Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lawrence Kawchuk | -- | 1908 | 2023-06-27 16:15:45 | | | |
| 2 | Conner Chen | Meta information modification | 1908 | 2023-06-29 07:36:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Koeppe, S.; Kawchuk, L.; Kalischuk, M. RNA Interference Past and Future Applications in Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/46133 (accessed on 02 March 2026).
Koeppe S, Kawchuk L, Kalischuk M. RNA Interference Past and Future Applications in Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/46133. Accessed March 02, 2026.
Koeppe, Sarah, Lawrence Kawchuk, Melanie Kalischuk. "RNA Interference Past and Future Applications in Plants" Encyclopedia, https://encyclopedia.pub/entry/46133 (accessed March 02, 2026).
Koeppe, S., Kawchuk, L., & Kalischuk, M. (2023, June 27). RNA Interference Past and Future Applications in Plants. In Encyclopedia. https://encyclopedia.pub/entry/46133
Koeppe, Sarah, et al. "RNA Interference Past and Future Applications in Plants." Encyclopedia. Web. 27 June, 2023.
Copy Citation
Antisense RNA was observed to elicit plant disease resistance and post-translational gene silencing (PTGS). The universal mechanism of RNA interference (RNAi) was shown to be induced by double-stranded RNA (dsRNA), an intermediate produced during virus replication. Plant viruses with a single-stranded positive-sense RNA genome have been instrumental in the discovery and characterization of systemic RNA silencing and suppression. Application of exogenously applied dsRNA is proving to be a potent strategy for delivery to improve crop performance.
RNAi
immunity
HIGS
1. Introduction
RNA silencing is a revolutionary innate immunity mechanism in eukaryotes that has greatly expanded our knowledge of gene expression and regulation in plants. RNA interference (RNAi) is an important regulatory mechanism that has become an invaluable tool for plant research, especially in terms of understanding the effects of gene regulation in response to abiotic and biotic stress. RNAi has enabled researchers to gain insight into gene function, pest resistance, and physiological processes in plants. Although RNA is known to play critical roles in biology, the extensive capabilities and complexity of this nucleic acid remained elusive and not fully understood. The intrinsic nature of the relatively labile, often single-stranded RNA molecule, and limited availability of RNA-dependent enzymes had slowed characterization and progress. Traditional research focused on messenger transcript (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA), but the universality of the molecule to life remained underestimated [1]. The occurrence of viruses with RNA genomes (gRNA), the dominant genome type of viruses in plants, provided an extraordinary platform for the study of RNA function and gene expression. In a relatively brief period of time, knowledge of the dynamic RNA molecule has dramatically increased, and our understanding of RNA capabilities and applications rapidly expanded.
2. RNA Interference
Remarkably, transformation of plants with the genus Polerovirus coat protein antisense RNA of the Potato leafroll virus produced similarly high levels of reduced virus titre and disease resistance as the corresponding sense mRNA [2][3]. The response was rapid and all transformed plants exhibited sequence-specific sustained high levels of immunity regardless of the virus inoculum concentration (Figure 1). Vector transmission of the virus by the green peach aphid Myzus persicae was reduced and disease symptoms in foliage and tubers were eliminated. This showed that RNA was capable of conferring resistance as a trigger molecule and was subsequently observed in other plant virus groups [4]. Replicative intermediates of RNA viruses include double-stranded RNA (dsRNA) and dsRNA secondary structures that are produced to regulate gene expression and are relatively stable compared to ssRNA due to the widespread occurrence of resilient ssRNA ribonucleases [5]. The disease resistance achieved with antisense RNA demonstrated an inherent ability of RNA to protect against pathogens.
