Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liangxin Fan | -- | 1590 | 2023-06-28 13:56:44 | | | |
| 2 | Jessie Wu | Meta information modification | 1590 | 2023-06-29 05:19:40 | | | | |
| 3 | Jessie Wu | Meta information modification | 1590 | 2023-06-29 05:21:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fan, L.; Zhu, X.; Liu, X.; He, F.; Yang, G.; Xu, C.; Yang, X. Intramolecular Cyclization. Encyclopedia. Available online: https://encyclopedia.pub/entry/46176 (accessed on 02 March 2026).
Fan L, Zhu X, Liu X, He F, Yang G, Xu C, et al. Intramolecular Cyclization. Encyclopedia. Available at: https://encyclopedia.pub/entry/46176. Accessed March 02, 2026.
Fan, Liangxin, Xinxin Zhu, Xingyuan Liu, Fangyu He, Guoyu Yang, Cuilian Xu, Xifa Yang. "Intramolecular Cyclization" Encyclopedia, https://encyclopedia.pub/entry/46176 (accessed March 02, 2026).
Fan, L., Zhu, X., Liu, X., He, F., Yang, G., Xu, C., & Yang, X. (2023, June 28). Intramolecular Cyclization. In Encyclopedia. https://encyclopedia.pub/entry/46176
Fan, Liangxin, et al. "Intramolecular Cyclization." Encyclopedia. Web. 28 June, 2023.
Copy Citation
3,n-fused (n = 4–7) tricyclic indoles are pervasive motifs, embedded in a variety of biologically active molecules and natural products. Thus, numerous catalytic methods have been developed for the synthesis of these skeletons.
palladium
tricyclic indoles
domino reaction
catalyst
total synthesis
1. Introduction
Indole is one of the most significant nitrogen heterocycles, owing to its unique biological activity and wide existence in numerous natural products, bioactive molecules, pharmaceuticals, and functional materials [1][2][3][4][5][6]. Among the numerous indole alkaloids, 3,n-fused (n = 4–7) tricyclic indoles have attracted much attention for their typical biological activities and synthetic challenges in recent years [7][8]. The tricyclic indole nucleus is found in a myriad of natural products and pharmaceutical molecules (Scheme 1), such as HKI 0231B [9], dehydrobufotenine [10], ambiguine H [11], tryptorubin A [12], celogentin C [13], chloropeptin I [14], and streptide [15]. Accordingly, a number of strategies have been established for the synthesis of these skeletons, including intramolecular Fischer indole synthesis, Witkop photocyclizations, Diels-Alder reactions, Friedel-Crafts reactions, Pictet-Spengler reactions, 6π-electrocyclizations, and transition-metal-catalyzed domino reactions.
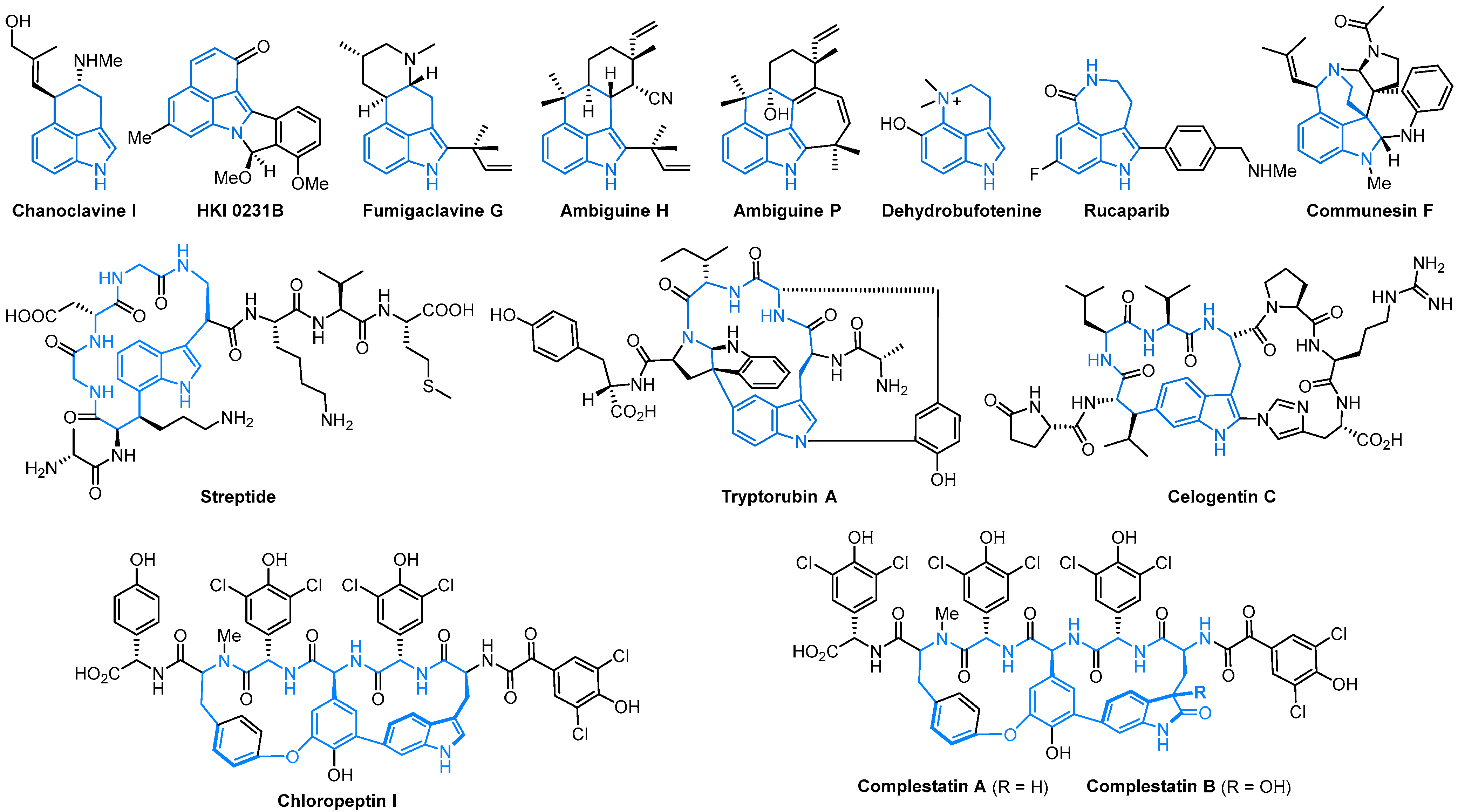
Scheme 1. Representative examples of biologically active molecules containing tricyclic indole cores.
