Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carla Pires | -- | 4626 | 2023-06-27 14:07:54 | | | |
| 2 | Fanny Huang | Meta information modification | 4626 | 2023-06-29 07:40:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pires, C. Superfoods for Type 2 Diabetes. Encyclopedia. Available online: https://encyclopedia.pub/entry/46125 (accessed on 07 February 2026).
Pires C. Superfoods for Type 2 Diabetes. Encyclopedia. Available at: https://encyclopedia.pub/entry/46125. Accessed February 07, 2026.
Pires, Carla. "Superfoods for Type 2 Diabetes" Encyclopedia, https://encyclopedia.pub/entry/46125 (accessed February 07, 2026).
Pires, C. (2023, June 27). Superfoods for Type 2 Diabetes. In Encyclopedia. https://encyclopedia.pub/entry/46125
Pires, Carla. "Superfoods for Type 2 Diabetes." Encyclopedia. Web. 27 June, 2023.
Copy Citation
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease affecting an estimated 537 million individuals worldwide. ‘Superfoods’ can be integrated into the diet of T2DM patients due to their health benefits.
T2DM
superfoods
HbA1c
metabolic control
glycaemia
1. Identified ‘Superfoods’ That Can Reduce Glycaemic Levels in Patients with Type 2 Diabetes Mellitus (T2DM)
The identified ‘superfoods’ that can reduce glycaemic levels in T2DM patients are summarised in Table 1.
Table 1. ‘Superfoods’ that can reduce glycaemic level in type 2 diabetic patients.
| ‘Superfoods’ | Examples | Reference(s) |
|---|---|---|
| Foods with polyphenols | Berries, tea, coffee, wine or grapes | [1][2][3][4][5][6][7] |
| Fermented dairy products | Yoghurt (fortified or not with vitamin D or probiotics) | [8][9][10][11][12][13][14] |
| Whole cereals/grains | Mixed grains, barley or oatmeal | [15][16][17][18] |
| Nuts | Almonds, Brazil nuts, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts | [19][20][21] |
| Proteins (especially, plant protein-rich diets) | Chickpeas (e.g., hummus), beans or other proteins–whey protein | [22][23][24][25] |
| Other foods | Sunflower seeds and flax seeds; cabbage; lupin, prickly pear cacti (Opuntia spp.) cladodes and honey (especially for short-term use) | [26][27][28][29][30] |
1.1. Foods with Polyphenols (e.g., Berries)
Polyphenols, and flavones such as luteolin (celery, parsley), quercetin (apple, broccoli), catechins (tea, chocolate), narigin (citrus fruit), genistein (soybean), cyanidin (grape, berries, onion), caffeic and ferulic acids (coffee, strawberries, wholegrain cereals), gallic acid (species, vinegar, grape), resveratrol (grape, wine), pinoresinol (cereals, sesame) and tannic acid (legumes, pomegranate) constitute bioactive compounds, which can (a) reduce carbohydrate digestion, triggered by the inhibition of intestinal enzymes (inhibition of α-glucosidase); (b) enhance glucose uptake (stimulating glucose transport into other functional tissue cells from the blood); (c) decrease gluconeogenesis; or (d) increase insulin secretion [5][6].
Besides a significant reduction in blood glucose, there was also an improvement in the lipidic profile and insulin resistance with these foods (e.g., with the daily consumption of coffee or tea). The daily consumption of naturally polyphenol-rich foods and beverages seems to provide a benefit in the control of cardiometabolic diseases. The diet should preferably contain different classes of polyphenols rather than a specific food or phenolic compound, since different classes of polyphenols seem to have a pleiotropic effect. For instance, the daily consumption of anthocyanidin-rich fruit (blueberries and apples) reduced the risk of T2DM by 23%. However, a positive association between the consumption of flavonoids and their subclasses and a reduced risk of T2DM was not found in all studies [6]. For instance, the light/moderate consumption of wine was associated with improved metabolic control in T2DM individuals due to the presence of non-alcoholic polyphenols, such as resveratrol [7]. However, the American Diabetes Association recommendations on alcohol intake should be followed: one drink a day for women and up to two per day for men (5 ounces of wine, a 12-ounce beer, or one and a half ounces of 80-proof spirits), because there are potential risks associated with alcohol consumption by T2DM patients, such as an increased hypoglycaemic risk, particularly when insulin and sulphonylureas are prescribed [31].
The bioactive compounds of berries in particular include powerful antioxidant anthocyanins (such as cyanidin and delphinidin), flavanols and phenolic acids (such as ellagitannins/ellagic acid), which can inhibit the enzymes amylase and glucosidase, preventing glucose absorption in the intestines (controlling postprandial glycaemia) and stimulating insulin secretion [2][3]. Berry anthocyanins enhance glucose uptake and metabolism through pAMPK/AMPK, GLUT-4 and SGLUT-1 activation; they also inhibit weight gain and pro-inflammatory responses, downregulating lipogenesis genes and pro-inflammatory cytokine production [4]. Additionally, anthocyanins support the positive modulation of plasma lipid levels, gut microbiota and endothelial function [1]. Findings between studies are not all in agreement with regard to the metabolic control effects in T2DM patients, which may be due to differences in the quantitative or qualitative anthocyanin intake. For instance, the profile of bioactive substances may vary with the product type or the variety and mixture of berries (e.g., wild blueberry, bilberry, cranberry, elderberry, raspberry seeds and strawberry). Additionally, health benefits may vary with the subjects’ baseline conditions (e.g., statistically significant alterations in glycaemia are unlikely in subjects with a normal glucose tolerance) [2]. Some studies propose that a moderate intake of berries (at least 50 mg anthocyanins, ⅓ cup of blueberries) is enough to mitigate the risk or to help in the metabolic control of T2DM, giving an example of the total anthocyanins (mg/100 g fresh) in blueberry highbush (387) and blueberry lowbush (487) [1][2]. However, other studies recommend a daily dose of 200 g to 400 g (70 kg; middle-aged person) [4].
1.2. Fermented Dairy Products (with or without Vitamin D or Probiotic Supplementation)
Fermented dairy products not supplemented with vitamin D or probiotics, especially yoghurts, seem to be capable of improving glycaemic markers. They potentially help the metabolic control of T2DM through increased satiety (decreased food intake), improved insulin sensitivity and insulin resistance, increased glucose tolerance, an altered gut hormone response, changed gut microbiota and enhanced body fat reduction [8][12].