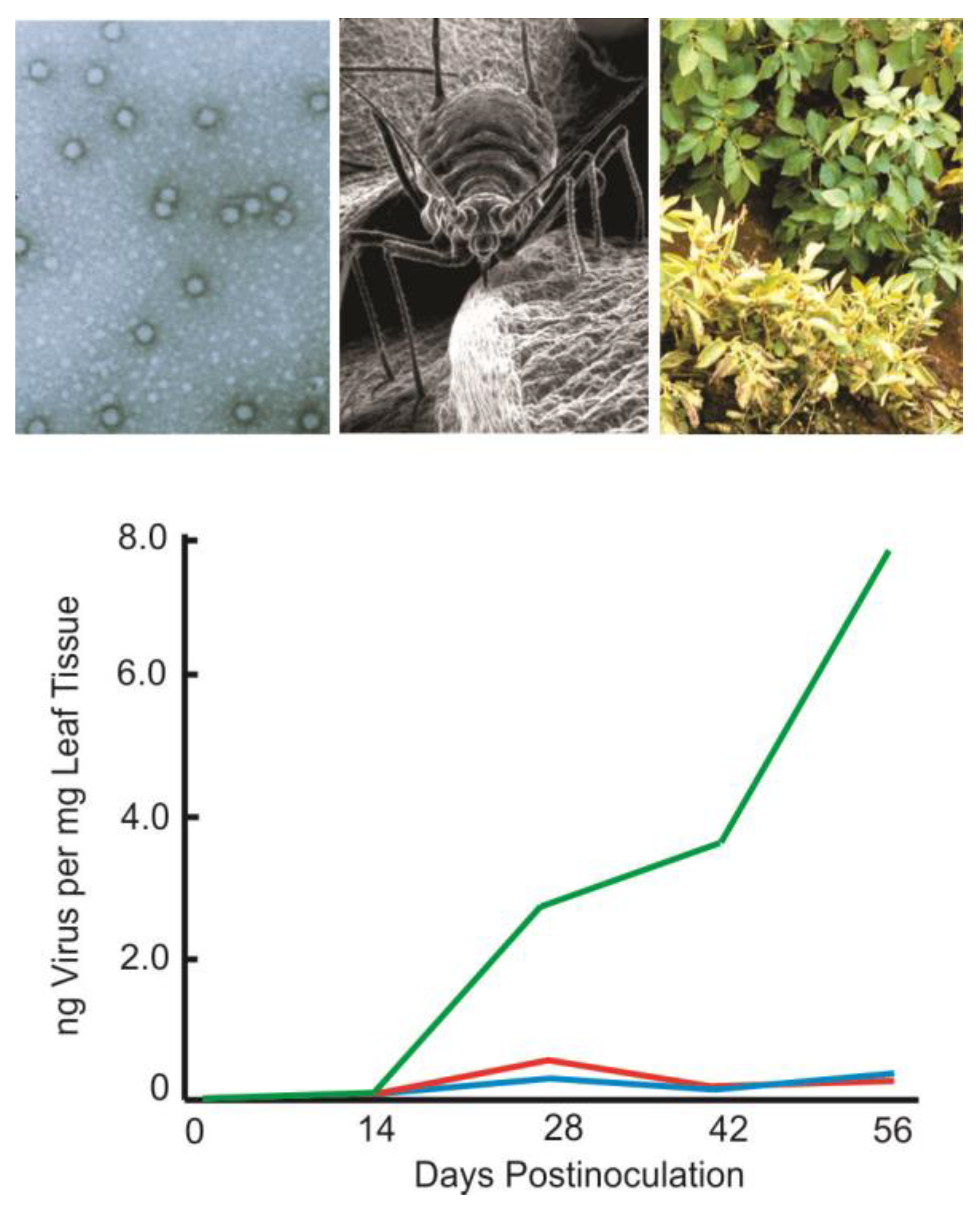
Figure 1. RNA interference (RNAi) against virus infection. Sense and antisense RNA protection against the single-stranded positive-sense RNA Potato leafroll virus (PLRV). Although phloem limited, members of the genus Polerovirus are transmitted in a persistent, non-propagative manner and cause considerable disease-related losses worldwide. The icosahedral virus is approximately 25 nm in size (top left transmission electron photomicrograph) and transmitted in an aphid-specific manner by the green peach aphid, Myzus persicae, approximately 2.5 mm in length (top middle scanning electron photomicrograph). Disease symptoms include stunting and chlorosis of infected plants (top right) that reduce yield and quality. Virus titres in plants expressing coat protein messenger RNA (mRNA, red line) or antisense RNA (aRNA, blue line) reduced virus levels significantly as compared to untransformed controls (green line), determined by double antibody sandwich (DAS) enzyme-linked immunosorbent assay (ELISA).
