
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Trovato | -- | 2604 | 2023-06-29 08:55:52 | | | |
| 2 | Lindsay Dong | Meta information modification | 2604 | 2023-06-29 11:09:52 | | |
Video Upload Options
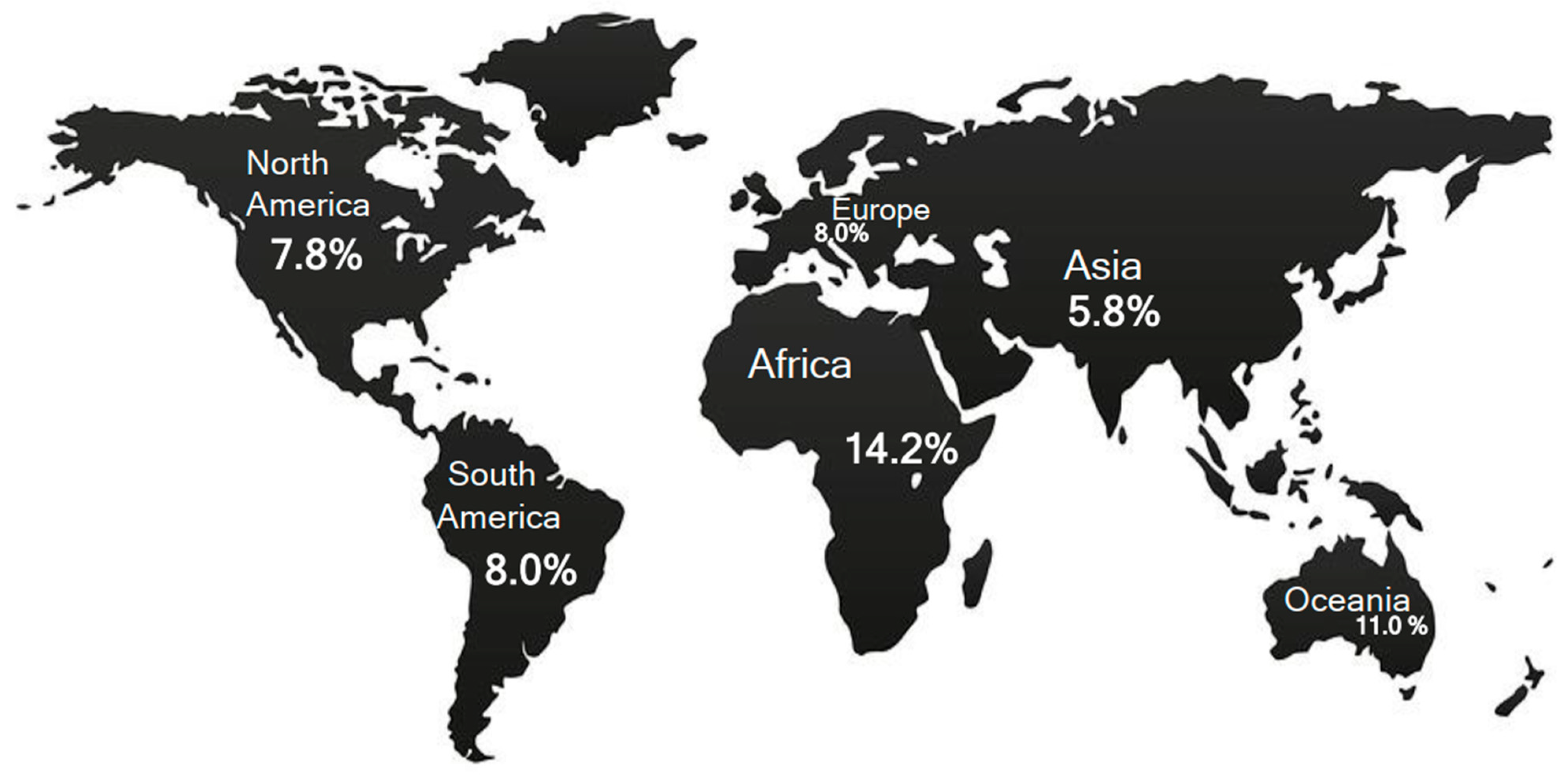
Hashimoto’s thyroiditis (HT) is a gender autoimmune disease that is manifested by chronic inflammation of the thyroid. Clinical trial studies (CTSs) use molecular biotechnologies (MB) to approach HT appearance.