Yoghurt naturally includes lactic acid bacteria, which can promote favourable changes in gut microbiota, the amelioration of glycaemic control and insulin resistance and an increase in glucagon-like peptide 1 (GLP-1), with an anorexigenic effect. The superior bioavailability of amino acids and insulinotropic peptides and the bacterial biosynthesis of vitamins such as vitamin K2 can explain the improvement of insulin sensitivity through the vitamin K-dependent protein osteocalcin, anti-inflammatory properties and lipid-lowering effects [13]. Milk and dairy products are strong insulin secretagogues, as their intake causes acute hyperinsulinaemia, and they also contain bioactive peptides, calcium and B-complex vitamins, which support regulation of the microbiome and the metabolic control of diabetes [8][12]. However, high-fat dairy and animal proteins are associated with greater insulin resistance and lower HDL cholesterol [9].
Dairy products supplemented/fortified with vitamin D (100 IU to 28,000 IU vitamin/day) can potentially control T2DM by suppressing the activation of T cells and systematic inflammatory markers [10]. The supplementation of dairy products with probiotics can also have a positive impact on the metabolic control of T2DM patients, since these patients tend to have a microbial imbalance or dysbiosis in the gastrointestinal tract, as well as systemic low-grade inflammation. This dysbiosis and inflammation can support an increased ratio of Firmicutes to Bacteroidetes (two dominant phyla within the gastrointestinal tract), and a reduced presence of lactic acid, producing species such as Lactobacillus, Bifidobacterium and Streptococcus. For instance, “Streptococcus thermophilus, Lactobacillus bulgaricus and/or Bifidobacterium lactis administered for 6 to 12 weeks may be efficacious for improving glycaemic control in adults with T2DM” [14]. Thus, yoghurt enriched with probiotic bacteria can reinforce gut health, reduce inflammation, regulate appetite, improve immune responses and ameliorate the intestinal barrier function and lipid profiles. Probiotics support the modulation of the gut microbiome by increasing GLP-1 and stimulating the production of short-chain fatty acids (SCFAs), which also promote GLP-1 secretion in obese subjects [11].
The findings between studies with regard to the integration of probiotics in dairy products in the diet of T2DM are not in agreement. A significant impact on fasting insulin, HbA1c or plasma C-reactive protein (CRP) were not reported in some studies [11][32][33], in opposition to other studies that reported positive outputs in the metabolic parameters of T2DM patients [11][12][13][14]. For instance, one study indicated that multi-strain probiotics with 7 million to 100 billion colony-forming units of Lactobacillus acidophilus, S. thermophilus, L. bulgaricus and/or B. lactis administered for 6–12 weeks may effectively control glycaemia in T2DM [14]. Additionally, one study indicated that the probiotic supplementation of dairy matrices can reduce lipid concentrations and anthropometric parameters [11].
1.3. Whole Cereals
The dietary fibre intake from whole grains decreases blood glucose, attenuates insulin responses and lowers inflammatory markers. Insoluble dietary fibres improve the whole-body insulin resistance after both a short-term and prolonged intake of cereal fibre. In general, fibre delays gastric emptying and decreases the intestinal absorption of glucose. Dietary fibre (both soluble and insoluble), phenolic compounds and other bioactive constituents may also decrease starch hydrolysis [15][16]. Whole grains are comprised of phenolic acids, such as hydroxybenzoic acids (p-hydroxybenzoic acid, gallic acid, vanillic acid and syringic acid) or hydroxycinnamic acids (ferulic acid, p-coumaric acid, caffeic acid and sinapic acid), which contribute to the overall antioxidant capacity (lowering oxidative stress), as well as having a high fibre content. Additionally, there is an inverse relationship between soluble dietary fibre intake and triglyceride levels [17]. The fermentation of fibre and resistant starch by microbiota in the large intestine leads to the production of SCFAs, which are linked to the secretion of gut hormones, glucose and lipid metabolism [15][16]. The consumption of whole grains therefore supports the regulation of intestinal microbiota, the synthesis of SCFAs, a reduction in transit time, the prevention of insulin resistance and the antioxidant activity of phenolic acids, which confer protection by binding carcinogens, as well as modulating the glycaemic response [17]. The consumption of whole grains also supports the control of long-term weight gain [15][16].
Whole grains should not be processed (e.g., barley, bulgur, farro, millet, quinoa, black rice, brown rice, red rice, wild rice, oatmeal, popcorn and whole-wheat flour), since this affects the dietary fibre composition and the release of phenolics. For instance, milling decreases the particle size and increases the surface area of cereals, which substantially increases starch hydrolysis and the glycaemic response [15].
1.4. Nuts
In general, nuts present a lower glycaemic index (GI), an elevated content of fibre and non-sodium minerals (potassium and magnesium) and high levels of antioxidant and anti-inflammatory compounds. In addition to macronutrients, nuts also contain micronutrients, water-soluble vitamins such as folate, non-sodium minerals and phenolics (e.g., flavonoids, phenolic acids, stilbenes, coumarins, lignans and tannins, among others). The presence of minerals (e.g., potassium, calcium and magnesium) and vegetable protein (arginine) potentially contributes to the maintenance of glucose control. Additionally, fatty acids are the predominant components of nuts, which explains their ability to lower cholesterol (e.g., almonds, Brazil nuts, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts) [19].
The intake of nuts is recommended as an alternative to the consumption of carbohydrates in individuals with T2DM because it improves glycaemic control and lipid risk factors [34]. Besides the potential to control glycaemia, the consumption of almonds has been related to weight control; decreased macronutrient bioavailability; reduced hunger; elevated satiety; increased resting energy expenditure; and a reduction of mean body mass, fat mass, diastolic blood pressure and low-density lipoprotein (LDL) cholesterol; as well as a possible improvement in colonic microbiota [20].
Additionally, the consumption of pistachios seems to result in better metabolic control (e.g., fasting blood glucose and Homeostatic Model Assessment of Insulin Resistance—HOMA-IR), with a simultaneous improvement in the cardiometabolic profile, endothelial dysfunction, inflammation and oxidative stress of patients with metabolic syndrome, as well as the possible amelioration of the lipid profile of obese patients. Thus, it seems that pistachio nuts may be an invaluable adjuvant therapy for the prevention and treatment of T2DM due to their high content of different types of antioxidants [21].
