Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zou Yining | -- | 1971 | 2023-06-28 10:30:02 | | | |
| 2 | Catherine Yang | Meta information modification | 1971 | 2023-06-29 04:32:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, T.; He, P.; Guo, D.; Chen, K.; Hu, Z.; Zou, Y.; Yining, Z. Immune Stimulation Mechanism of Aluminum Phosphate. Encyclopedia. Available online: https://encyclopedia.pub/entry/46163 (accessed on 07 February 2026).
Zhang T, He P, Guo D, Chen K, Hu Z, Zou Y, et al. Immune Stimulation Mechanism of Aluminum Phosphate. Encyclopedia. Available at: https://encyclopedia.pub/entry/46163. Accessed February 07, 2026.
Zhang, Ting, Peng He, Dejia Guo, Kaixi Chen, Zhongyu Hu, Yening Zou, Zou Yining. "Immune Stimulation Mechanism of Aluminum Phosphate" Encyclopedia, https://encyclopedia.pub/entry/46163 (accessed February 07, 2026).
Zhang, T., He, P., Guo, D., Chen, K., Hu, Z., Zou, Y., & Yining, Z. (2023, June 28). Immune Stimulation Mechanism of Aluminum Phosphate. In Encyclopedia. https://encyclopedia.pub/entry/46163
Zhang, Ting, et al. "Immune Stimulation Mechanism of Aluminum Phosphate." Encyclopedia. Web. 28 June, 2023.
Copy Citation
Aluminum phosphate is a compound of hydroxy-aluminum phosphate, similar to aluminum hydroxide, and the hydroxyl group on its surface can also lose or gain protons under different pH conditions, thus changing the surface charge. Aluminum phosphate can reduce the effective dose for inducing protective immune response against Lactobacillus mexicana of plasmid DNA.
aluminum phosphate adjuvants
immune stimulation mechanism
1. Depot Effect
In most cases, the antigens used in vaccine products are weak in immunogenicity, which can be enhanced by binding to adjuvants. After adsorption, the antigen accumulates on the surface of and inside the adjuvant particles, helping the antigen maintain its physical and chemical properties [1]. A huge number of studies have indicated that antigens can be combined with adjuvants and released slowly at the injection site to continuously stimulate the immune system and enhance immune response, which is called the depot effect. Harrison first demonstrated the existence of the depot effect by inoculating aluminium-containing nodules from one guinea pig into another guinea pig [2]. Taking AP for example, the antigen is mixed with aluminum phosphate into a gel state. When the mixture is injected into the body, it creates a kind of “reservoir” in the body. These insoluble gel particles adsorb and disperse the antigenic components, increasing the surface area of the antigen and forming a granuloma at the injection site. Antigens in the granuloma will slowly penetrate into the body and preserve the antigens that can only stay at the injection site temporarily for several weeks, so that the antigens will not be cleared by the liver in a short time and can achieve the purpose of long-term stimulating the immune system [3]. The depot effect at the vaccine injection site has long been considered one of the main action mechanisms of immune adjuvants. The depot effect is influenced by the physical properties of aluminum-containing adjuvants (surface area, charge, and morphologic structure). For example, a larger surface area may enhance antigen adsorption, promote antigen storage, and facilitate antigen presentation to antigen-presenting cells (APCs). Ultimately, the immune response is enhanced [4]. Antigens that are bound to aluminum-containing adjuvants are not only beneficial for presenting to antigen-presenting cells, but can also slowly release into tissues along with the decomposition of aluminum-containing adjuvants, leading to the delay of antigens consumption and their stimulation time to the immune system can also be prolonged. In addition, the prolonged interaction interval between antigens and presenting cells also enhances the induced immune response [5].
The depot effect is an important mechanism by which adjuvants play their role, but the depot effect alone cannot fully explain the mechanism by which aluminum-containing adjuvants enhance immune stimulation. Some studies have found that the depot is not a necessary condition for the function of an aluminum adjuvant: for example, Holt et al. [6] found that when diphtheria toxin was absorbed on an aluminum adjuvant and injected into guinea pigs, even if the inoculated tissue was removed seven days after inoculation, the effect caused by vaccination still existed. Hutchison et al. [7] found that removal of the inoculation site two hours after inoculation of the antigen–aluminum adjuvant mixture had no effect on the specific antibodies and T cell response produced by the body. In addition, Gupta et al. mixed C14-labeled tetanus toxoid with aluminum adjuvant for injection, and found that the antigen was not “stored”, but immediately released by monitoring the trace of C14 [8]. These results indicate that although the depot effect is one of the important mechanisms of immune adjuvants, it is not a necessary mechanism for all adjuvants to function. However, in the case of the antigen depot effect, further studies are required to validate its significance.
