
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hasan Shabbir | -- | 2742 | 2023-06-29 23:28:14 | | | |
| 2 | Wendy Huang | Meta information modification | 2742 | 2023-06-30 06:12:52 | | |
Video Upload Options
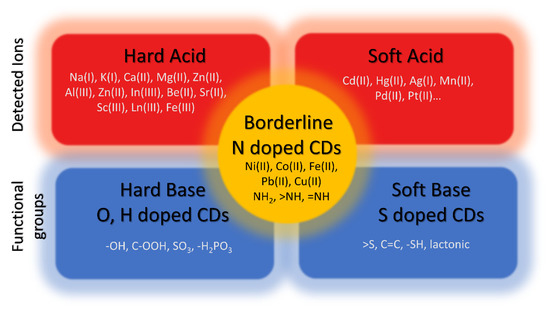
Carbon dots (CDs) are zero-dimensional nanomaterials composed of carbon and surface groups attached to their surface. CDs have a size smaller than 10 nm and have potential applications in different fields such as metal ion detection, photodegradation of pollutants, and bio-imaging, in this research, the capabilities of CDs in metal ion detection will be described. Quantum confinement is generally viewed as the key factor contributing to the uniqueness of CDs characteristics due to their small size and the lack of attention on the surface functional groups and their roles is given, however, in this research, the focus will be on the functional group and the composition of CDs. The surface functional groups depend on two parameters: (i) the oxidation of precursors and (ii) their composition.
1. Introduction
2. Origin of Optical Properties
3. Mercury Detection
4. Lead Detection
5. Silver Detection
6. Chromium Detection
7. Iron(III) and Iron(II) Detection
8. Copper(II) Detection
References
- Zhen Tian, Xutao Zhang, Di Li, Ding Zhou, Pengtao Jing, Dezhen Shen, Songnan Qu, Radek Zboril, Andrey L. Rogach Full-Color Inorganic Carbon Dot Phosphors for White-Light-Emitting Diodes. Advanced Optical Materials 2017, 5, 1, https://doi.org/10.1002/adom.201700416.
- Xiang Miao, Dan Qu, Dongxue Yang, Bing Nie, Yikang Zhao, Hongyou Fan and Zaicheng Sun Synthesis of Carbon Dots with Multiple Color Emission by Controlled Graphitization and Surface Functionalization. Advanced Materials 2018, 30, 1, https://doi.org/10.1002/adma.201704740.
- Ting Yuan, Ting Meng, Ping He,YuXin Shi, Yunchao Li, Xiaohong Li, Louzhen Fan ORCID logo and Shihe Yang Carbon quantum dots: an emerging material for optoelectronic applications. Journal of Materials Chemistry C 2019, 7, 6820-6835, DOI https://doi.org/10.1039/C9TC01730E.
- Yuan Xiong , Julian Schneider , Elena V. Ushakova , Andrey L. Rogach Influence of molecular fluorophores on the research field of chemically synthesized carbon dots. Nano Today 2018, 23, 124-139, https://doi.org/10.1016/j.nantod.2018.10.010.
- Jingjing Yu ,Chang Liu ,Kang Yuan ,Zunming Lu ,Yahui Cheng ,Lanlan Li ,Xinghua Zhang ,,Peng Jin ,Fanbin Meng and Hui Liu Luminescence Mechanism of Carbon Dots by Tailoring Functional Groups for Sensing Fe3+ Ions. https://doi.org/10.3390/nano8040233 2018, 8, 4, Nanomaterials.
- Hui Ding; Xue-Hua Li; Xiao-Bo Chen; Ji-Shi Wei; Xiao-Bing Li and Huan-Ming Xiong Surface states of carbon dots and their influences on luminescence. Journal of Applied Physics 2020, 127, 23, https://doi.org/10.1063/1.5143819.
- Hui Ding, Shang-Bo Yu, Ji-Shi Wei, and Huan-Ming Xiong Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 1, 484–491, https://doi.org/10.1021/acsnano.5b05406.