1.5. Proteins (Especially Plant Protein-Rich Diets)
Diets rich in plant protein, white meat or fish protein improve the levels of total cholesterol in people with T2DM compared with diets rich in red meat protein. Fasting glucose levels were found to improve with a plant protein diet, and improvements in blood pressure and body weight were achieved with weight-loss diets: 23% to 32% of energy intake as protein (for up to one year) [22].
With regard to plant protein foods, including leguminous proteins, the consumption of both beans and chickpeas (e.g., hummus) leads to an improvement of glycaemic control and insulin sensitivity in T2DM patients [23][24][35]. Leguminous plants such as soybeans and pulses (dried beans, dried peas, chickpeas and lentils) can reduce insulin resistance and other related T2DM parameters (e.g., HOMA-IR), with higher evidence for soybeans and chickpeas than other leguminous plants [23]. For instance, the consumption of beans leads to a healthy gut microbial population and diversity (SCFAs are produced from the fermentation of the complex dietary fibre and resistant starches of beans, increasing butyrate production), and the gut microbiome supports the reduction of body weight and body fat, as well as improving insulin sensitivity [23]. The better metabolic control of glucose following the consumption of hummus (in terms of an improvement in glucose tolerance and the enhancement of insulin secretion) can be explained by its low sugar and high fibre/protein content, slow digestibility, slow rates of absorption, amino acid profile, soluble fibres (slower gastric emptying), high monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) content, high levels of the resistant starch amylose which is digested and absorbed more slowly, and an increase in gut hormones such as GLP-1 and peptide YY because of the fermentation of amylose [24].
The consumption of the whey protein also supports the improvement of glycaemic control and insulin sensitivity in T2DM patients, which is explained by a rapid increase in bioactive peptides and amino acids (from the hydrolysation of the whey), increased insulin release and improved postprandial hyperglycaemia. Additionally, bioactive peptides activate the release of incretin hormones (gastric inhibitory polypeptide and GLP-1), which improve insulin resistance and inhibit dipeptidyl peptidase-IV, consequently reducing the degradation of gastric inhibitory polypeptide and GLP-1 [25]. Overall, whey supplementation was found to significantly decrease systolic blood pressure, diastolic blood pressure, HDL, waist circumference, triglycerides and fasting blood glucose in intervention groups when compared to control groups [36].
1.6. Sunflower Seeds and Flaxseeds
The bioactive components of sunflower seeds (chlorogenic acid) and flaxseeds (secoisolariciresinol diglucosoid) can improve insulin resistance and insulin production [26].
1.7. Cabbage and Lupin
According to epidemiological studies, crucifers such as cabbage may reduce the risk of T2DM due to the presence of bioactive compounds (e.g., vitamins, fibre, phenolic compounds, flavonoids, among others) which support the attenuation of oxidative stress, obesity, insulin resistance and hyperglycaemia [27]. However, according to the results of a recent systematic review and meta-analysis, brassica vegetables have no significant impact on the serum levels of triglycerides, LDL cholesterol, HDL cholesterol, fasting blood sugar and HbA1c in adults [37].
Lupin seems to be equally and possibly more effective among all the legumes in terms of the long-term health protection (e.g., satiety and glycaemic control, or serum lipid profile and blood pressure benefits) [28].
1.8. Prickly Pear Cacti (Opuntia spp.) Cladodes
The high fibre content of prickly pear cladodes may explain the amelioration of gut microbiota, with reduced glucose absorption, improved glycaemic control and reduced glucose peaks [29]. Nopal (Opuntia ficus indica) seems to suppress the inflammatory effect of lipopolysaccharides, which are responsible for inducing insulin resistance and glucose intolerance. For instance, in patients with T2DM, a significantly lower area under a curve (AUC) for glucose (287 ± 30) was achieved in the high-soy-protein breakfast + nopal group compared to the high-soy-protein breakfast only group (443 ± 49) [38].
1.9. Honey
The consumption of honey by T2DM patients may improve the glycaemic and lipid profile (although the effects of honey are acute/short term). These effects may be explained by its polyphenol content, the inhibition of α-amylase and α-glucosidase, a reduction of the inflammatory state and oxidative stress and the consequent protection from endothelial dysfunction and neurodegeneration [30]. The benefits of honey over isolated glucose as a sweetener were also confirmed in a study comparing the glycaemic effect of 75 g and 30 g of natural honey vs. 75 g of glucose in T2DM patients (n = 97), where results showed a mean rise in blood glucose of 85 mg/dL, 30 mg/dL and 170 mg/dL, respectively, (p < 0.005) after two hours [39].
2. Identified ‘Superfoods’ with the Potential to Reduce HbA1c in T2DM Patients
The number of reviews describing the potential reduction of HbA1c as a consequence of the consumption of certain ‘superfoods’ by T2DM patients is very limited and the results differ.
2.1. Foods with Polyphenols (e.g., Berries)
A reduction in HbA1c was reported in some studies following the consumption of berries (e.g., 50 g of freeze-dried strawberry powder, equivalent to 500 g fresh strawberries each day, or 320 mg day−1 anthocyanin capsules—bilberry and blackcurrant), with a potential 0.20% reduction in HbA1c [4][40][41]. The consumption of some foods containing polyphenols (e.g., green tea, catechins or total polyphenols) also produced improvements in the HbA1c levels according to some studies, with a net change of −0.30% (95% CI: −0.37, −0.22) [6][42]. For instance, three months of supplementation with resveratrol (250 mg/once daily) significantly reduced the mean HbA1c (mmol/L) (mean ± SD: 9.99 ± 1.50 vs. 9.65 ± 1.54; p < 0.05), systolic blood pressure (mm Hg) (mean ± SD: 139.71 ± 16.10 vs. 127.92 ± 15.37; p < 0.05) and total cholesterol (mg/dL) (mean ± SD: 4.70 ± 0.90 vs. 4.33 ± 0.76; p < 0.05), i.e., resveratrol supplementation improved the metabolic control of T2DM patients [43]. Additionally, the consumption of total polyphenols was associated with lower HbA1c concentrations in an observational study with T2DM subjects (n = 3000): people with the highest intake of energy-adjusted polyphenols (upper tercile) had a more favourable cardiovascular risk factor profile compared to people with the lowest intake (lower tercile) (7.70% vs. 7.67% HbA1c) [44].