2. Recruitment of Immune Cells
The immune system has three defense lines to protect human health: physical and chemical barriers, innate immunity, and adaptive immune responses. After intramuscularly injection, an aluminum adjuvant can stimulate the innate immunity of the body. The main participants of innate immunity include dendritic cells, macrophages, monocytes, neutrophils, eosinophils, basophils, mast cells, natural killer cells, interferon and complement proteins [9].
Both AH and AP activate immune system and immune system-related pathways in monocytes, and the in vitro immune response to AH is more pronounced compared to AP. At present, Kooijman et al. [10] proved through in vivo and in vitro experiments that AH and AP could recruit different kinds of cells and trigger different immune responses after injection. Because monocytes are the hub of innate immunity and adaptive immunity, the changes of monocytes are very important in studying the mechanism of adjuvant action. Both AH and AP can activate innate immunity in vivo. In vivo experiments show that only intramuscular immunity of AH can attract neutrophils, while AP can attract mononuclear cells and macrophages to the injection site [11]. These results indicate that AH and AP have different roles in innate immunity, which may be explained by that the different physical and chemical properties of these two adjuvants could cause different effects on cells. AH and AP adjuvanted antigens were injected into the muscle of mice, and proteomic analysis of the injection site showed that 67% of the up-regulated proteins near the injection site overlapped after the injection of the two aluminum-containing adjuvants, indicating that the two adjuvants had certain similarities in stimulating the immune response, compared with AP, AH induced more immune system-related pathways, and in vivo, more neutrophils were attracted to the injection site [10][12]. The recruitment of immune cells at the injection site provides favorable conditions for adaptive immunity.
3. Enhancement of Antigen Uptake by Antigen Presenting Cells (APCs)
A key step of inducing immune response is antigen uptake by APCs. Both the antigen adsorbated by aluminum adjuvant and the antigen free in the intercellular substance can be uptaken by APCs, but the former particles form can be taken up by APCs more easily [13]. The adsorption between antigens and adjuvants enables the antigen to maintain a high concentration at the injection site and release slowly, so as to prolong the time of antigen uptake by APCs and the effect of the antigen on the immune system [14].
Kooijman et al. [10] found that AH was superior to AP in aiding antigen presentation and processing. After 24 h incubation of AlPO4, antigen presentation and processing are down-regulated, and at this time, there is no down-regulation of AH incubation. This pathway can recruit neutrophils in AH adjuvanted antigens, but not in AP adjuvanted antigens. However, injection of antigens containing AP can recruit mononuclear/macrophage cells which are strongly up-regulated after 48 h incubation, and only with AP. This suggests some biological differences between AH and AP, and the choice of these adjuvants should be carefully considered in vaccine formulations. APCs include macrophages, B cells and dendritic cells (DCs). DCs can provide the widest range of exogenous antigens for T cells that play a key role in immune response. Only mature DCs can effectively activate T cells, and maturation of DCs is marked by the expression of co-stimulatory molecules such as CD80 and CD86 on the membrane surface [9]. DCs use protease in lysosomes and pH changes to process antigens into peptides, which are then expressed on cell membranes in the form of an antigen peptide-MHC Class II molecular complex and presented to CD4+ T cells to stimulate the differentiation of Th into Th2 [15]. An experiment performed by Sokolovska et al. showed that both AH- and AP-incubated CDs derived from mouse bone marrow could increase the expressions of CD80 and CD86 and promote the maturity of CDs. The effect of AH on increasing the expressions of CD80 and CD86 was significantly stronger than that of AP. These two adjuvants can also induce DCs to produce IL-1 and IL-18, while IL-1β and IL-18 can promote the differentiation of CD4+ T cells into Th2 cells as well [15][16].
4. Activation of NLRP3 Inflammasome Pro-Inflammatory Signaling Pathway
Aluminum-containing adjuvants can recruit leukocytes, promote differentiation of dendritic cells, and accelerate local tissue inflammation independent of Toll-like receptors. However, the cellular targets that release the pro-inflammatory activity of aluminum-containing adjuvants were not identified until recently. Data from various laboratories have shown that aluminum-containing adjuvants can target nucleotide-binding oligomerization domains (NOD), such as receptor protein 3 (NLRP3). NLRP3 is a member of the inflammasome, which can recognize danger signals transmitted into the cell. The main responsibility of macrophages is phagocytosis and processing antigens, and aluminum-containing adjuvants can induce and activate endogenous immune responses through NLRP3, thereby promoting the secretion of high levels pro-inflammatory cytokines, such as IL-1β and IL-18 from macrophages; however, they are not present when lacking NLRP3 inflammasome components in cells [17]. Kooijman et al. [10] found that aluminum adjuvants can participate in the innate and adaptive immunity induced by ovalbumin through activation of the NLRP3 inflammasome.