In recent years, numerous advances have been achieved via transition-metal-catalyzed domino reaction. Among them, palladium has been widely used as a versatile catalyst for their typical atom orbital to get a series of tricyclic indole skeletons. The seminal work on the synthesis of tryptophan derivatives was reported by Roberts in 1994 [16]. In this research, the palladium catalyst was employed to complete the bridge between C3 and C4. After that, especially within the last ten years, a variety of protocols for synthesis of these molecules have been developed and refined by researchers. Moreover, the palladium catalyst was also used extensively in cross-coupling reactions, such as Buchwald-Hartwig, Suzuki-Miyaura, Heck, Sonogashira, Negishi, and Stille to synthesize a variety of useful compounds [17][18].
2. Intramolecular Cyclization
The Heck reaction [19] is one of the most powerful methods for generating new C-C bonds, and has been used in numerous syntheses of high value-added chemicals and complex molecules. In 1999, Söderberg and co-workers [20] successfully developed a practical method for synthesizing 3,4-fused tricyclic indole skeletons via two consecutive palladium-catalyzed reactions, using an intramolecular Heck reaction followed by reductive N-heteroannulation. The reaction afforded various tricyclic indole products (3a–3d) in 41–78% yields with 5 mol% Pd(OAc)2 as catalyst, 12 mol% 1,3-bis(diphenylphosphino)propane (DPPP) as ligand, 60 psi carbon monoxide as reductant, and polar DMF as solvent (Scheme 2). Notably, compared to the previous Heck reaction, the relative rate of alkene insertion in this reaction is higher, likely due to the presence of the strong electron-withdrawing nitro group. Six years later, the same group [21] reported a similar method for preparation of 3,4-fused tricyclic indoles with 6–8-membered rings. With respect to the scope of substrates, nitrogen- and oxygen-containing rings work well in this reaction as well, except for the carbocyclic ring.
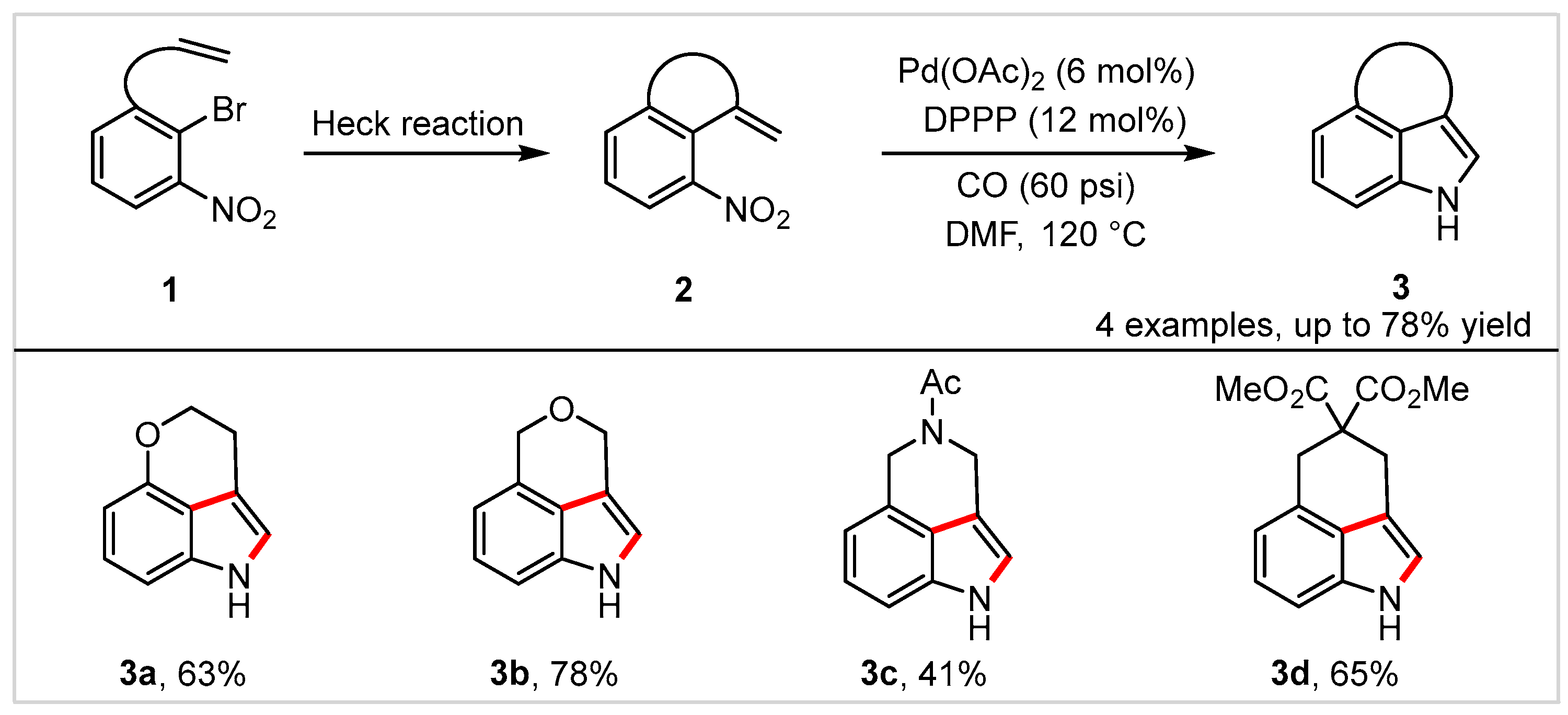
Scheme 2. Synthesis of 3,4-fused tricyclic indoles via two consecutive palladium-catalyzed reactions.
Medium-sized ring fused tricyclic indoles are quite useful structures in numerous natural products such as decursivine and serotobenine. In 2011, Van der Eycken and co-workers [22] reported a novel method for the construction of amide type of 3,4-fused tricyclic indole derivatives through a palladium-catalyzed intramolecular acetylene hydroarylation reaction with excellent regio- and stereoselectivity (Scheme 3). In this research, various nitrogen protecting groups and alkyne substituents, such as methyl (5a), ethyl (5b), tert-butyl (5c), phenyl (5d), and silyl groups were successfully tested and tolerated well, delivering the target products in moderate to good yields, and the structures were confirmed by 1H NMR, 13C NMR spectroscopy, HRMS (EI), and X-ray crystallography. The reaction is rapid, mild, regioselective, stereoselective and proceeds with good yields. It is worth noting that substrates bearing a free NH group (5c) also reacted smoothly in this reaction.