2.2. Fermented Dairy Products
The consumption of fermented dairy products was associated with a reduction in HbA1c in some studies [8][12][14]. For instance, a daily intake of 250 mL kefir significantly lowered HbA1c levels (providing a mean reduction of 1.34% HbA1c, from 8.54% (±1.56) to 7.20% (±1.12)) compared with the control group, who only received metformin [45].
Additionally, yoghurt enriched with vitamin D or probiotics produced a significant reduction in HbA1c. Over 12 weeks, yoghurt containing 170 mg calcium and 500 IU vitamin D/250 mL produced a reduction in HbA1c of around 1%, from 8.7% ± 1.8 to 7.8% ± 1.3, with a much lower improvement in HbA1c in the group consuming yoghurt that was not enriched with vitamin D (from 8.9% ± 1.6 to 8.5% ± 1.6) [46]. Meanwhile, 120 g/day fermented milk containing L. acidophilus La-5 and Bifidobacterium animalis subsp lactis BB-12 (109 colony-forming units/day, each) gave a mean reduction of −0.67% HbA1c, compared with a mean change of +0.31 HbA1c for the control group (conventional fermented milk) [47]. HbA1c (mmol/L) was also reduced from 7.61 ± 1.22 (before intervention) to 6.40 ± 1.91 (after intervention) at the end of an 8-week trial with fermented milk (kefir) enriched with probiotics (n = 60 T2DM patients) [48].
2.3. Whole Cereals
The arabinoxylan in whole cereals can contribute to the reduction of fasting glucose and HbA1c in diabetic fat rats [15][49]. The consumption of whole cereals can also contribute to better insulin sensitivity, glucose homeostasis, the amelioration of lipid disorders, weight control, the regulation of gut microbiota and/or better antioxidant and anti-inflammatory effects [15][16][17]. An increased fibre intake was found to reduce HbA1c (mean difference −2.00 mmol/mol (−2.3%) (95% CI: −3.30 to −0.71) from 33 controlled trials), comparing a daily dietary fibre intake of 35 g with the average intake of 19 g (increasing the daily fibre intake by 15 g or up to 35 g) in prospective studies or controlled trials in adults with prediabetes, gestational diabetes, type 1 diabetes and T2DM [18].
Additionally, HbA1c levels were decreased in the whole-grain germinated brown rice group (T2DM patients) in a randomised control trial (RCT), but without significance [50]. A low-GI legume diet reduced HbA1c values by −0.5% (95% CI: −0.6% to −0.4%), while a high wheat fibre diet reduced HbA1c values by −0.3% (95% CI: −0.4% to −0.2%) (n = 121 participants with T2DM, randomised into two groups: (i) low-GI legume diet—increasing legume intake (such as beans, chickpeas and lentils) by at least 1 cup per day, and (ii) high wheat fibre diet—increasing insoluble fibre by the consumption of whole wheat products; 3 months) [51]. However, no positive effect of the consumption of whole grains was found on HOMA-IR, HbA1c or 2 h glucose in a meta-analysis of 32 RCTs [52], which may be explained by the fact that only four out of the thirty-two RCTs specifically enrolled T2DM patients.
2.4. Nuts: Almonds and Pistachios
Overall, studies are not in agreement regarding the positive impact of nut consumption on the metabolic control of T2DM patients (e.g., fasting plasma glucose or HbA1c). Some studies describe a possible positive effect of some types of nuts, such as almonds and pistachios, on glycaemic control [19][20][21]. For instance, a reduction in HbA1c was reported following the consumption of almonds and pistachios in T2DM patients [20][21]. On the contrary, no favourable effects were found with regard to fasting blood glucose or HbA1c, as a consequence of consuming tree nuts or peanuts for 3 months [19][53], although only 14 out of the 40 RCTs specifically enrolled T2DM patients.
2.5. Proteins (Especially Plant Protein-Rich Diets)
A moderately greater weight loss was attained with a higher protein hypocaloric diet in T2DM patients compared to a lower protein hypocaloric diet. As expected, significant improvements in HbA1c were achieved with weight loss diets, which were not influenced by higher protein diets [22]. Depending on the magnitude of weight loss, greater reductions in HbA1c can be expected in T2DM patients. For instance, a weight loss of ≥15% reduced HbA1c by an average of 1.2% (1 year of follow-up) [54]. Meanwhile, a low-GI diet (190 g (1 cup) of legumes (cooked beans, chickpeas or lentils) per day for 3 months) significantly improved HbA1c (−0.5% absolute) [51]. Additionally, the consumption of black bean pasta meals (different protein concentrations) reduced postprandial glycaemia and insulinaemia when compared to the control in adults (white bread) [55].
The consumption of whey protein significantly reduced HbA1c (n = 6 studies) (weighted mean difference: −0.15; 95% CI: −0.29, −0.01) [25]. Whey can be given immediately before a meal (e.g., 55 g whey protein preload, given 30 min before a meal), since it reduces the postprandial glycaemic response by over a third, which can be explained by an increase in the early postprandial plasma insulin and GLP-1 responses [56][57]. Thus, T2DM patients who have relatively well-controlled fasting glucose and a mild-to-moderate elevation of HbA1c may be more likely to benefit from the postprandial glucose lowering effects of whey protein [57].
2.6. Flaxseed Supplementation
Flaxseed supplementation significantly reduced HbA1c (−0.19%; 95% CI: −0.38 to 0.00; p = 0.045; I2 = 12.8%) in participants with T2DM compared with the control group, but without an effect on fasting blood glucose (FBG) (−0.31 mmol/L; 95% CI: −0.86 to 0.25; p = 0.280; I2 = 82.9%). In particular, more significant reductions were reached for T2DM patients with a higher baseline level of HbA1c (e.g., HbA1c ≥ 7.0%) [58].
2.7. Lupin
A daily dose of a 10 g Lupinus mutabilis-based snack, consumed 30 min before lunch, significantly reduced HbA1c in a group of patients with HA1c levels <8.0% (mean value (%) ± standard deviation: 6.5 ± 0.6 (baseline) and 6.3 ± 0.7 (28 weeks); around 0.2% reduction), with no significant improvements in the group of patients with HbA1c levels >8.0%. In addition, body weight and both systolic and diastolic blood pressure significantly decreased, while HDL cholesterol significantly increased by the end of the study period. However, the fasting glucose levels (mg/dL) increased from 116.3 ± 27.8 (baseline) to 134.1 ± 38.71 (28 weeks) [59]. In contrast, in another studies, no significant effects on glycaemic control or blood pressure were found with the regular consumption of lupin-enriched foods in T2DM patients [60].