- Biao Yuan, Shanyue Guan, Xingming Sun, Xiaoming Li, Haibo Zeng, Zheng Xie, Ping Chen, and Shuyun Zhou Highly Efficient Carbon Dots with Reversibly Switchable Green–Red Emissions for Trichromatic White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 18, 16005–16014, https://doi.org/10.1021/acsami.8b02379.
- Venkatesh Gude, Ananya Das, Tanmay Chatterjeea and Prasun K. Mandal Molecular origin of photoluminescence of carbon dots: aggregation-induced orange-red emission. Physical Chemistry Chemical Physics 2016, 18, 28274-28280, DOI https://doi.org/10.1039/C6CP05321A.
- Meng Li Liu, Lin Yang, Rong Sheng Li, Bin Bin Chen, Hui Liu and Cheng Zhi Huang Large-scale simultaneous synthesis of highly photoluminescent green amorphous carbon nanodots and yellow crystalline graphene quantum dots at room temperature. Green Chemistry 2017, 19, 3611-3617, DOI https://doi.org/10.1039/C7GC01236E.
- Evgeny V. Kundelev, Nikita V. Tepliakov, Mikhail Yu. Leonov, Vladimir G. Maslov, Alexander V. Baranov, Anatoly V. Fedorov, Ivan D. Rukhlenko, and Andrey L. Rogach Amino Functionalization of Carbon Dots Leads to Red Emission Enhancement. The Journal of Physical Chemistry Letters 2019, 17, 5111–5116, https://doi.org/10.1021/acs.jpclett.9b01724.
- Ying Li, Hechun Lin, Chunhua Luo,Yunqiu Wang,Chunli Jiang,Ruijuan Qi, Rong Huang,Jadranka Travas-sejdic and Hui Peng Aggregation induced red shift emission of phosphorus doped carbon dots. RSC Advances 2017, 7, 32225-32228, DOI https://doi.org/10.1039/C7RA04781A.
- Velusamy Arul and Mathur Gopalakrishnan Sethuraman Facile green synthesis of fluorescent N-doped carbon dots from Actinidia deliciosa and their catalytic activity and cytotoxicity applications. Optical Materials 2017, 78, 181-190, https://doi.org/10.1016/j.optmat.2018.02.029.
- Siyu Lu, Laizhi Sui, Min Wu, Shoujun Zhu, Xue Yong and Bai Yang Graphitic Nitrogen and High-Crystalline Triggered Strong Photoluminescence and Room-Temperature Ferromagnetism in Carbonized Polymer Dots. Advanced Science 2018, 6, 2, https://doi.org/10.1002/advs.201801192.
- Zifei Wang, Fanglong Yuan, Xiaohong Li, Yunchao Li, Haizheng Zhong, Louzhen Fa and Shihe Yang 53% Efficient Red Emissive Carbon Quantum Dots for High Color Rendering and Stable Warm White-Light-Emitting Diodes. Advanced Materials 2017, 29, 37, https://doi.org/10.1002/adma.201702910.
- Lei Bao, Cui Liu, Zhi-Ling Zhang and Dai-Wen Pang Photoluminescence-Tunable Carbon Nanodots: Surface-State Energy-Gap Tuning. Advanced materials 2015, 27, 1663-1667, https://doi.org/10.1002/adma.201405070.
- Jin Gao,Mengmeng Zhu, Hui Huang, Yang Liu and Zhenhui Kang Advances, challenges and promises of carbon dots. Inorganic Chemistry Frontiers 2017, 4, 1963-1986, DOI https://doi.org/10.1039/C7QI00614D.
- Kai-Kai Liu, Shi-Yu Song, Lai-Zhi Sui, Si-Xuan Wu, Peng-Tao Jing, Ruo-Qiu Wang, Qing-Yi Li, Guo-Rong Wu, Zhen-Zhong Zhang, Kai-Jun Yuan and Chong-Xin Shan Efficient Red/Near-Infrared-Emissive Carbon Nanodots with Multiphoton Excited Upconversion Fluorescence. Advanced Science 2019, 6, 17, https://doi.org/10.1002/advs.201900766.