Experiments to transiently or stably increase endogenous gene expression often unexpectedly produced a decrease in mRNA. For example, attempts to overexpress chalcone synthase (CHS) in pigmented petunia petals blocked anthocyanin biosynthesis [6]. Developmental timing and expression of the CHS mRNA by the endogenous gene was not altered but the level of transcript was reduced by 50 fold. This posttranslational gene silencing (PTGS) highlighted a regulatory mechanism of gene expression involving RNA interference. Polygalacturonase involved in plant cell wall degradation and ripening was inhibited in transgenic tomato expressing antisense RNA [7]. Similarities between viral defense and gene silencing mechanisms suggested a common innate immunity in plants, including the systemic signalling in gene silencing contributing to the sequence-specific RNA interference [8][9].
3. Characterization of RNA Interference
RNA interference (RNAi) involves a sequence-specific suppression of gene expression by transcriptional or translational repression. The results of the RNAi characterization demonstrated that feeding or injecting gene-specific dsRNA into Caenorhabditis elegans resulted in the disappearance of the targeted message [10]. Silencing effects were observed with only a few molecules of unc-22 dsRNA per cell supporting a role as a trigger molecule. The RNAi mechanism is a naturally occurring process in most eukaryotes, conferring an ability of dsRNA to induce a sequence-specific systemic silencing process [3][6][7][10].
Exogenous dsRNA initiates RNAi by activating the ribonuclease Dicer enzymes that bind and cleave dsRNA into 21–24-base-pair small interfering RNA (siRNA) fragments with 3′ overhangs of 2–3 nucleotides (Figure 2). Dicer proteins have an RNA helicase domain, RNase III motifs, and nucleic-acid-binding PAZ domain [4]. The siRNA is converted to ssRNA when the sense complimentary RNA strand is degraded by Argonaute (AGO) enzymes and the antisense guide strand is incorporated into the RNA-induced silencing complex (RISC). Members of AGO possess a PAZ domain and a PIWI domain, resembling RNaseH, that are required for cleavage activity [4]. The RISC complex further uses this strand to bind and degrade additional copies of sense complimentary RNA. Systemic silencing occurs and the inherit specificity suggests that nucleic acid is the signal molecule in plants [4][9]. Amplification of even weak silencing signals indicates that RNA-dependent RNA-polymerase (RDRP) recognition and replication elicits effective silencing.