2.8. The Most Significant Reductions in HbA1c
Overall, the most significant reported reductions in HbA1c were achieved through (i) the consumption of dairy products (e.g., kefir or yoghurt) (HbA1c reduction of around 1%), with more significant reductions appearing to be attained with dairy products enriched with vitamin D or probiotics compared to non-enriched dairy products [46][47]; (ii) an increased fibre intake (by 15 g or up to 35 g) (HbA1c reduction of around 2%) [18] (Figure 1); or (iii) diets with weight reduction: a weight loss ≥15% reduced HbA1c by an average of 1.2% (1 year of follow-up) [54]. Thus, both dairy products, such as kefir or yoghurt (with or without vitamin D and/or probiotics) and an increased fibre intake (by 15 g or up to 35 g) [18][46][47] can be used as nutraceuticals, i.e., “a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of a disease” [61].
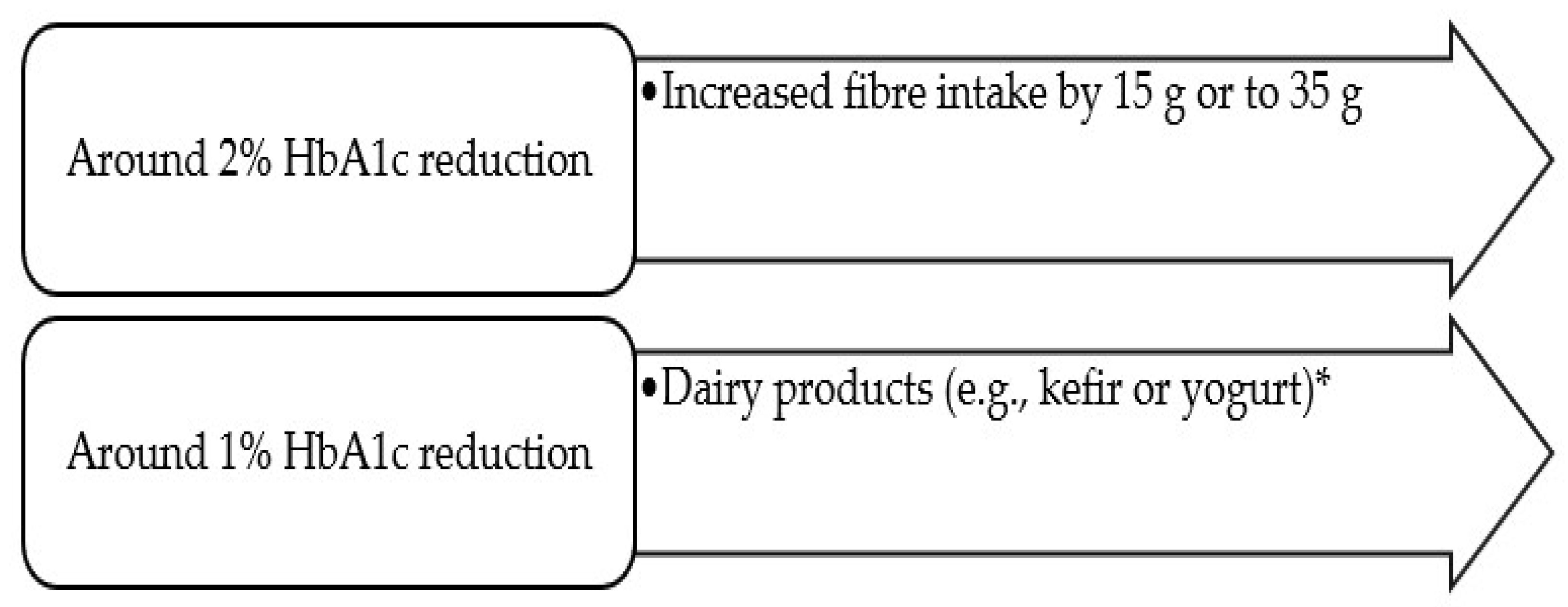
Figure 1. ‘Superfoods’ related to significant HbA1c reductions (≥1%)* Especially, dairy products enriched with vitamin D or probiotics.
3. New Proposed International Guidance on the Consumption of Identified ‘Superfoods’ by T2DM Patients
A proper medical nutrition therapy for T2DM patients may be more easily achieved and/or maintained with the concomitant use of ‘superfoods’, since they can improve or maintain metabolic control (glucose and lipid profiles, body mass weight, blood pressure and anti-inflammatory markers, among others). Thus, the following recommendations are proposed:
-
The isolated consumption of ‘superfoods’ should not be used to substitute a proper and successful diet or exercise plan for T2DM patients. The adoption of a certain diet, such as a Mediterranean-style, low-fat or low-carbohydrate diet, seems to be more relevant than the isolated consumption of ‘superfoods’ by T2DM patients, since it has been demonstrated that a successful medical nutrition therapy plan per se can reach a similar or greater reduction in HbA1c than medication for T2DM [62].
-
It is likely that the goals of a proper medical nutrition therapy for T2DM patients may be more easily achieved and/or maintained with the concomitant use of ‘superfoods’, since some ‘superfoods’ are likely to improve or maintain metabolic control (glucose and lipid profiles, body mass weight, blood pressure and anti-inflammatory markers, among others).
-
‘Superfoods’ should preferably be integrated into the diet plan of T2DM patients with the involvement of a nutritionist. For instance, ‘superfoods’ can be used to substitute foods from the same group of the food wheel, respecting the principles of a diversified and rational nutritional plan.
-
Metabolic supervision should be carried out in the months before and after the introduction of ‘superfoods’ into the diet of T2DM patients to identify and quantify eventual ameliorations.
-
‘Superfoods’ should be consumed in the right doses (quantitatively and qualitatively) to ensure that their bioactive properties are achieved, particularly given that ‘superfoods’ tend to be more expensive than other foods.
-
Less-controlled T2DM patients can benefit more from the inclusion of ‘superfoods’ in their diet than more-controlled T2DM patients.