- Di Li, Chao Liang, Elena V. Ushakova, Minghong Sun, Xiaodan Huang, Xiaoyu Zhang, Pengtao Jing, Seung Jo Yoo, Jin-Gyu Kim, Enshan Liu, Wei Zhang, Lihong Jing, Guichuan Xing, Weitao Zheng, Zikang Tang, Songnan Qu and Andrey L. Rogach Thermally Activated Upconversion Near-Infrared Photoluminescence from Carbon Dots Synthesized via Microwave Assisted Exfoliation. Small 2019, 15, 1, https://doi.org/10.1002/smll.201905050.
- Rigu Su,Qingwen Guan, Wei Cai, Wenjing Yang, Quan Xu, Yongjian Guo, Lipeng Zhang, Ling Fei and Meng Xu*d Multi-color carbon dots for white light-emitting diodes. RSC Advances 2019, 9, 9700-9708, https://doi.org/10.1039/C8RA09868A.
- LiQin Liu, YuanFang Li, Lei Zhan, Yue Liu and ChengZhi Huang One-step synthesis of fluorescent hydroxyls-coated carbon dots with hydrothermal reaction and its application to optical sensing of metal ions. Science China Chemistry 2011, 54, 1342–1347 , https://doi.org/10.1007/s11426-011-4351-6.
- N. Tsubokawa and M. Hosoya Reactive carbon black having acyl imidazole or acid anhydride groups: Preparation and reaction with functional polymers having hydroxyl or amino groups. Reactive Polymers 1990, 14, 33-40, https://doi.org/10.1016/0923-1137(91)90245-J.
- Takashi Ogi, Kana Aishima,Fitri Aulia Permatasari, Ferry Iskandar, Eishi Tanabec and Kikuo Okuyamaa Kinetics of nitrogen-doped carbon dot formation via hydrothermal synthesis. New Journal of Chemistry 2016, 40, 5555-5561, DOI https://doi.org/10.1039/C6NJ00009F.
- Xiaojuan Gong, Qingyan Zhang, Yifang Gao, Shaomin Shuang, Martin M. F. Choi, and Chuan Dong Phosphorus and Nitrogen Dual-Doped Hollow Carbon Dot as a Nanocarrier for Doxorubicin Delivery and Biological Imaging. ACS Applied Materials & Interfaces 2016, 18, 11288–11297, https://doi.org/10.1021/acsami.6b01577.
- Shanshan Wang, Dong-Sheng Yang and Fuqian Yang Nitrogen-induced shift of photoluminescence from green to blue emission for xylose-derived carbon dots. Nano Express 2020, 1, 2, 10.1088/2632-959X/aba771.
- Dr. Feng Li, Prof. Dayong Yang, Prof and Huaping Xu Non-Metal-Heteroatom-Doped Carbon Dots: Synthesis and Properties. Chemistry: A European Journal 2018, 25, 1165-1176, https://doi.org/10.1002/chem.201802793.
- Yeji Kim and Jongsung Kim Bioinspired thiol functionalized carbon dots for rapid detection of lead (II) ions in human serum. Optical Materials 2020, 99, 1, https://doi.org/10.1016/j.optmat.2019.109514.
- Jin Zhou, Xiaoyue Shan,Juanjuan Ma, Yamin Gu, Zhaosheng Qian, Jianrong Chena and Hui Feng Facile synthesis of P-doped carbon quantum dots with highly efficient photoluminescence. RSC Advances 2013, 4, 5465-5468, https://doi.org/10.1039/C3RA45294H.
- Lei Wang, Shou-Jun Zhu, Hai-Yu Wang, Song-Nan Qu, Yong-Lai Zhang, Jun-Hu Zhang, Qi-Dai Chen, Huai-Liang Xu, Wei Han, Bai Yang, and Hong-Bo Sun Common Origin of Green Luminescence in Carbon Nanodots and Graphene Quantum Dots. ACS Nano 2014, 3, 2541–2547, https://doi.org/10.1021/nn500368m.