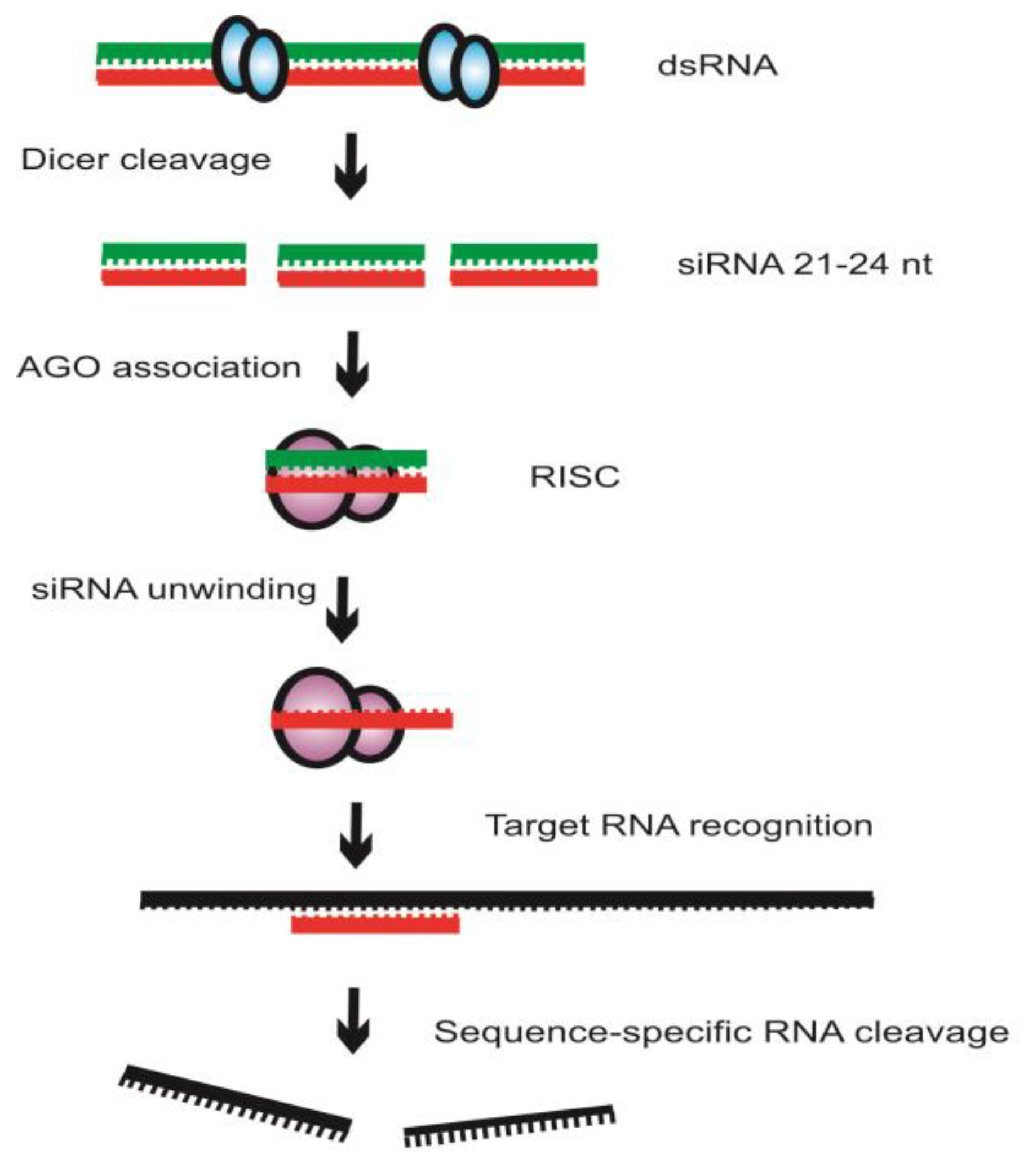
Figure 2. Mechanism of RNA interference (RNAi). RNAi is initiated by the enzyme Dicer that cleaves double-stranded RNA (dsRNA) into short fragments of approximately 21- to 24-nucleotide short interfering RNA (siRNA). The siRNA is unwound into single-stranded RNA and the sense RNA (green) is further cleaved and degraded by the enzyme Argonaute (AGO). The antisense RNA (red) is recruited into the RNA-induced silencing complex (RISC) that binds to the target sense RNA through the specificity of the complementary antisense RNA.
The occurrence of double-stranded RNA during viral RNA replication and hairpin RNA secondary structures regulating gene expression, indicated that ssRNA viruses have an inherent protective mechanism from RNAi [11][12]. Silencing suppressors were subsequently identified within RNA virus genomes that targeted different components of RISC, such as the DICER-LIKE (DCL) proteins, and inhibit innate RNA silencing [13]. Similar to the systemic nature of RNAi, silencing suppressors were also capable of systemic silencing suppression [14]. The application of RNA silencing suppressors, such as the Tomato bushy stunt tombusvirus p19 protein, are often required in preventing PTGS in plant studies expressing homologous or heterologous genes [11].