-
More significant HbA1c reductions seem to be achieved with the consumption of dairy products (giving a reduction of around 1%, with or without the enrichment of vitamin D and probiotics, according to diverse studies) [45][46][48] and with fibre supplementation (based on the comparison between a daily dietary fibre intake of 35 g and the average intake of 19 g: increasing the daily fibre intake by 15 g or up to 35 g resulted in a reduction of −2.00 mmol/mol (−2.3%) HbaA1c) [18]. Thus, these ‘superfoods’ are recommended in the diet of T2DM patients, although this does not exclude the need for metabolic control (before and after any alteration in the diet plan), since results are not in agreement between studies.
-
High-fat dairy products are not recommended [9][11]. The consumption of cheese can result in a 5 to 24% increase in the risk of developing T2DM according to some prospective cohort studies [11]. Thus, the consumption of high-fat dairy products should be limited (or eliminated) in the diets of T2DM patients.
-
Refined grain products should be substituted by whole grain foods, since diverse studies support an improvement in the metabolic metabolism and lipid profile of T2DM patients, a decreased risk of T2DM and a possible decreased risk of colon cancer, fatal coronary heart disease (CHD) and cardiovascular disease (CVD) mortality with the consumption of whole grain foods [16].
-
Different classes of foods with polyphenols (e.g., berries) are recommended rather than a specific food due to a likely pleiotropic effect, i.e., a potential contribution towards the regulation of glycaemic and lipidic metabolism (increased HDL cholesterol and decreased LDL cholesterol), blood pressure control and anti-obesity, as well as an improvement in the anti-inflammatory and oxidative stress plasma markers [2][4].
-
Proteins (especially plant protein-rich diets), such as beans/chickpeas, other leguminous plants (e.g., soybeans) and other proteins, such as whey protein, are recommended in the regular diet of T2DM patients, since they support an improvement in glycaemic control and insulin sensitivity [22][23][24].
-
Nuts (without added salt or sugar) are recommended, especially to control the lipidic profile (total cholesterol, LDL and triacylglycerols) [19].
-
Nuts seem to be an adequate alternative to the consumption of carbohydrates, with improved glycaemic control and lipid risk factors in individuals with T2DM [34].
-
Other ‘superfoods’, such as sunflower seeds, flaxseeds, cabbage, lupin, prickly pear cacti (Opuntia spp.) cladodes and honey (short term), may also improve the metabolic control of T2DM patients, but further studies are recommended.
References
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236.
- Chan, S.W.; Tomlinson, B. Effects of Bilberry Supplementation on Metabolic and Cardiovascular Disease Risk. Molecules 2020, 25, 1653.
- Derrick, S.A.; Kristo, A.S.; Reaves, S.K.; Sikalidis, A.K. Effects of Dietary Red Raspberry Consumption on Pre-Diabetes and Type 2 Diabetes Mellitus Parameters. Int. J. Environ. Res. Public Health 2021, 18, 9364.
- Hameed, A.; Galli, M.; Adamska-Patruno, E.; Krętowski, A.; Ciborowski, M. Select Polyphenol-Rich Berry Consumption to Defer or Deter Diabetes and Diabetes-Related Complications. Nutrients 2020, 12, 2538.
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555.
- Giacco, R.; Costabile, G.; Fatati, G.; Frittitta, L.; Maiorino, M.I.; Marelli, G.; Parillo, M.; Pistis, D.; Tubili, C.; Vetrani, C.; et al. Effects of polyphenols on cardio-metabolic risk factors and risk of type 2 diabetes. A joint position statement of the Diabetes and Nutrition Study Group of the Italian Society of Diabetology (SID), the Italian Association of Dietetics and Clinical Nutrition (ADI) and the Italian Association of Medical Diabetologists (AMD). Nutr. Metab. Cardiovasc. Dis. 2020, 30, 355–367.
- Restani, P.; Di Lorenzo, C.; Fradera, U.; Stockley, C.S.; Teissedre, P.L.; Ruf, J.C.; Iasiello, B.; Biella, S.; Colombo, F.; Kosti, R.I. Is it scientifically justifiable to exclude wine and/or unfermented grape derivatives from the diet of consumers with or at risk of developing type-2 diabetes? Food Funct. 2020, 11, 10266–10278.
- Awwad, S.F.; Abdalla, A.; Howarth, F.C.; Stojanovska, L.; Kamal-Eldin, A.; Ayyash, M.M. Invited review: Potential effects of short- and long-term intake of fermented dairy products on prevention and control of type 2 diabetes mellitus. J. Dairy Sci. 2022, 105, 4722–4733.
- Lombardo, M.; Bellia, C.; Moletto, C.; Aulisa, G.; Padua, E.; Della-Morte, D.; Caprio, M.; Bellia, A. Effects of Quality and Quantity of Protein Intake for Type 2 Diabetes Mellitus Prevention and Metabolic Control. Curr. Nutr. Rep. 2020, 9, 329–337.
- Emadzadeh, M.; Sahebi, R.; Khedmatgozar, H.; Sadeghi, R.; Farjami, M.; Sharifan, P.; Ravanshad, Y.; Ferns, G.A.; Ghayour-Mobarhan, M. A systematic review and meta-analysis of the effect of Vitamin D-fortified food on glycemic indices. Biofactors 2020, 46, 502–513.
- Companys, J.; Pla-Pagà, L.; Calderón-Pérez, L.; Llauradó, E.; Solà, R.; Pedret, A.; Valls, R.M. Fermented Dairy Products, Probiotic Supplementation, and Cardiometabolic Diseases: A Systematic Review and Meta-analysis. Adv. Nutr. 2020, 11, 834–863.
- Yanni, A.E.; Kartsioti, K.; Karathanos, V.T. The role of yoghurt consumption in the management of type II diabetes. Food Funct. 2020, 11, 10306–10316.
- Companys, J.; Pedret, A.; Valls, R.M.; Solà, R.; Pascual, V. Fermented dairy foods rich in probiotics and cardiometabolic risk factors: A narrative review from prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2021, 61, 1966–1975.
- Tiderencel, K.A.; Hutcheon, D.A.; Ziegler, J. Probiotics for the treatment of type 2 diabetes: A review of randomized controlled trials. Diabetes Metab. Res. Rev. 2020, 36, e3213.
- Wu, W.; Qiu, J.; Wang, A.; Li, Z. Impact of whole cereals and processing on type 2 diabetes mellitus: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1447–1474.