- Huifang Wu and Changlun Tong Nitrogen- and Sulfur-Codoped Carbon Dots for Highly Selective and Sensitive Fluorescent Detection of Hg2+ Ions and Sulfide in Environmental Water Samples. Journal of Agricultural and Food Chemistry 2019, 10, 2794–2800, https://doi.org/10.1021/acs.jafc.8b07176.
- Zhiguo Ye,Yanhui Zhang,Guixin Li and Baoxin Li Fluorescent Determination of Mercury(II) by Green Carbon Quantum Dots Synthesized from Eggshell Membrane. Analytical Letters 2020, 53, 18, https://doi.org/10.1080/00032719.2020.1759618.
- RETURN TO ISSUEPREVARTICLENEXT Pineapple Peel-Derived Carbon Dots: Applications as Sensor, Molecular Keypad Lock, and Memory Device Somasundaram Anbu Anjugam Vandarkuzhali, Sampathkumar Natarajan, Shanmugapriya Jeyabalan, Gandhi Sivaraman*, Subramanian Singaravadivel*, Shanmugam Muthusubramanian, and Balasubramanian Viswanathan Pineapple Peel-Derived Carbon Dots: Applications as Sensor, Molecular Keypad Lock, and Memory Device. ACS Omega 2018, 10, 12584–12592, https://doi.org/10.1021/acsomega.8b01146.
- Raji Atchudan , Thomas Nesakumar Jebakumar Immanuel Edison , Kanikkai Raja Aseer , Suguna Perumal , Namachivayam Karthik and Yong Rok Lee Highly fluorescent nitrogen-doped carbon dots derived from Phyllanthus acidus utilized as a fluorescent probe for label-free selective detection of Fe3+ ions, live cell imaging and fluorescent ink. Biosensors and Bioelectronics 2017, 99, 303-311, https://doi.org/10.1016/j.bios.2017.07.076.
- Wassana Yantasee , Yuehe Lin, Kitiya Hongsirikarn, Glen E Fryxell, Raymond Addleman and Charles Timchalk Electrochemical sensors for the detection of lead and other toxic heavy metals: the next generation of personal exposure biomonitors. Environ Health Perspect 2007, 12, 1683-90, DOI: 10.1289/ehp.10190.
- M S Bhatia and R Gupta, S Srivastava Migraine associated with water deprivation and progressive myopia. Cephalalgia . 2006, 6, 758-760, DOI: 10.1111/j.1468-2982.2006.01083.x.
- Papanikolaou NC , Hatzidaki EG , Belivanis S , Tzanakakis GN and Tsatsakis AM Lead toxicity update. A brief review. Med Sci Monit . 2005, 26, 1, doi: 10.2147/IMCRJ.S404885.
- Wassana Yantasee , Yuehe Lin, Kitiya Hongsirikarn, Glen E Fryxell, Raymond Addleman and Charles Timchalk Electrochemical sensors for the detection of lead and other toxic heavy metals: the next generation of personal exposure biomonitors. Environ Health Perspect . 2007, 12, 90, DOI: 10.1289/ehp.10190.
- Xiaofang Niu, Yanjun Liu, Fei Wang and D. Luo Highly sensitive and selective optical sensor for lead ion detection based on liquid crystal decorated with DNAzyme. Optics express 2019, 1, 1, DOI:10.1364/oe.27.030421.
- Federica Paladini and Mauro Pollini Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 16, 2540, doi: 10.3390/ma12162540.
- Dapeng Chen, X. Qiao, X. Qiu and Jianguo Chen less Synthesis and electrical properties of uniform silver nanoparticles for electronic applications. Journal of Materials Science 2009, 1, 1, DOI:10.1007/S10853-008-3204-Y.
- S. Syed Silver recovery aqueous techniques from diverse sources: Hydrometallurgy in recycling. Waste Management 2016, 50, 234-256, https://doi.org/10.1016/j.wasman.2016.02.006.
- Germarie Sánchez-Pomales , Thilak K Mudalige, Jin-Hee Lim and Sean W Linder Rapid determination of silver in nanobased liquid dietary supplements using a portable X-ray fluorescence analyzer. Journal of Agricultural and Food Chemistry 2013 , 30, 1, DOI: 10.1021/jf402018t.