4. Applications of RNA Interference
Stably or transiently expressed genes and nucleic acids in genetically engineered plants is often utilized in the study of gene function or the heterologous production of commercially valuable products. The use of full-length infectious clones (FLICs) of RNA viruses has facilitated the amplification of targeted genes, providing a convenient vector platform that can circumvent RNAi for site-directed mutations and increase or reduce gene expression to characterize PTGS and produce valuable heterologous commercial products. Application of virus-induced gene silencing (VIGS) has successfully utilized several RNA virus vectors including Tobacco rattle virus (TRV), Potato virus Y (PVY), TMV, and PLRV [15][16][17][18]. Different virus vectors confer specific advantages such as titre and tissue specificity. For example, field-grown plants are subjected to strict containment by regulatory agencies to limit unexpected transmission in the environment by vectors. Phloem-specific expression by the PLRV FLIC is not transmitted mechanically or by vector when the capsid readthrough protein is replaced by the heterologous nucleic acid, eliminating accidental movement of genetic materials (Figure 3).
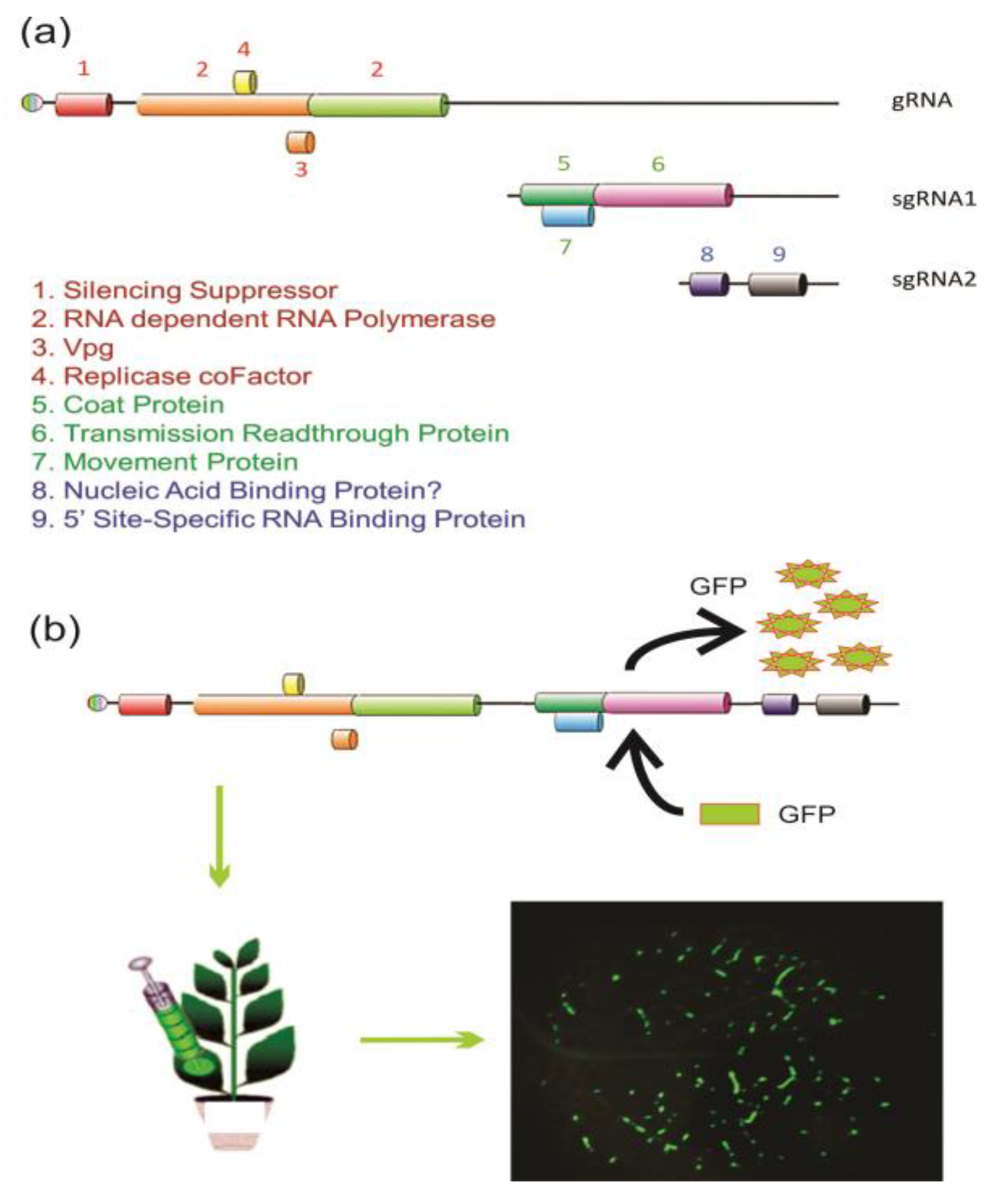
Figure 3. RNA virus replication and applications. (a) Genomic and subgenomic RNA for the replication and translational strategies of the Potato leafroll virus, including a silencing suppressor produced immediately following virus disassembly. Replication involves the production of antisense RNA and subsequent sense subgenomic RNAs (sgRNAs) and expression of proteins involves several translational strategies including leaky start and stop codons, proteolytic site-specific cleavage of genomic RNA (gRNA), and an internal ribosomal entry site (IRES) sequence. (b) A full-length infectious clone (FLIC) of the Potato leafroll virus RNA amplifies expression of heterologous sequences for virus-induced gene silencing (VIGS) or production of commercially valuable proteins as shown (magnification 0.25×) with green fluorescent protein (GFP).