1. Introduction
1.1. HT Biomarkers and Epidemiological Data
1.2. Prevalence of HT Diagnoses
1.3. Molecular Biotechnologies (MB) and HT
2. Medical Applications of Molecular Biotechnologies in Hashimoto’s Thyroiditis
HT may appear through different clinical and histological aspects, and thus, morphological and serum diagnoses of HT are not equivalent [4]. In addition, HT may be associated with benign and malignant follicular lesions as well as lymphomatous proliferations [38][41][42]. The exact etiology of HT still remains incompletely elucidated. Mainly, it has been related to interactions of different elements, such as genetic alterations, environmental and epigenetic factors [30][46][47]. MB are promising surveying methods to apply to the HT population.
References
- Yoo, W.S.; Chung, H.K. Recent Advances in Autoimmune Thyroid Diseases. Endocrinol. Metab. 2016, 31, 379.
- Bliddal, S.; Nielsen, C.H.; Feldt-Rasmussen, U. Recent Advances in Understanding Autoimmune Thyroid Disease: The Tallest Tree in the Forest of Polyautoimmunity. F1000Research 2017, 6, 1776.
- Antonelli, A.; Ferrari, S.M.; Corrado, A.; Di Domenicantonio, A.; Fallahi, P. Autoimmune Thyroid Disorders. Autoimmun. Rev. 2015, 14, 174–180.
- Trovato, M. A Historical Excursus of Diagnostic Methods for Hashimoto Thyroiditis and Graves’ Disease. Gazz. Med. Ital. Arch. Sci. Med. 2020, 179, 479–485.
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-Thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521.
- Bogusławska, J.; Godlewska, M.; Gajda, E.; Piekiełko-Witkowska, A. Cellular and Molecular Basis of Thyroid Autoimmunity. Eur. Thyroid J. 2022, 11, e210024.
- Thomas, T.; Sreedharan, S.; Khadilkar, U.N.; Deviprasad, D.; Kamath, M.P.; Bhojwani, K.M.; Alva, A. Clinical, Biochemical & Cytomorphologic Study on Hashimoto’s Thyroiditis. Indian J. Med. Res. 2014, 140, 729–735.
- Kahaly, G.J.; Gottwald-Hostalek, U. Use of Levothyroxine in the Management of Hypothyroidism: A Historical Perspective. Front. Endocrinol. 2022, 13, 1054983.
- Okosieme, O.; Gilbert, J.; Abraham, P.; Boelaert, K.; Dayan, C.; Gurnell, M.; Leese, G.; McCabe, C.; Perros, P.; Smith, V.; et al. Management of Primary Hypothyroidism: Statement by the British Thyroid Association Executive Committee. Clin. Endocrinol. 2016, 84, 799–808.
- Jordan, B.; Uer, O.; Buchholz, T.; Spens, A.; Zierz, S. Physical Fatigability and Muscle Pain in Patients with Hashimoto Thyroiditis. J. Neurol. 2021, 268, 2441–2449.
- Lei, Y.; Yang, J.; Li, H.; Zhong, H.; Wan, Q. Changes in Glucose-lipid Metabolism, Insulin Resistance, and Inflammatory Factors in Patients with Autoimmune Thyroid Disease. J. Clin. Lab. Anal. 2019, 33, e22929.
- Waliszewska-Prosół, M.; Bladowska, J.; Budrewicz, S.; Sąsiadek, M.; Dziadkowiak, E.; Ejma, M. The Evaluation of Hashimoto’s Thyroiditis with Event-Related Potentials and Magnetic Resonance Spectroscopy and Its Relation to Cognitive Function. Sci. Rep. 2021, 11, 2480.
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014, 24, 1670–1751.
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562.
- Chaker, L.; Razvi, S.; Bensenor, I.M.; Azizi, F.; Pearce, E.N.; Peeters, R.P. Hypothyroidism. Nat. Rev. Dis. Prim. 2022, 8, 30.
- Razvi, S.; Korevaar, T.I.M.; Taylor, P. Trends, Determinants, and Associations of Treated Hypothyroidism in the United Kingdom, 2005–2014. Thyroid 2019, 29, 174–182.
