
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cristina Russo | -- | 2948 | 2023-06-26 10:39:58 | | | |
| 2 | Sirius Huang | Meta information modification | 2948 | 2023-06-28 03:03:40 | | |
Video Upload Options
A variety of observational epidemiological studies have reported that vitamin D deficiency is often a crucial factor in many inflammatory diseases and autoimmune diseases, as well as the susceptibility to contract infectious diseases, including acute respiratory infections. Similarly, resveratrol regulates immunity, modifying the gene expression and the release of proinflammatory cytokines in the immune cells. Therefore, it plays an immunomodulatory role that can be beneficial in the prevention and development of non-communicable diseases associated with inflammation. Since both vitamin D and resveratrol also act as immunomodulators in inflammatory pathologies, many studies have paid particular attention to an integrated treatment of either vitamin D or resveratrol in the immune reaction against SARS-CoV-2 infections.
1. Vitamin D and SARS-CoV-2 Infection

2. ACE2 Receptor and Resveratrol and SARS-CoV-2 Infection
3. The Immune Response against SARS-CoV-2 Infection
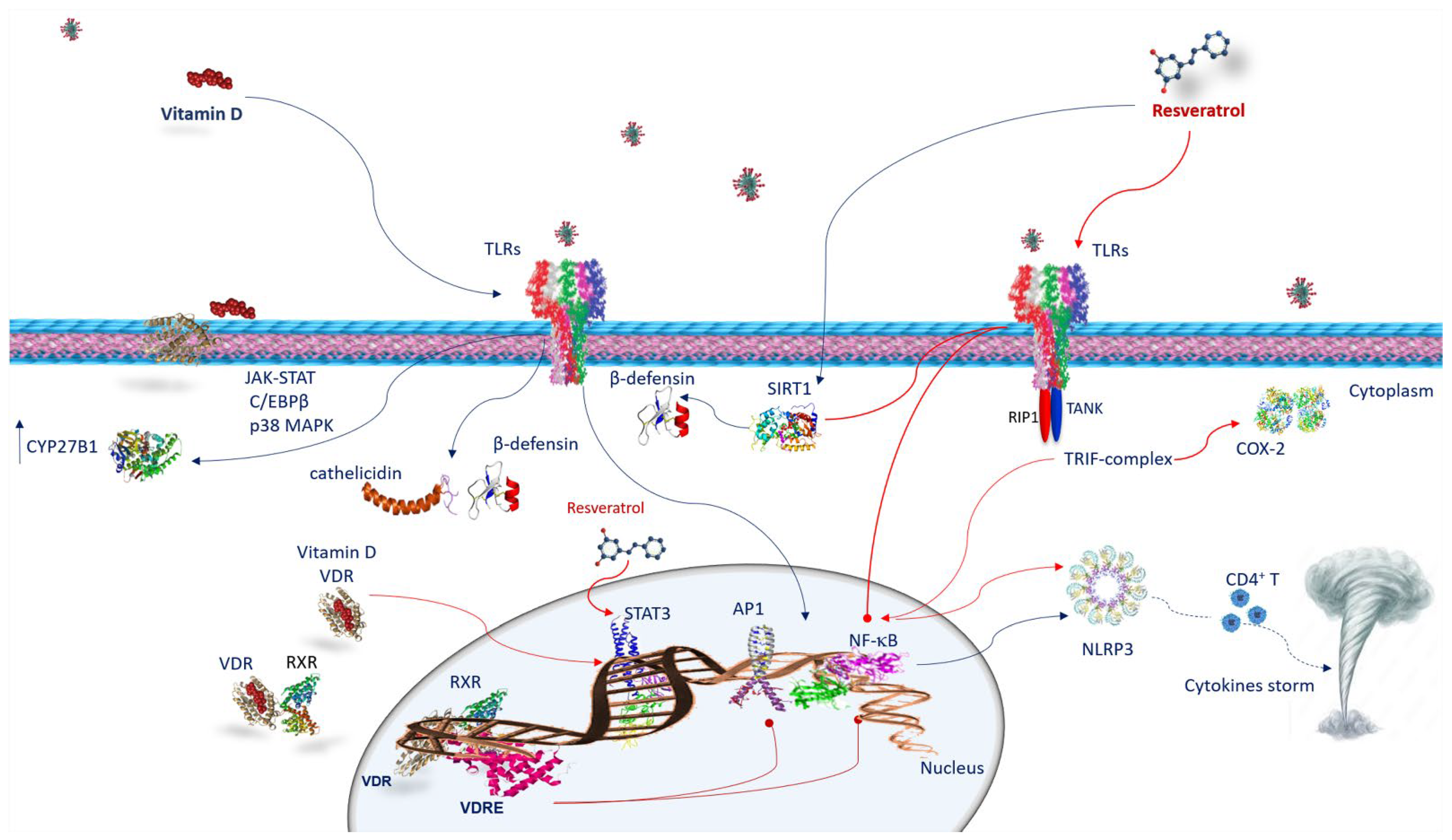
4. Anti-Inflammatory Activity and Immune Response of Vitamin D and Resveratrol against SARS-CoV-2 Infection
4.1. Vitamin D and Innate Immune Response against SARS-CoV-2 Infection
4.2. Vitamin D and Adaptive Immune Response against SARS-CoV-2 Infection
4.3. Resveratrol and Innate Immune Response against SARS-CoV-2 Infection
5. Vitamin D and Resveratrol Effects on Neutrophil Extracellular Traps (NETS) in SARS-CoV-2 Infection
References
- Shi, Y.; Liu, T.; Yao, L.; Xing, Y.; Zhao, X.; Fu, J.; Xue, X. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin-angiotensin system. Sci. Rep. 2017, 7, 3312.