- Tieri, M.; Ghelfi, F.; Vitale, M.; Vetrani, C.; Marventano, S.; Lafranconi, A.; Godos, J.; Titta, L.; Gambera, A.; Alonzo, E.; et al. Whole grain consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020, 71, 668–677.
- Khan, J.; Khan, M.Z.; Ma, Y.; Meng, Y.; Mushtaq, A.; Shen, Q.; Xue, Y. Overview of the Composition of Whole Grains’ Phenolic Acids and Dietary Fibre and Their Effect on Chronic Non-Communicable Diseases. Int. J. Environ. Res. Public Health 2022, 19, 3042.
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053.
- Alasalvar, C.; Salvadó, J.S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192.
- Dreher, M.L. A Comprehensive Review of Almond Clinical Trials on Weight Measures, Metabolic Health Biomarkers and Outcomes, and the Gut Microbiota. Nutrients 2021, 13, 1968.
- Nowrouzi-Sohrabi, P.; Hassanipour, S.; Sisakht, M.; Daryabeygi-Khotbehsara, R.; Savardashtaki, A.; Fathalipour, M. The effectiveness of pistachio on glycemic control and insulin sensitivity in patients with type 2 diabetes, prediabetes and metabolic syndrome: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 1589–1595.
- Pfeiffer, A.F.H.; Pedersen, E.; Schwab, U.; Risérus, U.; Aas, A.M.; Uusitupa, M.; Thanopoulou, A.; Kendall, C.; Sievenpiper, J.L.; Kahleová, H.; et al. The Effects of Different Quantities and Qualities of Protein Intake in People with Diabetes Mellitus. Nutrients 2020, 12, 365.
- Mullins, A.P.; Arjmandi, B.H. Health Benefits of Plant-Based Nutrition: Focus on Beans in Cardiometabolic Diseases. Nutrients 2021, 13, 519.
- Reister, E.J.; Belote, L.N.; Leidy, H.-J. The Benefits of Including Hummus and Hummus Ingredients into the American Diet to Promote Diet Quality and Health: A Comprehensive Review. Nutrients 2020, 12, 3678.
- Amirani, E.; Milajerdi, A.; Reiner, Ž.; Mirzaei, H.; Mansournia, M.A.; Asemi, Z. Effects of whey protein on glycemic control and serum lipoproteins in patients with metabolic syndrome and related conditions: A systematic review and meta-analysis of randomized controlled clinical trials. Lipids Health Dis. 2020, 19, 209.
- Rehman, A.; Saeed, A.; Kanwal, R.; Ahmad, S.; Changazi, S.H. Therapeutic Effect of Sunflower Seeds and Flax Seeds on Diabetes. Cureus 2021, 13, e17256.
- Uuh-Narvaez, J.J.; Segura-Campos, M.R. Cabbage (Brassica oleracea var. capitata): A food with functional properties aimed to type 2 diabetes prevention and management. J. Food Sci. 2021, 86, 4775–4798.
- Bryant, L.; Rangan, A.; Grafenauer, S. Lupins and Health Outcomes: A Systematic Literature Review. Nutrients 2022, 14, 327.
- Kashif, R.R.; D’Cunha, N.M.; Mellor, D.D.; Alexopoulos, N.I.; Sergi, D.; Naumovski, N. Prickly Pear Cacti (Opuntia spp.) Cladodes as a Functional Ingredient for Hyperglycemia Management: A Brief Narrative Review. Medicina 2022, 58, 300.
- Terzo, S.; Mulè, F.; Amato, A. Honey and obesity-related dysfunctions: A summary on health benefits. J. Nutr. Biochem. 2020, 82, 108401.
- American Diabetes Association. Alcohol & Diabetes. Available online: https://diabetes.org/healthy-living/medication-treatments/alcohol-diabetes (accessed on 29 March 2023).
- Hove, K.D.; Brøns, C.; Færch, K.; Lund, S.S.; Rossing, P.; Vaag, A. Effects of 12 weeks of treatment with fermented milk on blood pressure, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: A randomised double-blind placebo-controlled study. Eur. J. Endocrinol. 2015, 172, 11–20.
- Mohamadshahi, M.; Veissi, M.; Haidari, F.; Javid, A.Z.; Mohammadi, F.; Shirbeigi, E. Effects of probiotic yogurt consumption on lipid profile in type 2 diabetic patients: A randomized controlled clinical trial. J. Res. Med. Sci. 2014, 19, 531–536.
- Jenkins, D.J.A.; Kendall, C.W.C.; Lamarche, B.; Banach, M.S.; Srichaikul, K.; Vidgen, E.; Mitchell, S.; Parker, T.; Nishi, S.; Bashyam, B.; et al. Nuts as a replacement for carbohydrates in the diabetic diet: A reanalysis of a randomised controlled trial. Diabetologia 2018, 61, 1734–1747.
- Wallace, T.C.; Murray, R.; Zelma, K.M. The Nutritional Value and Health Benefits of Chickpeas and Hummus. Nutrients 2016, 8, 766.
- Badely, M.; Sepandi, M.; Samadi, M.; Parastouei, K.; Taghdir, M. The effect of whey protein on the components of metabolic syndrome in overweight and obese individuals; a systematic review and meta-analysis. Diabetes Metab. Syndr. 2019, 13, 3121–3131.
- Darand, M.; Alizadeh, S.; Mansourian, M. The effect of Brassica vegetables on blood glucose levels and lipid profiles in adults. A systematic review and meta-analysis. Phytother. Res. PTR 2022, 36, 1914–1929.
- López-Romero, P.; Pichardo-Ontiveros, E.; Avila-Nava, A.; Vázquez-Manjarrez, N.; Tovar, A.R.; Pedraza-Chaverri, J.; Torres, N. The effect of nopal (Opuntia ficus indica) on postprandial blood glucose, incretins, and antioxidant activity in Mexican patients with type 2 diabetes after consumption of two different composition breakfasts. J. Acad. Nutr. Diet. 2014, 114, 1811–1818.
- Nazir, L.; Samad, F.; Haroon, W.; Kidwai, S.S.; Siddiqi, S.; Zehravi, M. Comparison of glycaemic response to honey and glucose in type 2 diabetes. J. Pak. Med. Assoc. 2014, 64, 69–71.