- Wiersinga, W.M.; Duntas, L.; Fadeyev, V.; Nygaard, B.; Vanderpump, M.P.J. 2012 ETA Guidelines: The Use of L-T4 + L-T3 in the Treatment of Hypothyroidism. Eur. Thyroid J. 2012, 1, 55–71.
- Jonklaas, J.; Bianco, A.C.; Cappola, A.R.; Celi, F.S.; Fliers, E.; Heuer, H.; McAninch, E.A.; Moeller, L.C.; Nygaard, B.; Sawka, A.M.; et al. Evidence-Based Use of Levothyroxine/Liothyronine Combinations in Treating Hypothyroidism: A Consensus Document. Thyroid 2021, 31, 156–182.
- Lillevang-Johansen, M.; Abrahamsen, B.; Jørgensen, H.L.; Brix, T.H.; Hegedüs, L. Over- and Under-Treatment of Hypothyroidism Is Associated with Excess Mortality: A Register-Based Cohort Study. Thyroid 2018, 28, 566–574.
- Thayakaran, R.; Adderley, N.J.; Sainsbury, C.; Torlinska, B.; Boelaert, K.; Šumilo, D.; Price, M.; Thomas, G.N.; Toulis, K.A.; Nirantharakumar, K. Thyroid Replacement Therapy, Thyroid Stimulating Hormone Concentrations, and Long Term Health Outcomes in Patients with Hypothyroidism: Longitudinal Study. BMJ 2019, 366, l4892.
- Davies, T.F.; Morshed, S.A.; Mezei, M.; Latif, R. Brief Report—Monoclonal Antibodies Illustrate the Difficulties in Measuring Blocking TSH Receptor Antibodies. Front. Endocrinol. 2022, 13, 943459.
- Peterson, S.J.; Cappola, A.R.; Castro, M.R.; Dayan, C.M.; Farwell, A.P.; Hennessey, J.V.; Kopp, P.A.; Ross, D.S.; Samuels, M.H.; Sawka, A.M.; et al. An Online Survey of Hypothyroid Patients Demonstrates Prominent Dissatisfaction. Thyroid 2018, 28, 707–721.
- Perros, P.; Hegedüs, L.; Nagy, E.V.; Papini, E.; Hay, H.A.; Abad-Madroñero, J.; Tallett, A.J.; Bilas, M.; Lakwijk, P.; Poots, A.J. The Impact of Hypothyroidism on Satisfaction with Care and Treatment and Everyday Living: Results from E-Mode Patient Self-Assessment of Thyroid Therapy, a Cross-Sectional, International Online Patient Survey. Thyroid 2022, 32, 1158–1168.
- Bjerkreim, B.A.; Hammerstad, S.S.; Gulseth, H.L.; Berg, T.J.; Omdal, L.J.; Lee-Ødegård, S.; Eriksen, E.F. Effect of Liothyronine Treatment on Quality of Life in Female Hypothyroid Patients With Residual Symptoms on Levothyroxine Therapy: A Randomized Crossover Study. Front. Endocrinol. 2022, 13, 816566.
- Perros, P.; Nirantharakumar, K.; Hegedüs, L. Recent Evidence Sets Therapeutic Targets for Levothyroxine-Treated Patients with Primary Hypothyroidism Based on Risk of Death. Eur. J. Endocrinol. 2021, 184, C1–C3.
- Castello, R.; Caputo, M. Thyroid Diseases and Gender. Ital. J. Gend.-Specif. Med. 2019, 5, 136–141.
- Matana, A.; Popović, M.; Boutin, T.; Torlak, V.; Brdar, D.; Gunjača, I.; Kolčić, I.; Boraska Perica, V.; Punda, A.; Polašek, O.; et al. Genome-Wide Meta-Analysis Identifies Novel Gender Specific Loci Associated with Thyroid Antibodies Level in Croatians. Genomics 2019, 111, 737–743.
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ Thyroiditis: Epidemiology, Pathogenesis, Clinic and Therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367.
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Roliński, J. Immune Disorders in Hashimoto’s Thyroiditis: What Do We Know So Far? J. Immunol. Res. 2015, 2015, 979167.
- Vargas-Uricoechea, H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells 2023, 12, 918.
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global Prevalence and Epidemiological Trends of Hashimoto’s Thyroiditis in Adults: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 1020709.
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-Deficiency Disorders. Lancet 2008, 372, 1251–1262.