- Shlisky, J.; Mandlik, R.; Askari, S.; Abrams, S.; Belizan, J.M.; Bourassa, M.W.; Cormick, G.; Driller-Colangelo, A.; Gomes, F.; Khadilkar, A.; et al. Calcium deficiency worldwide: Prevalence of inadequate intakes and associated health outcomes. Ann. N. Y. Acad. Sci. 2022, 1512, 10–28.
- Malaguarnera, L. Vitamin D3 as Potential Treatment Adjuncts for COVID-19. Nutrients 2020, 12, 3512.
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury (ALI) via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438.
- Alruwaili, M.A.; Jarrar, Y. Effects of vitamin C and D on the mRNA expression of angiotensin converting enzyme 2 receptor, cathepsin L, and transmembrane serine protease in the mouse lungs. Libyan J. Med. 2022, 17, 2054111.
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620.
- Zhao, M.M.; Yang, W.L.; Yang, F.Y.; Zhang, L.; Huang, W.J.; Hou, W.; Fan, C.F.; Jin, R.H.; Feng, Y.M.; Wang, Y.C.; et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target. Ther. 2021, 6, 134.
- Daniloski, Z.; Jordan, T.X.; Wessels, H.H.; Hoagland, D.A.; Kasela, S.; Legut, M.; Maniatis, S.; Mimitou, E.P.; Lu, L.; Geller, E.; et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell 2021, 184, 92–105.e16.
- Quesada-Gomez, J.M.; Entrenas-Castillo, M.; Bouillon, R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166. J. Steroid Biochem. Mol. Biol. 2020, 202, 105719.
- Huang, J.; An, Q.; Ju, B.M.; Zhang, J.; Fan, P.; He, L.; Wang, L. Role of vitamin D/VDR nuclear translocation in down-regulation of NF-κB/NLRP3/caspase-1 axis in lupus nephritis. Int. Immunopharmacol. 2021, 100, 108131.
- Slominski, A.T.; Slominski, R.M.; Goepfert, P.A.; Kim, T.K.; Holick, M.F.; Jetten, A.M.; Raman, C. Reply to Jakovac and to Rocha et al.: Can vitamin D prevent or manage COVID-19 illness? Am. J. Physiol. Endocrinol. Metab. 2020, 319, E455–E457.
- Jang, I.A.; Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients 2018, 10, 1741.
- Ramdani, L.H.; Bachari, K. Potential therapeutic effects of Resveratrol against SARS-CoV-2. Acta Virol. 2020, 64, 276–280.
- Wang, C.; Ye, X.; Ding, C.; Zhou, M.; Li, W.; Wang, Y.; You, Q.; Zong, S.; Peng, Q.; Duanmu, D.; et al. Two Resveratrol Oligomers Inhibit Cathepsin L Activity to Suppress SARS-CoV-2 Entry. J. Agric. Food Chem. 2023, 71, 5535–5546.
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177.
- Clarke, N.E.; Belyaev, N.D.; Lambert, D.W.; Turner, A.J. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin. Sci. 2014, 126, 507–516.
- Moran, C.S.; Biros, E.; Krishna, S.M.; Wang, Y.; Tikellis, C.; Morton, S.K.; Moxon, J.V.; Cooper, M.E.; Norman, P.E.; Burrell, L.M.; et al. Resveratrol Inhibits Growth of Experimental Abdominal Aortic Aneurysm Associated with Upregulation of Angiotensin-Converting Enzyme 2. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2195–2203.
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 2007, 100, 1512–1521.
- De Ligt, M.; Hesselink, M.K.C.; Jorgensen, J.; Hoebers, N.; Blaak, E.E.; Goossens, G.H. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte 2021, 10, 408–411.
- Hosseini, A.; Hashemi, V.; Shomali, N.; Asghari, F.; Gharibi, T.; Akbari, M.; Gholizadeh, S.; Jafari, A. Innate and adaptive immune responses against coronavirus. Biomed. Pharmacother. 2020, 132, 110859.
- Malaguarnera, L. Vitamin D and microbiota: Two sides of the same coin in the immunomodulatory aspects. Int. Immunopharmacol. 2020, 79, 106112.
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A helpful immuno-modulator. Immunology 2011, 134, 123–139.
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946.
- Kong, X.F.; Zhu, X.H.; Pei, Y.L.; Jackson, D.M.; Holick, M.F. Molecular cloning, characterization, and promoter analysis of the human 25-hydroxyvitamin D3-1alpha-hydroxylase gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6988–6993.