NLRP3 is one of the members of NOD-like receptor family. Dead cells, silica, asbestos, aluminum adjuvant and other components can stimulate and activate NLRP3 [18][19]. NLRP3 inflammasome oligomerization can be achieved by caspase activation and recruitment domain (CARD). CARD interacts with aspartic protease 1 to form inflammatory bodies. When NLRP3 is stimulated, it combines with apoptosis-related specks such as protein ASC and pro-caspase-1 to assemble NLRP3 inflammasome. The activated NLRP3 inflammasome prompts ACS to lyse pro-caspase-1 into caspase-1. Caspase-1 then promotes the activation of IL-1β and IL-18 [18][19], which in turn promote the differentiation of CD4+ T cells into Th2 cells, enhancing the immune response, and Th2 cells stimulate the production of antibodies by B cells through secretion of cytokines to form humoral immunity [9][20].
Intracellular lysosomes can phagocyte aluminum-containing adjuvants ingested by APCs, and cathepsin B released after that can promote the assembly of NLRP3 inflammasome [21]. In addition, AH and AP are toxic to cells, and the injection of the aluminum adjuvant vaccine will cause cell damage and release of cellular DNA, ATP and uric acid [22]. AP is more toxic to monocytic leukemia cells (THP-1 cells) than AH. When the adjuvant concentration was 100.0 μg/mL, the mortality rate of THP-1 cells incubated with AP was more than 50%, while the result of AH was about 20% [23][24]. DNA, uric acid and other intracellular molecules released after cell death can act as damage-associated molecular patterns (DAMPs) to further activate innate immune cells [19][25].
The inhibition of caspase-1 showed that both AH and AP could induce caspase-1 activation, and then promote the maturation of IL-1β and IL-18. Ruwona et al. also showed that both AH and AP can promote IL-1β secretion by stimulating the NLRP3 inflammatorome, but AH can stimulate THP-1 cells to produce higher levels of IL-1β than AP, which may be related to the differences in the structure, density, surface charge and charge density between these two aluminum-containing adjuvants [26].
5. Other Immune Stimulatory Mechanisms
Traditionally, aluminum adjuvants have been thought to primarily induce a cellular immune response that involves the activation and proliferation of T cells. However, the results of Scholl et al. found that the aluminum adjuvant that adsorbed rNanH and rPknG proteins from Corynebacterium pseudotuberculosis vaccine produced partial protection in animals. Significant levels of total IgG, IgG1, and IgG2a antibodies against C. pseudotuberculosis were also produced. The levels of IgG2a isotypes were higher, and the antibodies produced were characterized by a mixed humoral response with a tendency toward Th1 type [27]. Rezende et al. adsorbed acid phosphatase rCP01850 with AH, it induced Th1/Th2 mixed immune response, which improved the survival rate after challenge [28].
Ramanathan et al. [29] found in guinea pigs that AH could activate complement in an alternative way and induce granulation formation and macrophage damage. In addition, dendritic cells can recruit aluminum-adsorbed antigen-antibodies, and complement factors can regulate receptors on B cells to form aluminum-adsorbed antigen-antibodies, thereby promoting immune response. Thus, aluminum-containing adjuvants can activate complements and further enhance the immune response via B cells and dendritic cells [14][30]. Modes of action of aluminum-containing adjuvants are summarized in Figure 1.