- Andersson, M.; Karumbunathan, V.; Zimmermann, M.B. Global Iodine Status in 2011 and Trends over the Past Decade. J. Nutr. 2012, 142, 744–750.
- Zimmermann, M.B. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408.
- Devdhar, M.; Drooger, R.; Pehlivanova, M.; Singh, G.; Jonklaas, J. Levothyroxine Replacement Doses Are Affected by Gender and Weight, But Not Age. Thyroid 2011, 21, 821–827.
- Poste, G.; Carbone, D.P.; Parkinson, D.R.; Verweij, J.; Hewitt, S.M.; Jessup, J.M. Leveling the Playing Field: Bringing Development of Biomarkers and Molecular Diagnostics up to the Standards for Drug Development. Clin. Cancer Res. 2012, 18, 1515–1523.
- Troch, M.; Woehrer, S.; Streubel, B.; Weissel, M.; Hoffmann, M.; Müllauer, L.; Chott, A.; Raderer, M. Chronic Autoimmune Thyroiditis (Hashimoto’s Thyroiditis) in Patients with MALT Lymphoma. Ann. Oncol. 2008, 19, 1336–1339.
- Trovato, M.; Giuffrida, G.; Seminara, A.; Fogliani, S.; Cavallari, V.; Ruggeri, R.M.; Campennì, A. Coexistence of Diffuse Large B-Cell Lymphoma and Papillary Thyroid Carcinoma in a Patient Affected by Hashimoto’s Thyroiditis. Arch. Endocrinol. Metab. 2017, 61, 643–646.
- Anil, C.; Goksel, S.; Gursoy, A. Hashimoto’s Thyroiditis Is Not Associated with Increased Risk of Thyroid Cancer in Patients with Thyroid Nodules: A Single-Center Prospective Study. Thyroid 2010, 20, 601–606.
- Chen, Y.-K.; Lin, C.-L.; Cheng, F.T.-F.; Sung, F.-C.; Kao, C.-H. Cancer Risk in Patients with Hashimoto’s Thyroiditis: A Nationwide Cohort Study. Br. J. Cancer 2013, 109, 2496–2501.
- Resende De Paiva, C.; Grønhøj, C.; Feldt-Rasmussen, U.; Von Buchwald, C. Association between Hashimoto’s Thyroiditis and Thyroid Cancer in 64,628 Patients. Front. Oncol. 2017, 7, 53.
- Pavlidis, E.T.; Pavlidis, T.E. A Review of Primary Thyroid Lymphoma: Molecular Factors, Diagnosis and Management. J. Investig. Surg. 2019, 32, 137–142.
- Rodríguez-Sevilla, J.J.; Salar, A. Recent Advances in the Genetic of MALT Lymphomas. Cancers 2021, 14, 176.
- Rossi, E.D.; Vielh, P. Thyroid and Molecular Testing. Advances in Thyroid Molecular Cytopathology. J. Mol. Pathol. 2021, 2, 77–92.
- Trovato, M. Update on International Medical Taxonomies of Biomarkers and Their Applications in Management of Thyroid Cancers. Diagnostics 2022, 12, 662.
- Lee, H.J.; Stefan–Lifshitz, M.; Li, C.W.; Tomer, Y. Genetics and Epigenetics of Autoimmune Thyroid Diseases: Translational Implications. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101661.
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; De Vincentiis, M.; Greco, A. Hashimoto’s Thyroiditis: An Update on Pathogenic Mechanisms, Diagnostic Protocols, Therapeutic Strategies, and Potential Malignant Transformation. Autoimmun. Rev. 2020, 19, 102649.
- Strachan, D.P. Hay Fever, Hygiene, and Household Size. BMJ 1989, 299, 1259–1260.
- Tomer, Y.; Davies, T.F. Infection, Thyroid Disease, and Autoimmunity. Endocr. Rev. 1993, 14, 107–120.
- Desailloud, R.; Hober, D. Viruses and Thyroiditis: An Update. Virol. J. 2009, 6, 5.
- Morohoshi, K.; Takahashi, Y.; Mori, K. Viral Infection and Innate Pattern Recognition Receptors in Induction of Hashimoto’s Thyroiditis. Discov. Med. 2011, 12, 505–511.
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.-F. The ‘Hygiene Hypothesis’ for Autoimmune and Allergic Diseases: An Update. Clin. Exp. Immunol. 2010, 160, 1–9.