Innate immunity is more complex than originally envisioned and the RNAi regulatory mechanism is independent of other recognition and signalling pathways. Identification of genes for gene receptors and avirulence proteins has advanced our understanding of cellular resistance to a wide range of pathogens, including Pseudomonas syringae, Cladosporium fulvum, and Verticillium species [19][20][21][22]. Mechanisms for signal amplification and recognition by receptors of sessile plants has improved our understanding of an important component of innate immunity [23][24]. Cross-protection and intracellular communication has expanded with the discovery of RNAi and its role in innate immunity and gene regulation through extracellular plant and fungal RNA [25][26]. Together the different sources of innate immunity provide complementary strategies in controlling historically devastating crop losses and emerging new threats to food production.
Exogenously introduced dsRNA to target plant pests began with the introduction of dsRNAs through microinjections [27][28]. Microinjections are a favoured laboratory technique because incredibly precise amounts of dsRNA can be introduced into the target organism, allowing for precise delivery [29]. Although an adequate delivery method for lab- and smaller-scale applications, microinjection unfortunately is not suitable for field-level control of plant pests and pathogens [27][28]. Another delivery method involves the soaking of an organism in a suspension that contains the target dsRNA or directly spraying it with a solution containing the dsRNA [29]. This method may not be as exact as microinjection; however, it is often used because of its ease of use and overall convenience. Many other methods of RNA delivery have been examined and application choice is often influenced by several factors including efficacy and economics.
Investigators have created transgenic plants that express desired dsRNAs to cause RNAi-induced gene silencing in the target organism when it ingests plant material, referred to as Host-Induced Gene Silencing (HIGS) [28][30]. One example of HIGS was transgenic Zea mays (corn) called SmartStax Pro that was created to target corn rootworm (Diabrotica vwirgifera virgifera) that was approved for commercial use by the U.S. Environmental Protection Agency, the U.S. Food and Drug Administration, and the U.S. Department of Agriculture [28][30][31]. Commercial acceptancet of transgenic plants has been challenging due to general public concerns related to genetic engineering, especially the stable insertion of nucleic acid from other organisms [30][32]. A similar pest control efficacy was achieved with exogenously applied dsRNA in plants, representing a friendlier environmental and regulatory strategy for protection and production improvements [27].
