Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natalia Kruglova | -- | 1870 | 2023-06-26 08:52:37 | | | |
| 2 | Fanny Huang | Meta information modification | 1870 | 2023-06-26 09:12:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kruglova, N.; Zinatullina, A.; Yegorova, N. Histological Features of Primary Morphogenic Calli. Encyclopedia. Available online: https://encyclopedia.pub/entry/46025 (accessed on 07 February 2026).
Kruglova N, Zinatullina A, Yegorova N. Histological Features of Primary Morphogenic Calli. Encyclopedia. Available at: https://encyclopedia.pub/entry/46025. Accessed February 07, 2026.
Kruglova, Natalia, Anna Zinatullina, Natalia Yegorova. "Histological Features of Primary Morphogenic Calli" Encyclopedia, https://encyclopedia.pub/entry/46025 (accessed February 07, 2026).
Kruglova, N., Zinatullina, A., & Yegorova, N. (2023, June 26). Histological Features of Primary Morphogenic Calli. In Encyclopedia. https://encyclopedia.pub/entry/46025
Kruglova, Natalia, et al. "Histological Features of Primary Morphogenic Calli." Encyclopedia. Web. 26 June, 2023.
Copy Citation
The use of in vitro callus cultures as experimental model systems allows us to get closer to understanding the patterns and features of morphogenesis in intact plants. In this regard, the problem of realizing the morphogenetic potential of callus cells due to their pluri- and totipotency properties is of great interest. To solve this problem, it is important to use the histological approach, which involves studying the structures of developing tissues, organs and organisms in their interactions and relationships.
primary morphogenic calli
plant morphogenesis
1. Introduction
Plant morphogenesis is defined as the integrated process of spatial and temporal development of tissues, organs and embryos, controlled by hormones and regulated by a network of genes acting sequentially or in concert (in [1][2]).
Morphogenesis remains the most complex fundamental problem of plant biology. The complexity of this phenomenon in plants is caused by the holistic nature of morphogenetic processes and their dependence on many interacting internal and external factors. In addition, plant morphogenesis occurs during the entire ontogenesis of an individual by the constant functioning of vegetative and floral (reproductive) meristems [3][4].
The model approach of plant cells, tissues and organs in in vitro cultivation allows us to get closer to understanding the patterns and features of morphogenesis in intact plants. This approach makes it possible, with some simplification, to study the details of integrated morphogenetic processes and the mechanisms of their regulation under controlled experimental conditions [5][6][7]. The basis for the use of such models is the similarity of the main morphogenetic events in plants in planta and in vitro [8][9].
In vitro callus cultures are promising model experimental systems for studying plant morphogenesis. The first publications devoted to obtaining calli in in vitro conditions from the isolated tissue segments of some plants appeared in the late 1930s (in [10]). To date, the ability to form calli in vitro has been revealed in representatives of many plant families. Numerous studies have established that it is possible to use various vegetative, generative and embryonic organs as explants for callus obtaining. A large number of experimental works have been published concerning the study of both in vitro callus formation and the morphogenesis events in them that lead to the formation of regenerants. Especially great success has been achieved in identifying the molecular aspects of these processes, namely the participation of a number of specific gene systems in them [9][11][12][13][14][15]. Important theoretical generalizations have been made. Thus, the ability to form calli in vitro and produce full-fledged regenerants from them is regarded as one of the manifestations of plant development plasticity, largely due to the attached lifestyle [11][16][17]. Biotechnologies for the mass production of economically valuable regenerants have been developed based on the use of callus cultures in vitro [6][18][19].
At the same time, the problem of implementation of the morphogenetic potential of callus cells in in vitro conditions is far from a final solution. To solve this problem, the histological approach should be used, involving investigation of the structures in developing tissues, organs and organisms in their interactions and relationships.
The importance of using the histological approach is already demonstrated in a definition of the term “callus”. In classical studies on in vitro plant tissue cultivation, Skoog and Miller [20] briefly called the “callus” an unorganized growing tissue represented by a mass of dedifferentiated cells. The more complete definition of a callus also reflects its histological characteristics: a callus is an integrated system that is formed as a result of the proliferation of surface or depth cells of various plant tissues; such a system is initially composed of homogeneous cells that are gradually transformed into groups of heterogeneous cells with species-specific morphogenetic potencies, which are realized via various morphogenesis pathways (in [21]).
Attempts have been made to create periodization of callus formation and their development in vitro. In these processes, critical stages [21] and phases of induction and expression [22] are distinguished. In general, this issue is debatable. Apparently, in this single integrated process, one should be able to distinguish (i) the formation of primary morphogenic calli (“callus formation”) and the complication of their structure (“callusogenesis”), as a rule, on the in vitro induction medium, and (ii) the development of groups of callus cells according to various morphogenesis pathways (“morphogenesis pathways in calli”), as a rule, on the in vitro regeneration medium.
2. Histological Features of Primary Morphogenic Calli
The problem of the competence of explant cells both in in vitro callus formation and callus cells in in vitro morphogenesis was raised in the earliest studies of these processes. Thus, in 1902, Gottlieb Haberlandt (in [23]) proposed the concept of plant cell totipotency as the ability to form a new organism. The modern interpretation of this concept and the used terms are presented, for example, in works [24][25]. Later, the concept of plant cell pluripotency as the ability to form a new organ was elaborated (detailed more in [11][16][17][26][27]). Concepts such as multi-, omni-, oligo- and unipotency of cells have also been proposed and developed (detailed more in [28]). At the same time, researchers believe that the properties of cell totipotency and pluripotency in the above interpretations should be considered basic during investigation of callus formation, callusogenesis and morphogenesis in calli in in vitro conditions.