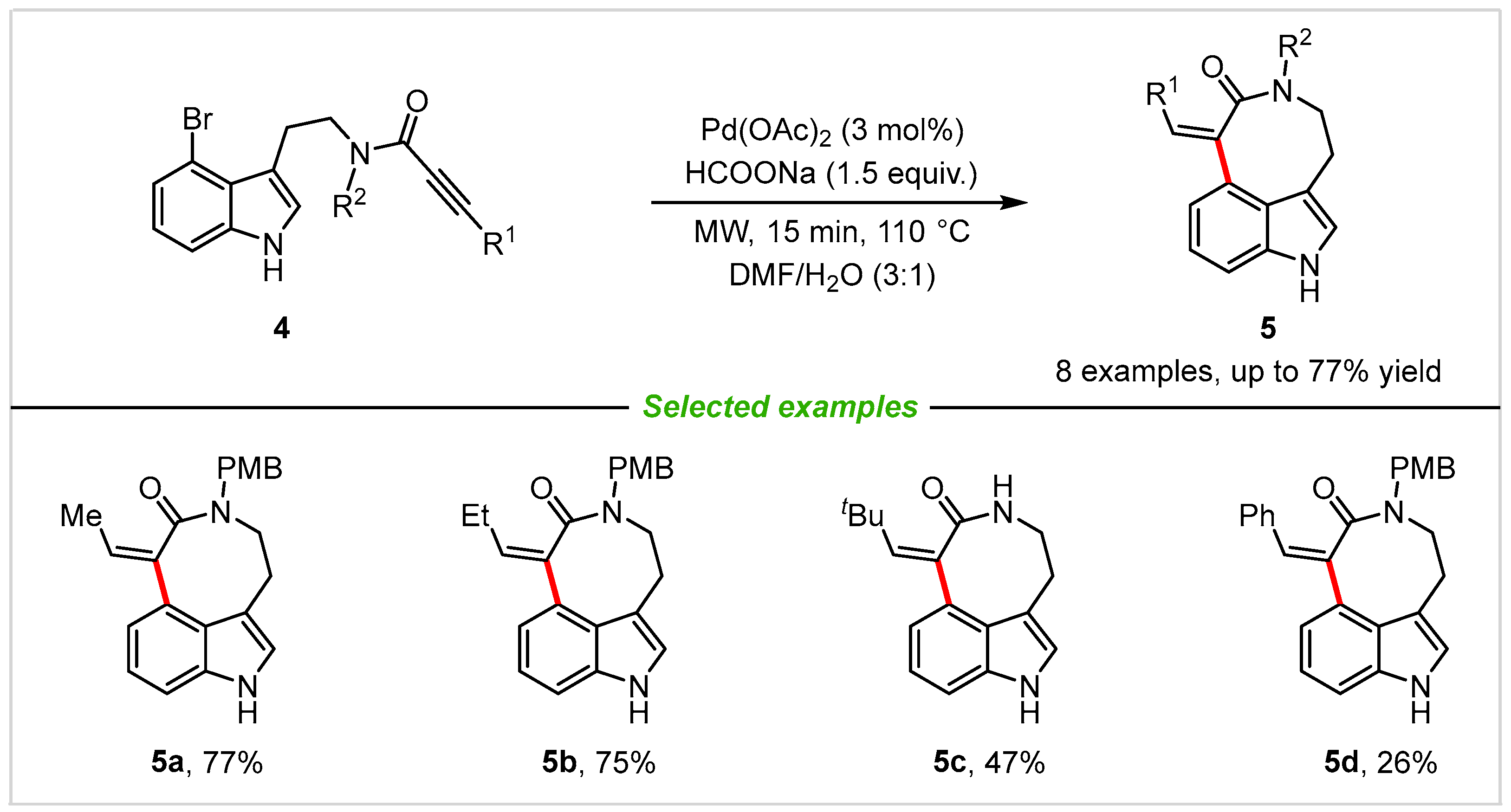
Scheme 3. Synthesis of 3,4-fused tricyclic indoles through palladium-catalyzed intramolecular acetylene hydroarylation.
As we know, Larock indole synthesis is one of the most efficient methods for the rapid construction of indole skeletons from halo-anilines and alkynes with palladium catalyst [23][24]. In 2013, Boger and co-workers [25] successfully reported the first example for rapid assembly of 3,(4-6)-tricyclic indoles 7 via intramolecular Larock indole annulation with a powerful Pd2(dba)3/DtPBF catalyst system, and catalyst and other reaction parameters were examined in detail (Scheme 4). This novel transformation features excellent functional-group tolerance (18 examples), good yields (up to 89% yield), and provides an efficient strategy to afford natural product chloropeptin I, chloropeptin II DEF ring system and key isomers directly. The ring size ranges from 6 to 28, which includes peptide chain and conventional carbon chain. More importantly, the TES or TMS group are easily removed from the molecules, leading to the formation of various indoline skeletons. It is worth noting that the established method is a good complement to the Stille or Suzuki cross-coupling reactions for the synthesis of cyclic or macrocyclic ring indole systems [26][27].