Microorganisms transformed to contain target dsRNA have also been evaluated as a method for exogenous application. One notable example showed that bacteria transformed to contain the target dsRNA could be fed to insects to induce RNAi [33]. These genetically modified bacteria in some cases were even able to colonize the gut of the host and continue to deliver dsRNA directly to it through the gut. Another example of the ingestion of a transformed microorganism is the study of transformed Saccharomyces cerevisiae (yeast) containing dsRNA targeting spotted wing fruit fly Drosophila suzukii [34]. This type of yeast naturally occurs on the surface of rotting fruit that D. suzukii consumes, and therefore was seen as a viable vector to induce oral ingestion of the dsRNA. They had success and found that locomotor activity, survivorship, and reproductive fitness were all negatively impacted by the complimentary dsRNA [34]. Acceptance of products derived from transgenic platforms are subjected to elevated regulatory and consumer acceptance concerns.
References
- Gheysen, G.; Vanholme, B. RNAi from plants to nematodes. Trends Biotechnol. 2007, 25, 89–92.
- Kawchuk, L.M.; Martin, R.R.; McPherson, J. Resistance in transgenic potato expressing the potato leafroll virus coat protein gene. Mol. Plant Microbe Interact. 1990, 3, 301–307.
- Kawchuk, L.M.; Martin, R.R.; McPherson, J. Sense and antisense RNA-mediated resistance to potato leafroll virus in Russet Burbank potato plants. Mol. Plant Microbe Interact. 1991, 4, 247–253.
- Waterhouse, P.M.; Wang, M.B.; Lough, T. Gene silencing as an adaptive defence against viruses. Nature 2001, 411, 834–842.
- Jaag, H.M.; Kawchuk, L.; Rohde, W.; Fischer, R.; Emans, N.; Prüfer, D. An unusual internal ribosomal entry site of inverted symmetry directs expression of a potato leafroll polerovirus replication-associated protein. Proc. Natl. Acad. Sci. USA 2003, 100, 8939–8944.
- Van der Krol, A.R.; Lenting, P.E.; Veenstra, J.; van der Meer, I.M.; Koes, R.E.; Gerats, A.G.M.; Mol, J.N.M.; Stuitje, A.R. An anti-sense chalcone synthase gene in transgenic plants inhibits flower pigmentation. Nature 1988, 333, 866–869.
- Smith, C.J.S.; Watson, C.F.; Ray, J.; Bird, C.R.; Morris, P.C.; Schuch, W.; Grierson, D. Antisense RNA inhibition of polygalacturonase gene expression in transgenic tomatoes. Nature 1988, 334, 724–726.
- Ratcliff, F.; Harrison, B.D.; Baulcombe, D.C. A similarity between viral defense and gene silencing in plants. Science 1997, 276, 1558–1560.
- Voinnet, O.; Baulcombe, D.C. Systemic signalling in gene silencing. Nature 1997, 389, 553.
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Science 1998, 391, 806–811.
- Voinnet, O.; Pinto, Y.M.; Baulcombe, D.C. Suppression of gene silencing: A general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci. USA 1999, 96, 14147–14152.
- Anandalakshmi, R.; Pruss, G.J.; Ge, X.; Marathe, R.; Mallory, A.C.; Smith, T.H.; Vance, V.B. A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA 1998, 95, 13079–13084.
- Fusaro, A.F.; Correa, R.I.; Nakasugi, K.; Jackson, C.; Kawchuk, L.; Vaslin, M.F.S.; Waterhouse, P.M. The Enamovirus P0 protein is a silencing suppressor which inhibits local and systemic RNA silencing through AGO1 degradation. Virology 2012, 426, 178–187.
- Fusaro, A.F.; Barton, D.A.; Nakasugi, K.; Jackson, C.; Kalischuk, M.L.; Kawchuk, L.M.; Vaslin, M.F.S.; Correa, R.L.; Waterhouse, P.M. The luteovirus P4 movement protein is a suppressor of systemic RNA silencing. Viruses 2017, 9, 294.