- Versini, M.; Jeandel, P.-Y.; Bashi, T.; Bizzaro, G.; Blank, M.; Shoenfeld, Y. Unraveling the Hygiene Hypothesis of Helminthes and Autoimmunity: Origins, Pathophysiology, and Clinical Applications. BMC Med. 2015, 13, 81.
- Garn, H.; Potaczek, D.P.; Pfefferle, P.I. The Hygiene Hypothesis and New Perspectives—Current Challenges Meeting an Old Postulate. Front. Immunol. 2021, 12, 637087.
- Kondrashova, A.; Seiskari, T.; Ilonen, J.; Knip, M.; Hyöty, H. The ‘Hygiene Hypothesis’ and the Sharp Gradient in the Incidence of Autoimmune and Allergic Diseases between Russian Karelia and Finland. APMIS 2013, 121, 478–493.
- Detels, R.; Brody, J.A.; Edgar, A.H. Multiple Sclerosis among American, Japanese and Chinese Migrants to California and Washington. J. Chronic Dis. 1972, 25, 3–10.
- Leibowitz, U.; Kahana, E.; Alter, M. The Changing Frequency of Multiple Sclerosis in Israel. Arch. Neurol. 1973, 29, 107–110.
- Bodansky, H.J.; Staines, A.; Stephenson, C.; Haigh, D.; Cartwright, R. Evidence for an Environmental Effect in the Aetiology of Insulin Dependent Diabetes in a Transmigratory Population. BMJ 1992, 304, 1020–1022.
- Staines, A.; Hanif, S.; Ahmed, S.; McKinney, P.A.; Shera, S.; Bodansky, H.J. Incidence of Insulin Dependent Diabetes Mellitus in Karachi, Pakistan. Arch. Dis. Child. 1997, 76, 121–123.
- Hammond, S.R. The Age-Range of Risk of Developing Multiple Sclerosis: Evidence from a Migrant Population in Australia. Brain 2000, 123, 968–974.
- Bach, J.-F. Revisiting the Hygiene Hypothesis in the Context of Autoimmunity. Front. Immunol. 2021, 11, 615192.
- Krassas, G.E.; Tziomalos, K.; Pontikides, N.; Lewy, H.; Laron, Z. Seasonality of Month of Birth of Patients with Graves’ and Hashimoto’s Diseases Differ from That in the General Population. Eur. J. Endocrinol. 2007, 156, 631–636.
- Attard, C.C.; Sze, W.C.C.; Vella, S. Predictors of Autoimmune Thyroid Disease. Bayl. Univ. Med. Cent. Proc. 2022, 35, 608–614.
- Flicek, P.; Amode, M.R.; Barrell, D.; Beal, K.; Brent, S.; Chen, Y.; Clapham, P.; Coates, G.; Fairley, S.; Fitzgerald, S.; et al. Ensembl 2011. Nucleic Acids Res. 2011, 39, D800–D806.
- Borchers, C.H.; Kast, J.; Foster, L.J.; Siu, K.W.M.; Overall, C.M.; Binkowski, T.A.; Hildebrand, W.H.; Scherer, A.; Mansoor, M.; Keown, P.A. The Human Proteome Organization Chromosome 6 Consortium: Integrating Chromosome-Centric and Biology/Disease Driven Strategies. J. Proteom. 2014, 100, 60–67.
- Kulski, J.K.; Suzuki, S.; Shiina, T. Human Leukocyte Antigen Super-Locus: Nexus of Genomic Supergenes, SNPs, Indels, Transcripts, and Haplotypes. Hum. Genome Var. 2022, 9, 49.
- Zaletel, K.; Gaberšček, S. Hashimoto’s Thyroiditis: From Genes to the Disease. Curr. Genom. 2011, 12, 576–588.
- Douillard, V.; Castelli, E.C.; Mack, S.J.; Hollenbach, J.A.; Gourraud, P.-A.; Vince, N.; Limou, S. Approaching Genetics Through the MHC Lens: Tools and Methods for HLA Research. Front. Genet. 2021, 12, 774916.
- De Santis, D.; Truong, L.; Martinez, P.; D’Orsogna, L. Rapid High-resolution HLA Genotyping by MinION Oxford Nanopore Sequencing for Deceased Donor Organ Allocation. HLA 2020, 96, 141–162.