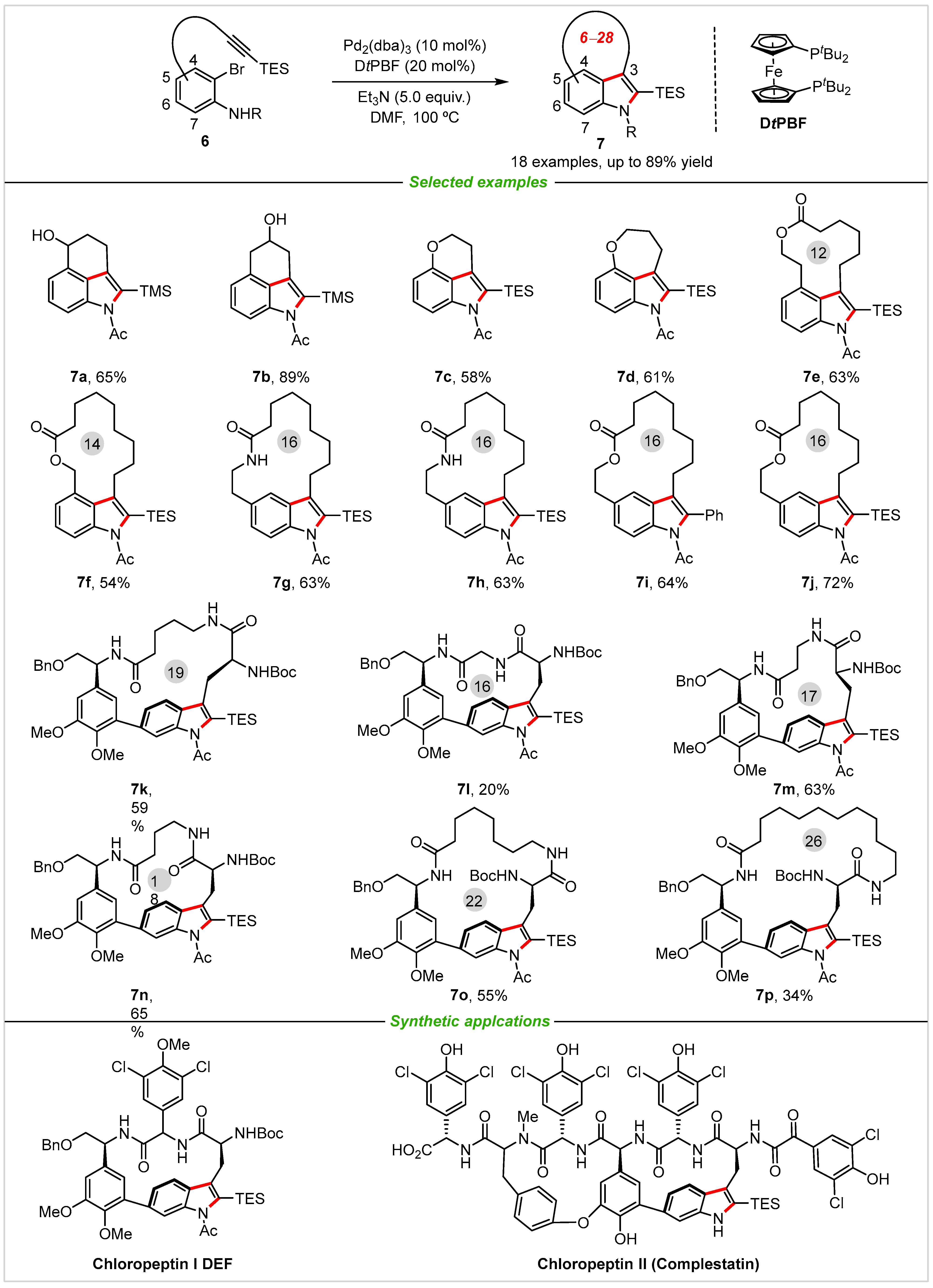
Scheme 4. Synthesis of diversely functionalized 3,4-, 3,5-, and 3,6-fused tricyclic indoles 7 with the alkyne tethered ortho-bromoanilines.
In the same year, Jia and co-workers [28] successfully disclosed a new and general method for the synthesis of 3,4-fused tricyclic indole derivatives through intramolecular Larock indole annulation from internal alkyne tethered ortho-iodoaniline derivatives, and these skeletons are often embedded in numerous natural products and bioactive molecules (Scheme 5). After extensive condition studies, the optimal reaction conditions are as follows: Pd(OAc)2 (20 mol%), PPh3 (40 mol%), K2CO3 (2.0 equiv.), and LiCl (1.0 equiv.) in DMF at 100 °C under nitrogen atmosphere. Applying the Pd/PPh3 catalyst system to the indole-synthesized reaction successfully produced 6–18-membered ring fused tricyclic indoles 9 in good to excellent yields (up to 99% yield), and various linkers, including carbon- (9c,9g,9i)), oxygen- (9a,9b,9d,9e,9h), or nitrogen- (9f) are all tolerated well. In addition, the method was highlighted by the total synthesis of the natural product fargesine, which was isolated from the root and stem of Evodia fargesii Dode. Notably, the authors also explored the reaction with the easily accessible ortho-bromoaniline-type substrates. Considering the lower reactivity of the C-Br bond, some representative ligands were tested to complete the ideal conversion, results showed that electron rich Me-phos or DPPP gave the best yields. Later, the method was also extended to the synthesis of 3,5-fused tricyclic indole scaffolds by the same group [29], giving a variety of 3,5-fused macrocyclic with good yields, and the method was also used to afford the tetrahydropyrrolo [4,3,2-de]quinolone ring.
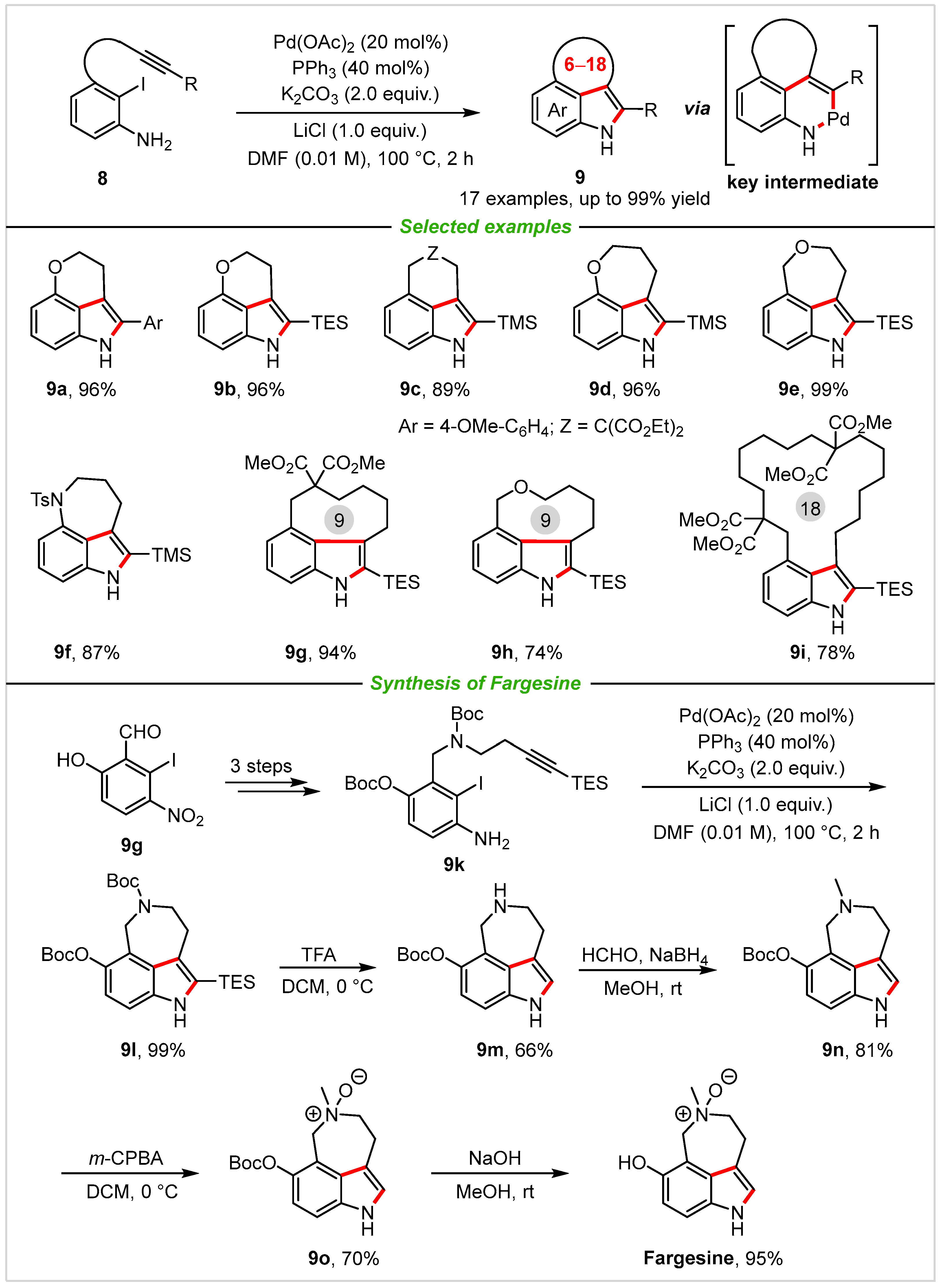
Scheme 5. Synthesis of 3,4-fused tricyclic indoles via palladium-catalyzed intramolecular annulation of internal alkyne tethered ortho-iodoanilines and total synthesis of fargesine.