- Ratcliff, F.; Martin-Hernandez, A.M.; Baulcombe, D.C. Technical Advance. Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J. 2001, 25, 237–245.
- Kumagai, M.H.; Donson, J.; Della-Cioppa, G.; Harvey, D.; Grill, L.K. Cytoplasmic inhibition of carotenoid biosynthesis with Virus-derived RNA. Proc. Natl. Acad. Sci. USA 1995, 92, 1679–1683.
- Ruiz, M.T.; Voinnet, O.; Baulcombe, D.C. Initiation and maintenance of virus-induced gene silencing. Plant Cell 1998, 10, 937–946.
- Kawchuk, L.; Jaag, H.M.; Toohey, K.; Martin, R.; Rohde, W.; Prüfer, D. In planta agroinfection by Canadian and German Potato leafroll virus full-length cDNAs. Can. J. Plant. Path. 2002, 24, 239–243.
- Martin, G.B. Map-based cloning of a protein-kinase gene conferring disease resistance in tomato. Science 1993, 262, 1432.
- Jones, D.A.; Thomas, C.M.; Hammond-Kosack, K.E.; Balint-Kurti, P.J.; Jones, J.D.G. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 1994, 266, 789–793.
- Kawchuk, L.M.; Hachey, J.; Lynch, D.R.; Kulcsar, F.; Van Rooijen, G.; Waterer, D.R.; Robertson, A.; Kokko, E.; Byers, R.; Howard, R.J.; et al. Tomato Ve disease resistance genes encode cell surface-like receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 6511–6515.
- Förderer, A.; Li, E.; Lawson, A.W.; Deng, Y.; Sun, Y.; Logemann, E.; Zhang, X.; Wen, J.; Han, Z.; Chang, J.; et al. Wheat resistosome defines common principles of immune receptor channels. Nature 2022, 610, 532–539.
- Kalischuk, M.; Müller, B.; Fusaro, A.F.; Wijekoon, C.P.; Waterhouse, P.M.; Prüfer, D.; Kawchuk, L. Amplification of cell signaling and disease resistance by an immunity receptor Ve1Ve2 heterocomplex in plants. Comm. Biol. 2022, 5, 497.
- Kourelis, J.; Marchal, C.; Posbeyikian, A.; Harant, A.; Kamoun, S. NLR immune receptor–nanobody fusions confer plant disease resistance. Science 2023, 379, 934.
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.D.; Jin, H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123.
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin1, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129.
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016, 7, 553.
- Sang, H.; Kim, J.I. Advanced strategies to control plant pathogenic fungi by host-induced gene silencing (HIGS) and spray-induced gene silencing (SIGS). Plant Biotechnol. Rep. 2020, 14, 1–8.
- Yu, N.; Christiaens, O.; Liu, J.; Niu, J.; Cappelle, K.; Caccia, S.; Huvenne, H.; Smagghe, G. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci. 2013, 1, 4–14.
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically modified organism-free RNA interference: Exogenous application of RNA molecules in plants. Plant Physiol. 2020, 182, 38–50.
- Gordon, K.H.J.; Waterhouse, P.M. RNAi for insect-proof plants. Nat. Biotechnol. 2007, 25, 1231–1232.
- Puyam, A.; Kaur, K. Exploiting RNA interference mechanism in plants for disease resistance. In Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches. Sustainability in Plant and Crop Protection; Ul Haq, I., Ijaz, S., Eds.; Springe: Cham, Switzerland, 2020; Volume 13, pp. 217–236.
- Murphy, K.A.; West, J.D.; Kwok, R.S.; Chiu, J.C. Accelerating research on Spotted Wing Drosophila management using genomic technologies. J. Pest Sci. 2016, 89, 631–641.
- Murphy, K.A.; Tabuloc, C.A.; Cervantes, K.R.; Chiu, J.C. Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci. Rep. 2016, 6, 22587.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
935
Revisions:
2 times
(View History)
Update Date:
29 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