- Zawadzka-Starczewska, K.; Tymoniuk, B.; Stasiak, B.; Lewiński, A.; Stasiak, M. Actual Associations between HLA Haplotype and Graves’ Disease Development. J. Clin. Med. 2022, 11, 2492.
- Stasiak, M.; Zawadzka-Starczewska, K.; Tymoniuk, B.; Stasiak, B.; Lewiński, A. Significance of HLA in the Development of Graves’ Orbitopathy. Genes Immun. 2023, 24, 32–38.
- Liao, W.-L.; Liu, T.-Y.; Cheng, C.-F.; Chou, Y.-P.; Wang, T.-Y.; Chang, Y.-W.; Chen, S.-Y.; Tsai, F.-J. Analysis of HLA Variants and Graves’ Disease and Its Comorbidities Using a High Resolution Imputation System to Examine Electronic Medical Health Records. Front. Endocrinol. 2022, 13, 842673.
- Mori, K.; Yoshida, K. Viral Infection in Induction of Hashimotoʼs Thyroiditis: A Key Player or Just a Bystander? Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 418–424.
- Weider, T.; Genoni, A.; Broccolo, F.; Paulsen, T.H.; Dahl-Jørgensen, K.; Toniolo, A.; Hammerstad, S.S. High Prevalence of Common Human Viruses in Thyroid Tissue. Front. Endocrinol. 2022, 13, 938633.
- Wang, J.; Zhang, W.; Liu, H.; Wang, D.; Wang, W.; Li, Y.; Wang, Z.; Wang, L.; Zhang, W.; Huang, G. Parvovirus B19 Infection Associated with Hashimoto’s Thyroiditis in Adults. J. Infect. 2010, 60, 360–370.
- Heidari, Z.; Jami, M. Parvovirus B19 Infection Is Associated with Autoimmune Thyroid Disease in Adults. Int. J. Endocrinol. Metab. 2021, 19, e115592.
- Pastore, F. Hepatitis C Virus Infection and Thyroid Autoimmune Disorders: A Model of Interactions between the Host and the Environment. WJH 2016, 8, 83.
- Caselli, E.; Zatelli, M.C.; Rizzo, R.; Benedetti, S.; Martorelli, D.; Trasforini, G.; Cassai, E.; Degli Uberti, E.C.; Di Luca, D.; Dolcetti, R. Virologic and Immunologic Evidence Supporting an Association between HHV-6 and Hashimoto’s Thyroiditis. PLoS Pathog. 2012, 8, e1002951.
- Seyyedi, N.; Dehbidi, G.R.; Karimi, M.; Asgari, A.; Esmaeili, B.; Zare, F.; Farhadi, A.; Dabbaghmanesh, M.H.; Saki, F.; Behzad-Behbahani, A. Human Herpesvirus 6A Active Infection in Patients with Autoimmune Hashimoto’s Thyroiditis. Braz. J. Infect. Dis. 2019, 23, 435–440.
- Trovato, M.; Sciacchitano, S.; Facciolà, A.; Valenti, A.; Visalli, G.; Di Pietro, A. Interleukin-6 Signalling as a Valuable Cornerstone for Molecular Medicine (Review). Int. J. Mol. Med. 2021, 47, 107.
- Trovato, M.; Ruggeri, R.M.; Sciacchitano, S.; Vicchio, T.M.; Picerno, I.; Pellicanò, G.; Valenti, A.; Visalli, G. Serum Interleukin-6 Levels Are Increased in HIV-Infected Patients That Develop Autoimmune Disease during Long-Term Follow-Up. Immunobiology 2018, 223, 264–268.
- Lin, C.-Y.; Chung, Y.-H.; Shi, Y.-F.; Tzang, B.-S.; Hsu, T.-C. The VP1 Unique Region of Human Parvovirus B19 and Human Bocavirus Induce Lung Injury in Naïve Balb/c Mice. PLoS ONE 2018, 13, e0202667.
- Canuti, M.; Eis-Huebinger, A.M.; Deijs, M.; De Vries, M.; Drexler, J.F.; Oppong, S.K.; Müller, M.A.; Klose, S.M.; Wellinghausen, N.; Cottontail, V.M.; et al. Two Novel Parvoviruses in Frugivorous New and Old World Bats. PLoS ONE 2011, 6, e29140.