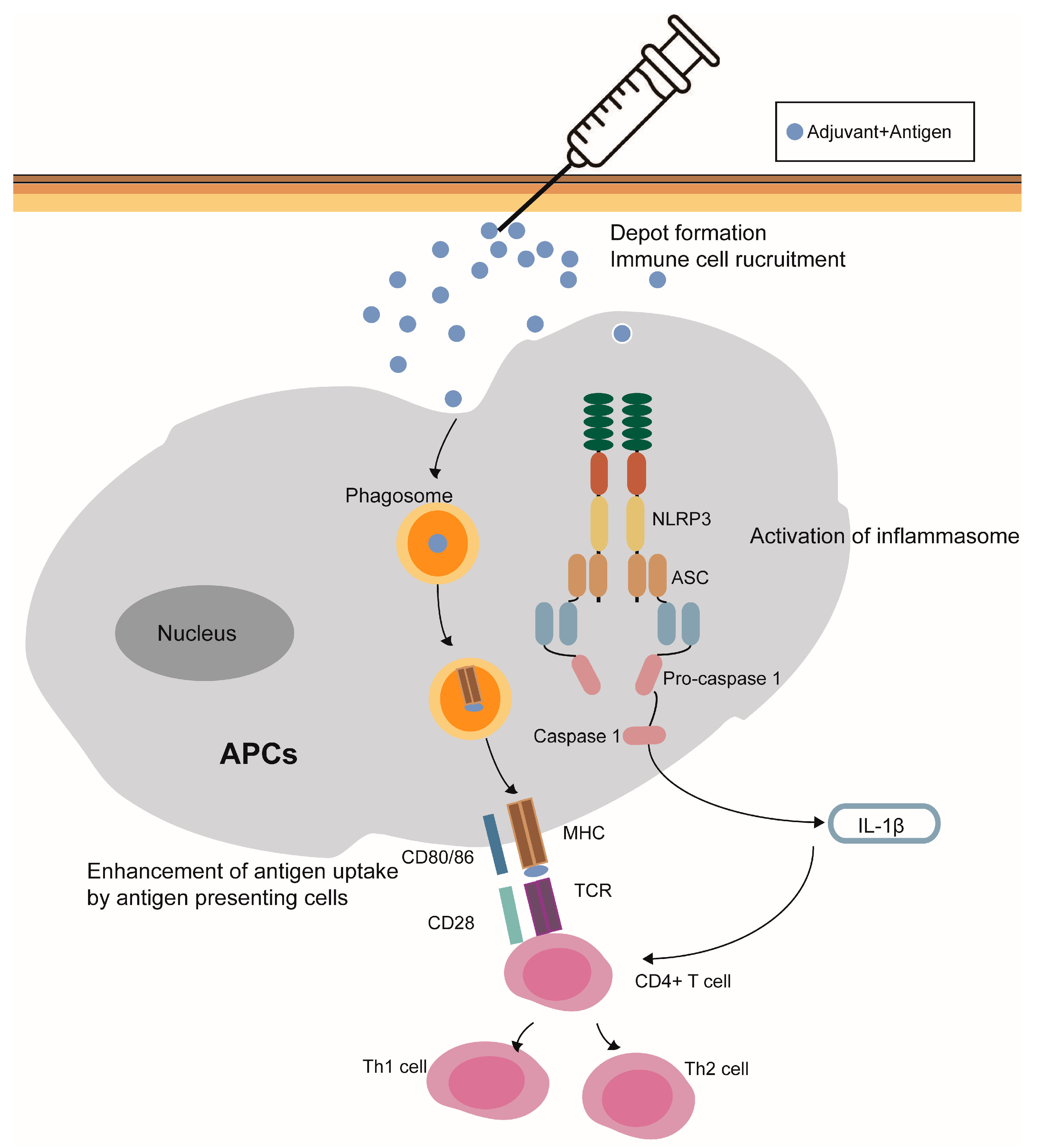
Figure 1. Modes of action of aluminum-containing adjuvants. (1) Depot effect; (2) recruitment of immune cells; (3) enhancement of antigen uptake by antigen presenting cells; (4) the NLRP3 inflammasome activation; and (5) potentiality to stimulate the humoral immune responses.
References
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488.
- Harrison, W.T. Some Observations on the Use of Alum Precipitated Diphtheria Toxoid. Am. J. Public Health Nation’s Health 1935, 25, 298–300.
- Cox, J.C.; Coulter, A.R. Adjuvants—A classification and review of their modes of action. Vaccine 1997, 15, 248–256.
- Shi, Y.; HogenEsch, H.; Regnier, F.E.; Hem, S.L. Detoxification of endotoxin by aluminum hydroxide adjuvant. Vaccine 2001, 19, 1747–1752.
- Matheis, W.; Zott, A.; Schwanig, M. The role of the adsorption process for production and control combined adsorbed vaccines. Vaccine 2001, 20, 67–73.
- Holt, L.B. Developments in diphtheria prophylaxis. JAMA 1950, 144, 1415.
- Hutchison, S.; Benson, R.A.; Gibson, V.B.; Pollock, A.H.; Garside, P.; Brewer, J.M. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012, 26, 1272.