The strategy of diversity oriented synthesis (DOS) is of great importance in organic synthesis, medicine chemistry, agrochemical chemistry, and material sciences, which aims to efficient collections of small molecules with diverse appendages, functional groups, stereochemistry, and skeletons [30]. In 2013, the You group [31] developed an efficient, Pd(0)-catalyzed allylic alkylation protocol for the synthesis of 3,4-fused tricyclic indole derivatives from readily available 3-subsituted indoles at 50 °C (Scheme 6). The phosphine ligand was crucial for this Pd-catalyzed allylic alkylation reaction, results showed that the 2-(2-(diphenylphosphanyl)phenyl)-4,4-dimethyl-4,5-dihydrooxazole (L1) proved to be the best ligand and led to the formation of tricyclic indole product (11a) in 70% yield. Substituents at the nitrogen such as benzyl and allyl could be well-tolerated, and delivered tricyclic indoles in moderate yields. Indole substrates substituted with fluorine (11c) and chlorine (11d) at the 7-position generated the products with 57% and 60% yield, respectively. Moreover, various 3-substituted substrates were used to examine the substrate scope of the Pd-catalyzed allylic dearomatization reaction under slightly modified conditions. In the transformation, screening of various chiral ligands showed that planar chiral ferrocene-based ligand (L2) gave the highest value of ee. Substrates bearing methyl, allyl, benzyl, and various nucleophilic substituents at the 3-position of indole could be applied in the reaction and gave the desired products in good yields with moderate enantioselectivity (12a–12h). Notably, when an excess of trifluoroacetic acid (TFA) was added to (12e) at room temperature, tetracyclic product (12i) was achieved with 80% yield and 73% ee via deprotection of the Boc group followed by addition to imine.
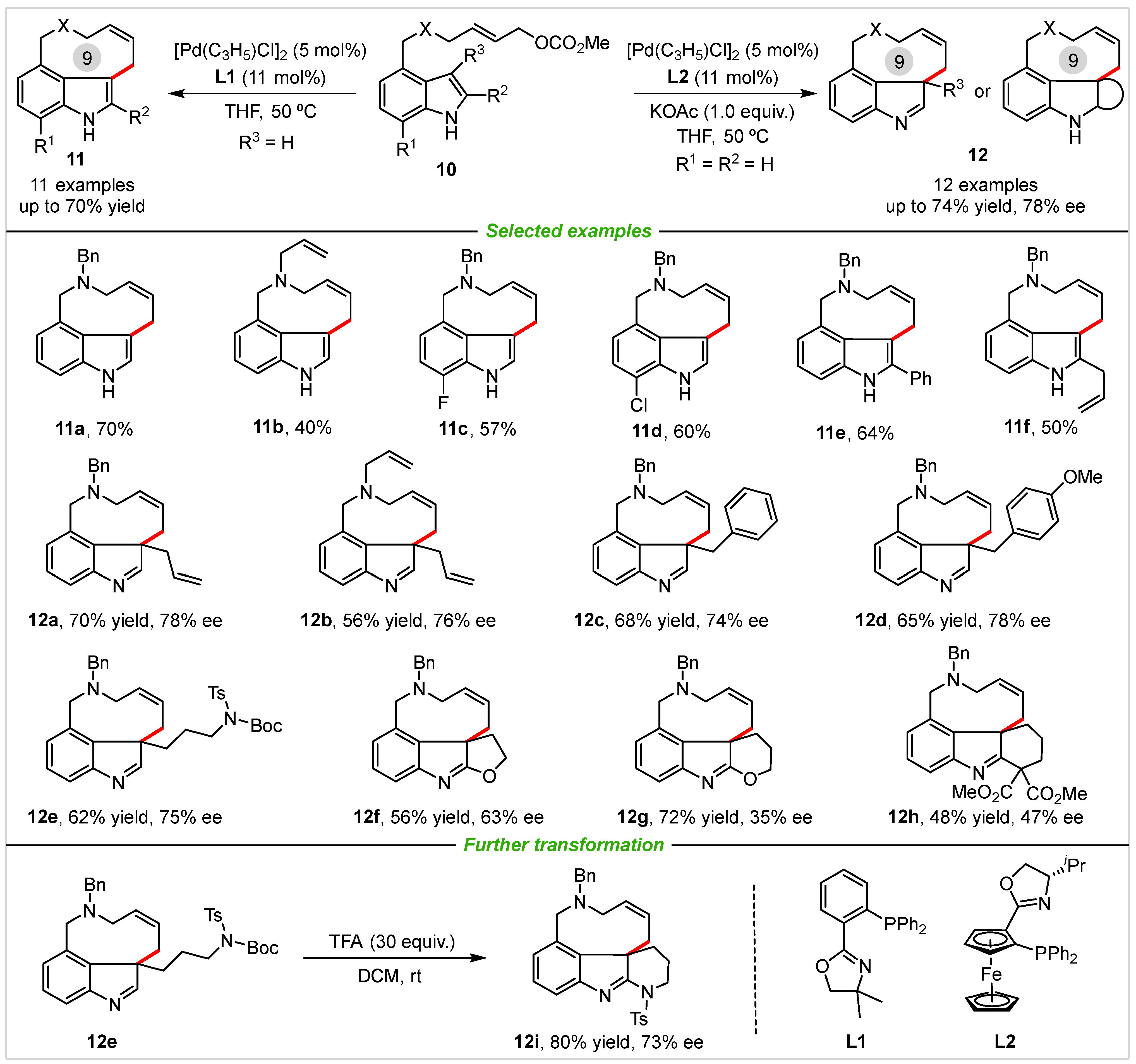
Scheme 6. Diversity oriented synthesis of 3,4-fused tricyclic indole and their derivatives via palladium-catalyzed allylic alkylation reactions.
