destabilization of human period I clock protein by human casein kinase I epsilon. NeuroReport 2000, 11, 951–955.
- Waschek, J.A.; Baca, S.M.; Akerman, S.S. Pacap and migraine headache: Immunomodulation of neural circuits in autonomic ganglia and brain parenchyma. J. Headache Pain 2018, 19, 23.
- Ernstsen, C.; Christensen, S.L.; Rasmussen, R.H.; Nielsen, B.S.; Jansen-Olesen, I.; Olesen, J.; Kristensen, D.M. The PACAP pathway is independent of CGRP in mouse models of migraine: Possible new drug target? Brain 2022, 145, 2450–2460.
- Guo, S.; Jansen-Olesen, I.; Olesen, J.; Christensen, S.L. Role of PACAP in migraine: An alternative to CGRP? Neurobiol. Dis. 2023, 176, 105946.
- Schytz, H.W.; Birk, S.; Wienecke, T.; Kruuse, C.; Olesen, J.; Ashina, M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 2009, 132, 16–25.
- Ghanizada, H.; Al-Karagholi, M.A.; Arngrim, N.; Olesen, J.; Ashina, M. PACAP27 induces migraine-like attacks in migraine patients. Cephalalgia 2020, 40, 57–67.
More
Information
Subjects:
Neurosciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
704
Revisions:
2 times
(View History)
Update Date:
03 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