- Gupta, R.K.; Chang, A.-C.; Griffin, P.; Rivera, R.; Siber, G.R. In vivo distribution of radioactivity in mice after injection of biodegradable polymer microspheres containing 14C-labeled tetanus toxoid. Vaccine 1996, 14, 1412–1416.
- Li, X.; Wang, X.; Ito, A. Tailoring inorganic nanoadjuvants towards next-generation vaccines. Chem. Soc. Rev. 2018, 47, 4954–4980.
- Kooijman, S.; Vrieling, H.; Verhagen, L.; de Ridder, J.; de Haan, A.; van Riet, E.; Heck, A.J.; Kersten, G.F.; Pennings, J.L.; Metz, B. Aluminum hydroxide and aluminum phosphate adjuvants elicit a different innate immune response. J. Pharm. Sci. 2022, 111, 982–990.
- Ghimire, T.R. The mechanisms of action of vaccines containing aluminum adjuvants: An in vitro vs in vivo paradigm. Springerplus 2015, 4, 181.
- Danielsson, R.; Eriksson, H. Aluminium adjuvants in vaccines–A way to modulate the immune response. Semin. Cell Dev. Biol. 2021, 115, 3–9.
- Mannhalter, J.; Neychev, H.; Zlabinger, G.; Ahmad, R.; Eibl, M.M. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: Effect on antigen uptake and antigen presentation. Clin. Exp. Immunol. 1985, 61, 143.
- HogenEsch, H. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine 2002, 20, S34–S39.
- Sokolovska, A.; Hem, S.L.; Hogenesch, H. Activation of dendritic cells and induction of CD4(+) T cell differentiation by aluminum-containing adjuvants. Vaccine 2007, 25, 4575–4585.
- Terhune, T.D.; Deth, R.C. A role for impaired regulatory T cell function in adverse responses to aluminum adjuvant-containing vaccines in genetically susceptible individuals. Vaccine 2014, 32, 5149–5155.
- HogenEsch, H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front. Immunol. 2013, 3, 406.
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265.
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 2010, 28, 321–342.
- Eisenbarth, S.C.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008, 453, 1122–1126.
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856.
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011, 17, 996–1002.
- Mold, M.; Shardlow, E.; Exley, C. Insight into the cellular fate and toxicity of aluminium adjuvants used in clinically approved human vaccinations. Sci. Rep. 2016, 6, 31578.
- Shardlow, E.; Mold, M.; Exley, C. The interaction of aluminium-based adjuvants with THP-1 macrophages in vitro: Implications for cellular survival and systemic translocation. J. Inorg. Biochem. 2020, 203, 110915.
- Zhang, J.-G.; Czabotar, P.E.; Policheni, A.N.; Caminschi, I.; San Wan, S.; Kitsoulis, S.; Tullett, K.M.; Robin, A.Y.; Brammananth, R.; van Delft, M.F.; et al. The Dendritic Cell Receptor Clec9A Binds Damaged Cells via Exposed Actin Filaments. Immunity 2012, 36, 646–657.
- Ruwona, T.B.; Xu, H.; Li, X.; Taylor, A.; Shi, Y.C.; Cui, Z. Toward understanding the mechanism underlying the strong adjuvant activity of aluminum salt nanoparticles. Vaccine 2016, 34, 3059–3067.
- Scholl, N.R.; Silva, M.T.d.O.; Barbosa, T.N.; de Pinho, R.B.; Alves, M.S.D.; Portela, R.W.; Azevedo, V.A.d.C.; Borsuk, S. Evaluation of the Association of Recombinant Proteins NanH and PknG from Corynebacterium pseudotuberculosis Using Different Adjuvants as a Recombinant Vaccine in Mice. Vaccines 2023, 11, 519.
- Rezende, A.; Brum, A.; Bezerra, F.; Braite, D.; Sá, G.; Thurow, H.; Seixas, F.; Azevedo, V.; Portela, R.; Borsuk, S. Assessment of the acid phosphatase CP01850 from Corynebacterium pseudotuberculosis in DNA and subunit vaccine formulations against caseous lymphadenitis. Arq. Bras. Med. Vet. E Zootec. 2020, 72, 199–207.
- Ramanathan, V.; Badenoch-Jones, P.; Turk, J. Complement activation by aluminium and zirconium compounds. Immunology 1979, 37, 881.
- Egwang, T.; Befus, A. The role of complement in the induction and regulation of immune responses. Immunology 1984, 51, 207.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
767
Revisions:
2 times
(View History)
Update Date:
29 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