As important raw materials, allenes, which possess unique reactivity compared to alkenes, alkynes, and others, owing to the presence of two cumulative orthogonal π-bonds [32][33][34][35][36], have proven to be key synthetic intermediates in construction of various organic molecules. In particular, allenes generally react with aryl halide to produce π-allylpalladium(II) species in the presence of Pd(0) catalyst through a classic Heck insertion process. In 2015, Nemoto and co-workers [37] successfully demonstrated a novel Pd-catalyzed cascade cyclization with allene tethered ortho-iodoanilines 13 as starting materials. This enabled rapidly assembled 3,4-fused tricyclic 3-alkylidene indoline scaffolds through a sequence of oxidative addition, allene insertion, and allylic amination, thus yielding a new class of 3,4-tricyclic 3-alkylidene indoline skeletons in good to excellent yields with broad substrates scope (Scheme 7). Furthermore, the 3,4-tricyclic 3-alkylidene indolines were divergently transformed into three types of 3,4-fused tricyclic indoles in 94% to quantitative yield with rather simple operation, successfully demonstrating the utility of this cascade process. Alternatively, the desired 3,4-fused tricyclic indoles could also be obtained by oxidation of products 14 with DDQ or PCC.
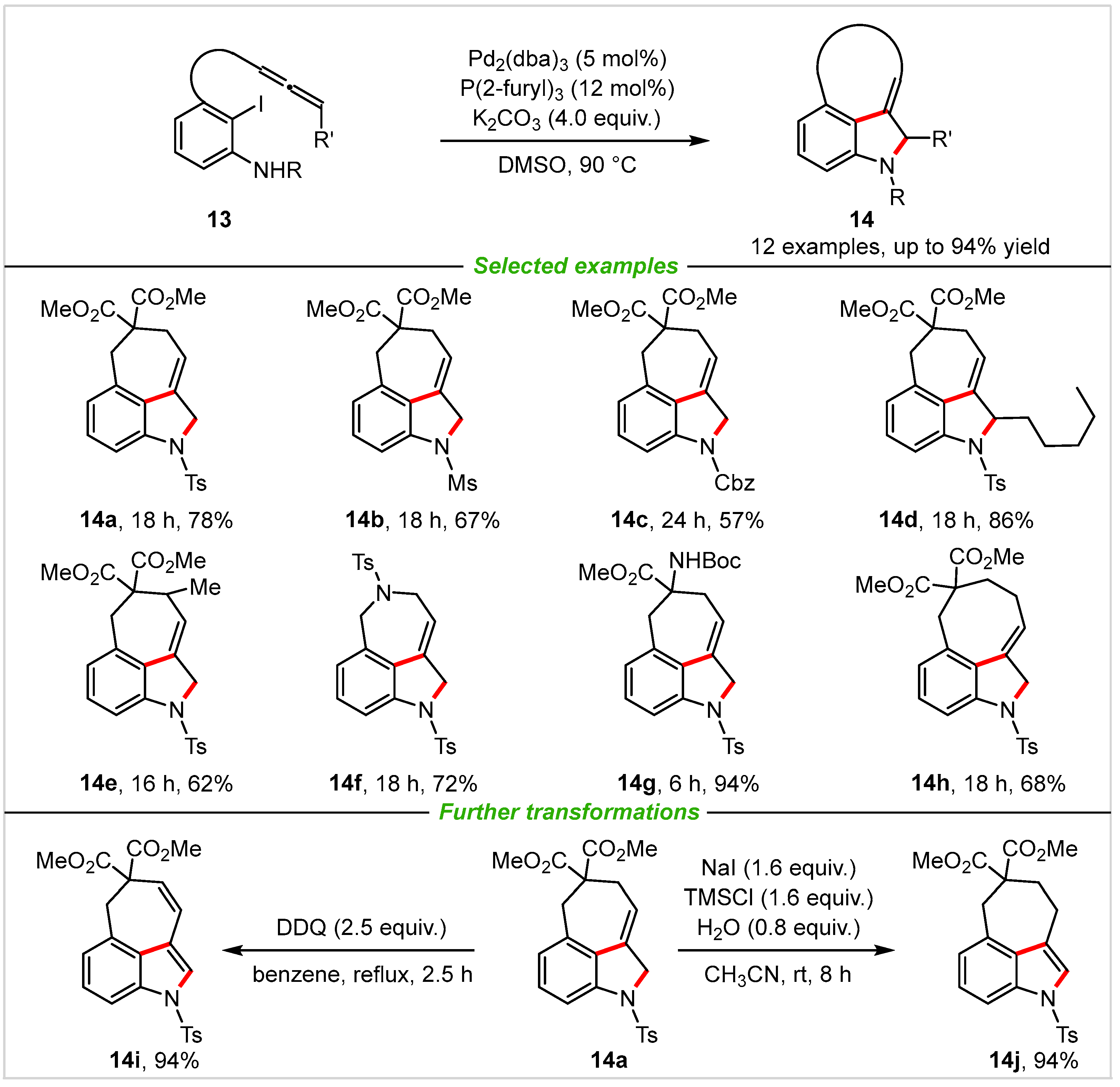
Scheme 7. Palladium-catalyzed intramolecular annulation of allene tethered ortho-iodoanilines.
In many macrocyclic natural products, peptide-based macrocycles are pervasive motifs in medicine molecules [38][39][40]. Among them, drugs, such as anticancer agent octreotide, antibiotic vancomycin, and immunosuppressant cyclosporin are typical molecules. Therefore, a concise method for the synthesis of these skeletons is highly attractive and challenging. In 2018, Chen, Liu, Shen, Qi, He, and co-workers [41] described an amide and pyridine directed β-C(sp3)-H arylation reaction to access diverse peptide macrocycles under Pd(II) catalysis (Scheme 8). In this transformation, 3,5-fused tricyclic indole (16a) and 3,7-fused tricyclic indole (16b) were obtained in 87% and 71% yields, respectively.