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Xue, X. Effects of Berries Consumption on Cardiovascular Risk Factors: A Meta-analysis with Trial Sequential Analysis of Randomized Controlled Trials. Sci. Rep. 2016, 6, 23625.
- Calvano, A.; Izuora, K.; Oh, E.C.; Ebersole, J.L.; Lyons, T.J.; Basu, A. Dietary berries, insulin resistance and type 2 diabetes: An overview of human feeding trials. Food Funct. 2019, 10, 6227–6243.
- Liu, K.; Zhou, R.; Wang, B.; Chen, K.; Shi, L.Y.; Zhu, J.D.; Mi, M.T. Effect of green tea on glucose control and insulin sensitivity: A meta-analysis of 17 randomized controlled trials. Am. J. Clin. Nutr. 2013, 98, 340–348.
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541.
- Vitale, M.; Vaccaro, O.; Masulli, M.; Bonora, E.; Del Prato, S.; Giorda, C.B.; Nicolucci, A.; Squatrito, S.; Auciello, S.; Babini, A.C.; et al. Polyphenol intake and cardiovascular risk factors in a population with type 2 diabetes: The TOSCA.IT study. Clin. Nutr. 2017, 36, 1686–1692.
- El-Bashiti, T.A.; Zabut, B.M.; Abu Safia, F.F. Effect of Probiotic Fermented Milk (Kefir) on Some Blood Biochemical Parameters Among Newly Diagnosed Type 2 Diabetic Adult Males in Gaza Governorate. Curr. Res. Nutr. Food Sci. 2019, 7, 568–575.
- Shab-Bidar, S.; Neyestani, T.R.; Djazayery, A.; Eshraghian, M.R.; Houshiarrad, A.; Gharavi, A.; Kalayi, A.; Shariatzadeh, N.; Zahedirad, M.; Khalaji, N.; et al. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: A randomized double-blind clinical trial. BMC Med. 2011, 9, 125.
- Tonucci, L.B.; Santos, K.M.O.; de Oliveira, L.L.; Ribeiro, S.M.R.; Martino, H.S.D. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92.
- Ostadrahimi, A.; Taghizadeh, A.; Mobasseri, M.; Farrin, N.; Payahoo, L.; Beyramalipoor Gheshlaghi, Z.; Vahedjabbari, M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Iran. J. Public Health 2015, 44, 228–237.
- Hartvigsen, M.L.; Jeppesen, P.B.; Lærke, H.N.; Njabe, E.N.; Knudsen, K.E.; Hermansen, K. Concentrated arabinoxylan in wheat bread has beneficial effects as rye breads on glucose and changes in gene expressions in insulin-sensitive tissues of Zucker diabetic fatty (ZDF) rats. J. Agric. Food Chem. 2013, 61, 5054–5063.
- Ding, Q.; Ren, J.; Zhou, Y.; Bai, Z.; Yan, J.; Na, G.; Shan, Y. Whole grain germinated brown rice regulates intestinal immune homeostasis and gastrointestinal hormones in type 2 diabetic patients-a randomized control trial. Food Funct. 2022, 13, 8274–8282.
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Mitchell, S.; Sahye-Pudaruth, S.; Blanco Mejia, S.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 1653–1660.
- Li, Z.; Yan, H.; Chen, L.; Wang, Y.; Liang, J.; Feng, X.; Hui, S.; Wang, K. Effects of whole grain intake on glycemic control: A meta-analysis of randomized controlled trials. J. Diabetes Investig. 2022, 13, 1814–1824.
- Tindall, A.M.; Johnston, E.A.; Kris-Etherton, P.M.; Petersen, K.S. The effect of nuts on markers of glycemic control: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 297–314.
- Shinde, S.; Thieu, V.; Kwan, A.; Houghton, K.F.; Schapiro, D.; Meyers, J. 952-P: The Relationship between Weight Loss and HbA1c in People with Type 2 Diabetes. Diabetes 2022, 71 (Suppl. S1), 952–P.
- Winham, D.M.; Thompson, S.V.; Heer, M.M.; Davitt, E.D.; Hooper, S.D.; Cichy, K.A.; Knoblauch, S.T. Black Bean Pasta Meals with Varying Protein Concentrations Reduce Postprandial Glycemia and Insulinemia Similarly Compared to White Bread Control in Adults. Foods 2022, 11, 1652.
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602.
- Mignone, L.E.; Wu, T.; Horowitz, M.; Rayner, C.K. Whey protein: The “whey” forward for treatment of type 2 diabetes? World J. Diabetes 2015, 6, 1274–1284.
- Xi, H.; Zhou, W.; Sohaib, M.; Niu, Y.; Zhu, R.; Guo, Y.; Wang, S.; Mao, J.; Wang, X.; Guo, L. Flaxseed supplementation significantly reduces hemoglobin A1c in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Nutr. Res. 2023, 110, 23–32.
- Fornasini Salvador, M.V.; Abril-Ulloa, S.V.; Beltrán Carreño, J.P.; Villacrés, E.; Cuadrado-Merino, L.; Robalino, F.; Sánchez, R.; Ricaurte Ortiz, P.S.; Muñoz, E.B.; Benítez Loza, N.B.; et al. Efficacy of a Lupinus mutabilis Sweet snack as complement to conventional type 2 diabetes mellitus treatment. Eficacia de un tentempié de Lupinus mutabilis Sweet como complemento al tratamiento convencional de la diabetes mellitus tipo 2. Nutr. Hosp. 2019, 36, 905–911.
- Ward, N.C.; Mori, T.A.; Beilin, L.J.; Johnson, S.; Williams, C.; Gan, S.K.; Puddey, I.B.; Woodman, R.; Phillips, M.; Connolly, E.; et al. The effect of regular consumption of lupin-containing foods on glycaemic control and blood pressure in people with type 2 diabetes mellitus. Food Funct. 2020, 11, 741–747.
- DeFelice, S.L.; FIM Rationale and Proposed Guidelines for the Nutraceutical Research & Education Act—NREA, 10 November 2002. Foundation for Innovation in Medicine. Available online: http://www.fimdefelice.org/archives/arc.researchact.html (accessed on 23 May 2023).
- Jones, K.; McArdle, P. The ADA nutrition therapy consensus report: A quick guide. J. Diabetes Nurs. 2019, 23, 103.
More
Information
Subjects:
Nutrition & Dietetics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
581
Revisions:
2 times
(View History)
Update Date:
29 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