- Melissa May Fung Chang, Irine Runnie Ginjom, Maria Ngu-Schwemlein and Sing Muk Ng Synthesis of yellow fluorescent carbon dots and their application to the determination of chromium(III) with selectivity improved by pH tuning. Microchimica Acta 2016, 183, 1899–1907 , DOI 10.1007/s00604-016-1819-2.
- Elizabeta Nemeth and Tomas Ganz The role of hepcidin in iron metabolism. Acta Haematol 2009, 2, 78-86, DOI: 10.1159/000243791.
- Judith A Simcox and Donald A McClain Iron and diabetes risk. Cell Metab 2013, 3, 329-41, DOI: 10.1016/j.cmet.2013.02.007.
- Guanxiong Liu , Baoqiang Li , Ying Liu , Yujie Feng , Dechang Jia and , Yu Zhou Rapid and high yield synthesis of carbon dots with chelating ability derived from acrylamide/chitosan for selective detection of ferrous ions. Applied Surface Science 2009, 487, 1167-1175, https://doi.org/10.1016/j.apsusc.2019.05.069.
- Swarnkar, S. R.; Gupta, B. L. and Sekharan, R. Dhana Iron control in zinc plant residue leach solution. Hydrometallurgy, 1996, 42, 21-26, 10.1016/0304-386X(95)00077-T .
- Jing Shi, G. Ni, Jinchun Tu, Xiaoyong Jin and Juan Peng less Green synthesis of fluorescent carbon dots for sensitive detection of Fe2+ and hydrogen peroxide. Journal of Nanoparticle Research 2017, 1, 1, DOI:10.1007/s11051-017-3888-5.
- Daniel López de Romaña , Manuel Olivares, Ricardo Uauy and Magdalena Araya Risks and benefits of copper in light of new insights of copper homeostasis. Journal of Trace Elements in Medicine and Biology 2011, 1, 3-13, doi: 10.1016/j.jtemb.2010.11.004..
- Peng Wang , Yonghui Yuan , Ke Xu , Hongshan Zhong , Yinghui Yang , Shiyu Jin , Ke Yang and Xun Qi Biological applications of copper-containing materials. Bioactive Materials 2021, 6, 916-927, https://doi.org/10.1016/j.bioactmat.2020.09.017.
- Alfonso Salinas-Castillo, Maria Ariza-Avidad, Christian Pritz, Maria Camprubí-Robles, Belen Fernández, Maria J. Ruedas-Rama, Alicia Megia-Fernández, Alejandro Lapresta-Fernández, Francisco Santoyo-Gonzalez, Annelies Schrott-Fischer and Luis F. Capitan-Vallvey Carbon dots for copper detection with down and upconversion fluorescent properties as excitation sources. Chemical Communications 2012, 49, 1103-1105, DOI https://doi.org/10.1039/C2CC36450F.
- Van Dien Dang, Akhilesh Babu Ganganboina, and Ruey-An Doong Bipyridine- and Copper-Functionalized N-doped Carbon Dots for Fluorescence Turn Off–On Detection of Ciprofloxacin. ACS Appl. Mater. Interfaces 2020, 29, 32247–32258, https://doi.org/10.1021/acsami.0c04645.
- Van Dien Dang, Akhilesh Babu Ganganboina, and Ruey-An Doong Bipyridine- and Copper-Functionalized N-doped Carbon Dots for Fluorescence Turn Off–On Detection of Ciprofloxacin. ACS Appl. Mater. Interfaces 2020, 29, 32247–32258, https://doi.org/10.1021/acsami.0c04645.
- Dr. Xiaochun Zheng, Prof. Wenjun Liu, Dr. Qixiao Gai, Prof. Zhaoshuo Tian and Prof. Shoutian Ren A Carbon-Dot-Based Fluorescent Probe for the Sensitive and Selective Detection of Copper(II) Ions. ChemistrySelect 2019, 4, 2392-2397, https://doi.org/10.1002/slct.201803584.