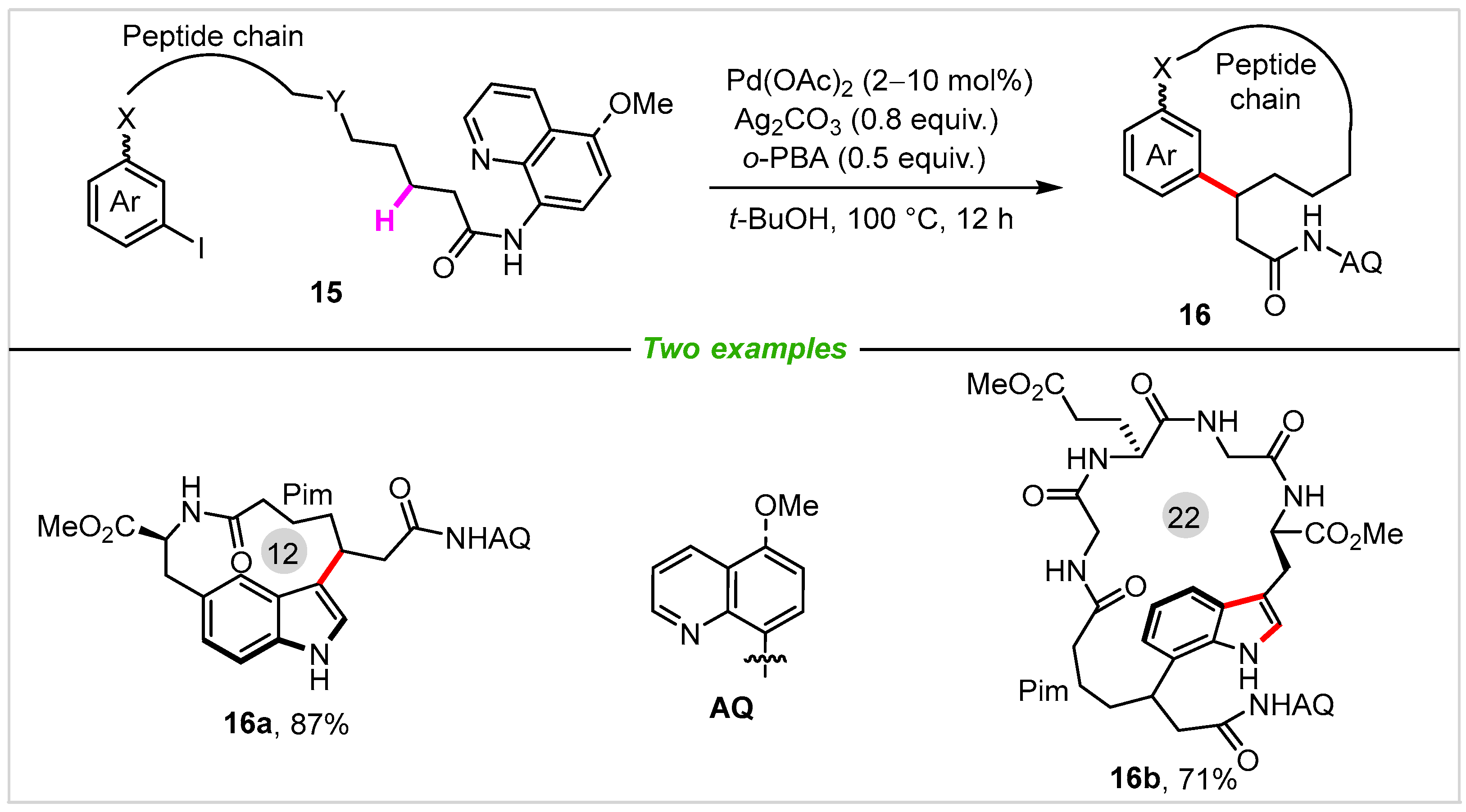
Scheme 8. Synthesis of peptide-based macrocycles via palladium-catalyzed intramolecular C(sp3)-H arylation.
References
- Cacchi, S.; Fabrizi, G. Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2005, 105, 2873–2920.
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875–2911.
- Gribble, G.W. Recentdevelopments in indole ring synthesis—Methodology and applications. J. Chem. Soc. Perkin Trans. 2000, 1, 1045–1075.
- Prabagar, B.; Yang, Y.; Shi, Z. Site-selective C-H functionalization to access the arene backbone of indoles and quinolines. Chem. Soc. Rev. 2021, 50, 11249–11269.
- Nagaraju, K.; Ma, D. Oxidative coupling strategies for the synthesis of indole alkaloids. Chem. Soc. Rev. 2018, 47, 8018–8029.
- Bariwal, J.; Voskressensky, L.G.; Van der Eycken, E.V. Recent advances in spirocyclization of indole derivatives. Chem. Soc. Rev. 2018, 47, 3831–3848.
- Nemoto, T.; Harada, S.; Nakajima, M. Synthetic methods for 3,4-fused tricyclic indoles via indole ring formation. Asian J. Org. Chem. 2018, 7, 1730–1742.
- Connon, R.; Guiry, P.J. Recent advances in the development of one-pot/multistep syntheses of 3,4-annulated indoles. Tetrahedron Lett. 2020, 61, 151696.
- Scopton, A.; Kelly, T.R. Synthesis of HKI 0231B. J. Org. Chem. 2005, 70, 10004–10012.
- Ginnon, W.F.; Benigni, J.D.; Suzuki, J.; Daly, J.W. The synthesis of dehydrobufotenine. Tetrahedron Lett. 1967, 8, 1531–1533.
- Sahu, S.; Das, B.; Maji, M.S. Stereodivergent total synthesis of hapalindoles, fischerindoles, hapalonamide H, and ambiguine H alkaloids by developing a biomimetic, redox-neutral, cascade Prins-type cyclization. Org. Lett. 2018, 20, 6485–6489.
- Wyche, T.P.; Ruzzini, A.C.; Schwab, L.; Currie, C.R.; Clardy, J. Tryptorubin A: A polycyclic peptide from a fungus-derived streptomycete. J. Am. Chem. Soc. 2017, 139, 12899–12902.
- Ma, B.; Litvinov, D.N.; He, L.; Banerjee, B.; Castle, S.L. Total synthesis of celogentin C. Angew. Chem. Int. Ed. 2009, 48, 6104–6107.