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.-M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J. Gen. Virol. 2019, 100, 367–368.
- Pénzes, J.J.; Söderlund-Venermo, M.; Canuti, M.; Eis-Hübinger, A.M.; Hughes, J.; Cotmore, S.F.; Harrach, B. Reorganizing the Family Parvoviridae: A Revised Taxonomy Independent of the Canonical Approach Based on Host Association. Arch. Virol. 2020, 165, 2133–2146.
- Lozano-Fernandez, J.; Carton, R.; Tanner, A.R.; Puttick, M.N.; Blaxter, M.; Vinther, J.; Olesen, J.; Giribet, G.; Edgecombe, G.D.; Pisani, D. A Molecular Palaeobiological Exploration of Arthropod Terrestrialization. Phil. Trans. R. Soc. B 2016, 371, 20150133.
- Lozano-Fernandez, J.; Tanner, A.R.; Puttick, M.N.; Vinther, J.; Edgecombe, G.D.; Pisani, D. A Cambrian–Ordovician Terrestrialization of Arachnids. Front. Genet. 2020, 11, 182.
- Pénzes, J.J.; De Souza, W.M.; Agbandje-McKenna, M.; Gifford, R.J. An Ancient Lineage of Highly Divergent Parvoviruses Infects Both Vertebrate and Invertebrate Hosts. Viruses 2019, 11, 525.
- Cossart, Y.E.; Cant, B.; Field, A.M.; Widdows, D. Parvovirus-like particles in human sera. Lancet 1975, 305, 72–73.
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. Cloning of a Human Parvovirus by Molecular Screening of Respiratory Tract Samples. Proc. Natl. Acad. Sci. USA 2005, 102, 12891–12896.
- Lehmann, H.W.; Von Landenberg, P.; Modrow, S. Parvovirus B19 Infection and Autoimmune Disease. Autoimmun. Rev. 2003, 2, 218–223.
- Adamson, L.A.; Fowler, L.J.; Clare-Salzler, M.J.; Hobbs, J.A. Parvovirus B19 Infection in Hashimoto’s Thyroiditis, Papillary Thyroid Carcinoma, and Anaplastic Thyroid Carcinoma. Thyroid 2011, 21, 411–417.
- Wang, J.H.; Zhang, W.P.; Liu, H.X.; Wang, D.; Li, Y.F.; Wang, W.Q.; Wang, L.; He, F.R.; Wang, Z.; Yan, Q.G.; et al. Detection of Human Parvovirus B19 in Papillary Thyroid Carcinoma. Br. J. Cancer 2008, 98, 611–618.
- Gravelsina, S.; Nora-Krukle, Z.; Svirskis, S.; Cunskis, E.; Murovska, M. Presence of B19V in Patients with Thyroid Gland Disorders. Medicina 2019, 55, 774.
- Wang, X.; Xu, P.; Cheng, F.; Li, Y.; Wang, Z.; Hao, S.; Wang, J.; Ning, K.; Ganaie, S.S.; Engelhardt, J.F.; et al. Cellular Cleavage and Polyadenylation Specificity Factor 6 (CPSF6) Mediates Nuclear Import of Human Bocavirus 1 NP1 Protein and Modulates Viral Capsid Protein Expression. J. Virol. 2020, 94, e01444-19.
- Sowd, G.A.; Serrao, E.; Wang, H.; Wang, W.; Fadel, H.J.; Poeschla, E.M.; Engelman, A.N. A Critical Role for Alternative Polyadenylation Factor CPSF6 in Targeting HIV-1 Integration to Transcriptionally Active Chromatin. Proc. Natl. Acad. Sci. USA 2016, 113, E1054–E1063.
- Zheng, Y.; Schubert, H.L.; Singh, P.K.; Martins, L.J.; Engelman, A.N.; D’Orso, I.; Hill, C.P.; Planelles, V. Cleavage and Polyadenylation Specificity Factor 6 Is Required for Efficient HIV-1 Latency Reversal. mBio 2021, 12, e01098-21.
- Mattola, S.; Hakanen, S.; Salminen, S.; Aho, V.; Mäntylä, E.; Ihalainen, T.O.; Kann, M.; Vihinen-Ranta, M. Concepts to Reveal Parvovirus–Nucleus Interactions. Viruses 2021, 13, 1306.