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37–47.
- Russo, C.; Morello, G.; Malaguarnera, R.; Piro, S.; Furno, D.L.; Malaguarnera, L. Candidate genes of SARS-CoV-2 gender susceptibility. Sci. Rep. 2021, 11, 21968.
- Agraz-Cibrian, J.M.; Giraldo, D.M.; Urcuqui-Inchima, S. 1,25-Dihydroxyvitamin D(3) induces formation of neutrophil extracellular trap-like structures and modulates the transcription of genes whose products are neutrophil extracellular trap-associated proteins: A pilot study. Steroids 2019, 141, 14–22.
- Pasquereau, S.; Nehme, Z.; Haidar Ahmad, S.; Daouad, F.; Van Assche, J.; Wallet, C.; Schwartz, C.; Rohr, O.; Morot-Bizot, S.; Herbein, G. Resveratrol Inhibits HCoV-229E and SARS-CoV-2 Coronavirus Replication In Vitro. Viruses 2021, 13, 354.
- Zakaria, M.Y.; Georghiou, P.E.; Banoub, J.H.; Beshay, B.Y. Inclusion of a Phytomedicinal Flavonoid in Biocompatible Surface-Modified Chylomicron Mimic Nanovesicles with Improved Oral Bioavailability and Virucidal Activity: Molecular Modeling and Pharmacodynamic Studies. Pharmaceutics 2022, 14, 905.
- McCreary, M.R.; Schnell, P.M.; Rhoda, D.A. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Sci. Rep. 2022, 12, 10978.
- Wahedi, H.M.; Ahmad, S.; Abbasi, S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 3225–3234.
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000, 164, 6509–6519.
- Holmes-McNary, M.; Baldwin, A.S., Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000, 60, 3477–3483.
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023.
- Valle, M.S.; Russo, C.; Casabona, A.; Crimi, N.; Crimi, C.; Colaianni, V.; Cioni, M.; Malaguarnera, L. Anti-inflammatory role of vitamin D in muscle dysfunctions of patients with COPD: A comprehensive review. Minerva Med. 2022. Online ahead of print.
- Valle, M.S.; Russo, C.; Malaguarnera, L. Protective role of vitamin D against oxidative stress in diabetic retinopathy. Diabetes Metab. Res. Rev. 2021, 37, e3447.
- Arbore, G.; West, E.E.; Spolski, R.; Robertson, A.A.B.; Klos, A.; Rheinheimer, C.; Dutow, P.; Woodruff, T.M.; Yu, Z.X.; O’Neill, L.A.; et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4⁺T cells. Science 2016, 352, aad1210.
- De la Lastra, C.A.; Villegas, I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol. Nutr. Food Res. 2005, 49, 405–430.
- Euba, B.; López-López, N.; Rodríguez-Arce, I.; Fernández-Calvet, A.; Barberán, M.; Caturla, N.; Martí, S.; Díez-Martínez, R.; Garmendia, J. Resveratrol therapeutics combines both antimicrobial and immunomodulatory properties against respiratory infection by non-typeable Haemophilus influenzae. Sci. Rep. 2017, 7, 12860.
- Lin, L.; Wen, S.H.; Guo, S.Z.; Su, X.Y.; Wu, H.J.; Chong, L.; Zhang, H.L.; Zhang, W.X.; Li, C.C. Role of SIRT1 in Streptococcus pneumoniae-induced human β-defensin-2 and interleukin-8 expression in A549 cell. Mol. Cell. Biochem. 2014, 394, 199–208.
- Park, K.; Elias, P.M.; Hupe, M.; Borkowski, A.W.; Gallo, R.L.; Shin, K.O.; Lee, Y.M.; Holleran, W.M.; Uchida, Y. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J. Investig. Dermatol. 2013, 133, 1942–1949.
- Vasamsetti, S.B.; Karnewar, S.; Gopoju, R.; Gollavilli, P.N.; Narra, S.R.; Kumar, J.M.; Kotamraju, S. Resveratrol attenuates monocyte-to-macrophage differentiation and associated inflammation via modulation of intracellular GSH homeostasis: Relevance in atherosclerosis. Free Radic. Biol. Med. 2016, 96, 392–405.
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768.
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147.
- Lescure, F.X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706.
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287.
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999.
- Fakhoury, H.M.A.; Kvietys, P.R.; Shakir, I.; Shams, H.; Grant, W.B.; Alkattan, K. Lung-Centric Inflammation of COVID-19: Potential Modulation by Vitamin D. Nutrients 2021, 13, 2216.
- De Souza Andrade, M.M.; Leal, V.N.C.; Fernandes, I.G.; Gozzi-Silva, S.C.; Beserra, D.R.; Oliveira, E.A.; Teixeira, F.M.E.; Yendo, T.M.; Sousa, M.D.G.T.; Teodoro, W.R.; et al. Resveratrol Downmodulates Neutrophil Extracellular Trap (NET) Generation by Neutrophils in Patients with Severe COVID-19. Antioxidants 2022, 11, 1690.