- Garfunkle, J.; Kimball, F.S.; Trzupek, J.D.; Takizawa, S.; Shimamura, H.; Tomishima, M.; Boger, D.L. Total synthesis of chloropeptin II (complestatin) and chloropeptin I. J. Am. Chem. Soc. 2009, 131, 16036–16038.
- Isley, N.A.; Endo, Y.; Wu, Z.-C.; Covington, B.C.; Bushin, L.B.; Seyedsayamdost, M.R.; Boger, D.L. Total synthesis and stereochemical assignment of streptide. J. Am. Chem. Soc. 2019, 141, 17361–17369.
- Horwell, D.C.; Nichols, P.D.; Ratcliffe, G.S.; Roberts, E. Synthesis of conformationally constrained tryptophan derivatives. J. Org. Chem. 1994, 59, 4418–4423.
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295.
- Shetgaonkar, S.E.; Mamgain, R.; Kikushima, K.; Dohi, T.; Singh, F.V. Palladium-catalyzed organic reactions involving hypervalent iodine reagents. Molecules 2022, 27, 3900.
- Heck, R.F. Acylation, methylation, and carboxyalkylation of olefins by Group VIII metal derivative. J. Am. Chem. Soc. 1968, 90, 5518–5526.
- Söderberg, B.C.; Rector, S.R.; O’Neil, S.N. Palladium-catalyzed synthesis of fused indoles. Tetrahenron Lett. 1999, 40, 3657–3660.
- Söderberg, B.C.; Hubbard, J.W.; Rector, S.R.; O’Neil, S.N. Synthesis offused indoles by sequential palladium-catalyzed Heck reaction and N-heteroannulation. Tetrahedron 2005, 61, 3637–3649.
- Peshkov, V.A.; Van Hove, S.; Donets, P.A.; Pereshivko, O.P.; Van Hecke, K.; Van Meervelt, L.; Van der Eycken, E.V. Synthesis of the azocinoindole framework through Pd-catalyzed intramolecular acetylene hydroarylation. Eur. J. Org. Chem. 2011, 2011, 1837–1840.
- Larock, R.C.; Yum, E.K. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J. Am. Chem. Soc. 1991, 113, 6689–6690.
- Larock, R.C.; Yum, E.K.; Refvik, M.D. Synthesis of 2,3-disubstituted indoles via palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 1998, 63, 7652–7662.
- Breazzano, S.P.; Poudel, Y.B.; Boger, D.L. A Pd(0)-mediated indole (macro)cyclization reaction. J. Am. Chem. Soc. 2013, 135, 1600–1606.
- Mohr, P.J.; Halcomb, R.L. Total synthesis of (+)-phomactin A using a B-alkyl Suzuki macrocyclization. J. Am. Chem. Soc. 2003, 125, 1712–1713.
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489.
- Shan, D.; Gao, Y.; Jia, Y. Intramolecular Larock indole synthesis: Preparation of 3,4-fused tricyclic indoles and total synthesis of fargesine. Angew. Chem. Int. Ed. 2013, 52, 4902–4905.
- Gao, Y.; Shan, D.; Jia, Y. Intramolecular Larock indole synthesis for the preparation of tricyclic indoles and its application in the synthesis of tetrahydropyrroloquinoline and fargesine. Tetrahedron 2014, 70, 5136–5141.
- Zeng, J.; Sun, G.; Yao, W.; Zhu, Y.; Wang, R.; Cai, L.; Liu, K.; Zhang, Q.; Liu, X.-W.; Wan, Q. 3-Aminodeoxypyranoses in glycosylation: Diversity-oriented synthesis and assembly in oligosaccharides. Angew. Chem. Int. Ed. 2017, 56, 5227–5231.
- Xu, Q.-L.; Dai, L.-X.; You, S.-L. Diversity oriented synthesis of indole-based peri-annulated compounds via allylic alkylation reactions. Chem. Sci. 2013, 4, 97–102.
- Byun, S.; Farah, A.O.; Wise, H.R.; Katchmar, A.; Cheong, P.H.Y.; Scheidt, K.A. Enantioselective copper-catalyzed borylative amidation of allenes. J. Am. Chem. Soc. 2022, 144, 22850–22857.
- Li, M.; Dixon, D.J. Stereoselective spirolactam synthesis via palladium catalyzed arylative allene carbocyclization cascades. Org. Lett. 2010, 12, 3784–3787.
- Ma, S.; Zhao, S. Pd(0)-catalyzed insertion−cyclization reaction of 2,3-allenols with aryl or alkenyl halides. Diastereoselective synthesis of highly optically active trans-2,3-disubstituted vinylic oxiranes. J. Am. Chem. Soc. 1999, 121, 7943–7944.
- Chen, S.; Gao, Z.; Zhao, H.; Li, B. Palladium-catalyzed regio- and stereoselective arylesterification of ferrocenylallene: A synthetic route to ferrocene-containing disubstituted E-allylic esters. J. Org. Chem. 2014, 79, 1481–1486.
- Lee, M.; Nguyen, M.; Brandt, C.; Kaminsky, W.; Lalic, G. Catalytic Hydroalkylation of allenes. Angew. Chem. Int. Ed. 2017, 56, 15703–15707.
- Nakano, S.; Inoue, N.; Hamada, Y.; Nemoto, T. Pd-catalyzed cascade cyclization by intramolecular Heck insertion of an allene-allylic amination sequence: Application to the synthesis of 3,4-fused tricyclic indoles. Org. Lett. 2015, 17, 2622–2625.
- Walsh, C.T.; O’Brien, R.V.; Khosla, C. Nonproteinogenic amino acid building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Angew. Chem. Int. Ed. 2013, 52, 7098–7124.
- Cardote, T.A.F.; Ciulli, A. Cyclic and macrocyclic peptides as chemical tools to recognise protein surfaces and probe protein–protein interactions. ChemMedChem 2016, 11, 787–794.
- Marsault, E.; Peterson, M.L. Macrocycles are great cycles: Applications, opportunities, and challenges of synthetic macrocycles in drug discovery. J. Med. Chem. 2011, 54, 1961–2004.
- Zhang, X.; Lu, G.; Sun, M.; Mahankali, M.; Ma, Y.; Zhang, M.; Hua, W.; Hu, Y.; Wang, Q.; Chen, J.; et al. A general strategy for synthesis of cyclophane-braced peptide macrocycles via palladium-catalysed intramolecular sp3 C-H arylation. Nat. Chem. 2018, 10, 540–548.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Revisions:
3 times
(View History)
Update Date:
29 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