It has been experimentally revealed that the success of in vitro callus formation is determined by a complex of interrelated endogenous and exogenous factors. Modern research in this area is largely devoted to the gene regulatory networks and epigenetic regulators involved in callus formation in vitro, including specific mutants of Arabidopsis thaliana (e.g., [8]). From a histological standpoint, the presence of targeted morphogenetically competent cells in explant tissues should be considered the main endogenous factor; such cells can be called “initial callus cells”. Exogenous factors (mainly hormones in the induction nutrient medium, as well as the effects of stress, e.g., wounds) induce the reprogramming of such cells in the direction of in vitro callus formation. The principal issue is this: do the initial cells already have the competence to form calli under in planta conditions, or is it the conditions of in vitro cultivation that induce their reprogramming with the acquisition of competence? This and other issues related to callus formation from explant cells should be considered debatable.
Be that as it may, under adequate in vitro conditions, explant cells give rise to primary morphogenic calli. The morphological characteristics of calli that have appeared on the surfaces of various explants and are capable of morphogenesis during further in vitro culturation are quite similar in many plants; they are compact nodular structures [29][30][31][32] (Figure 1(1)).
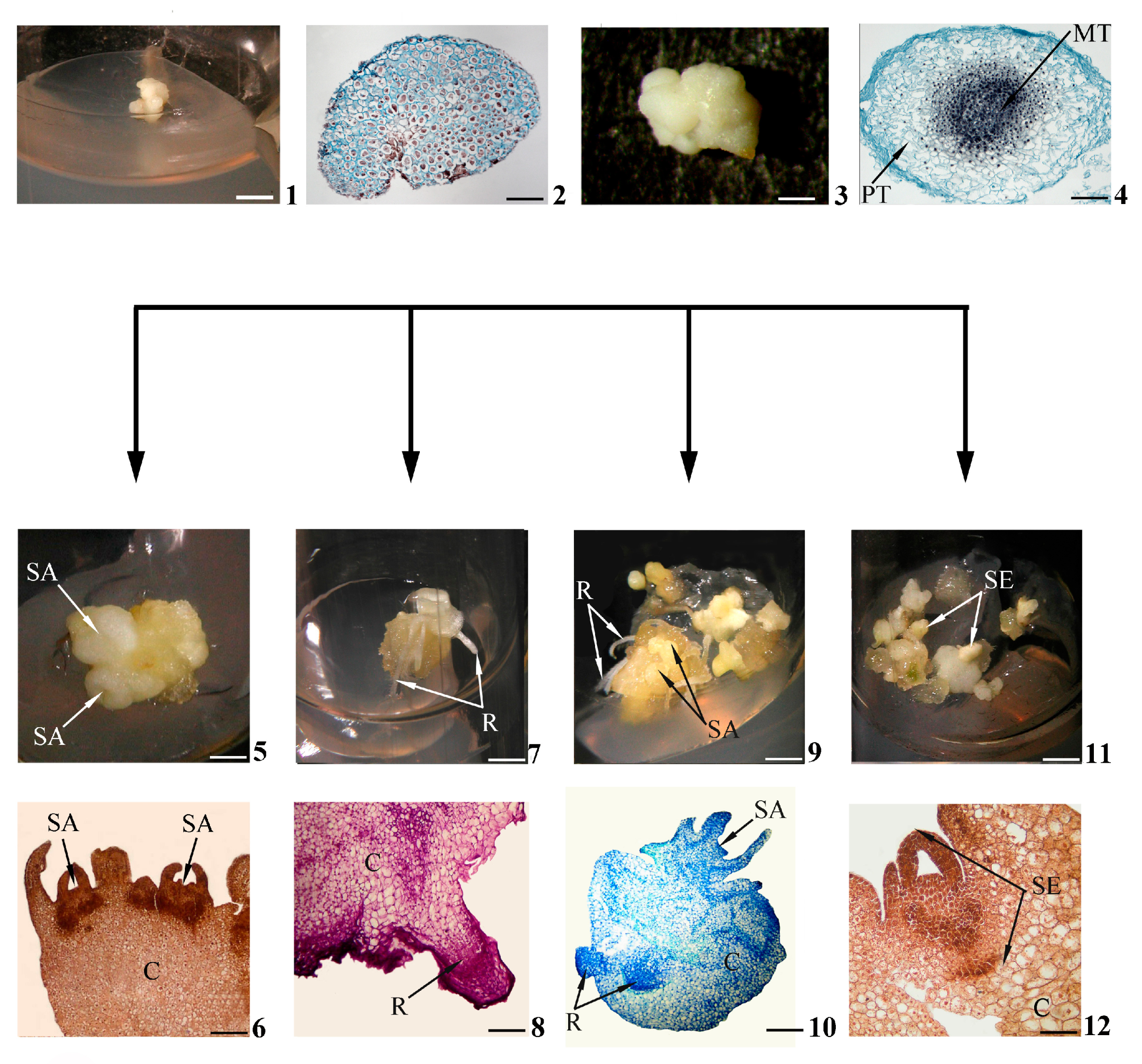
Figure 1. Stages of in vitro morphogenesis in calli. Primary morphogenic callus at initial stage of development according to morphological (1) and histological (2) data; developed primary morphogenic callus according to morphological (3) and histological (4) data; shoots (buds) in the morphogenic callus according to morphological (5) and histological (6) data; roots in the morphogenic callus according to morphological (7) and histological (8) data; gemmorhizogenic structures in the morphogenic callus according to morphological (9) and histological (10) data; somatic embryos in the morphogenic callus according to morphological (11) and histological (12) data. Symbols: C—callus, MT—meristematic tissue, PT—parenchymatic tissue, R—root, SA—shoot apex, SE—somatic embryo. Scale bars: (1) = 150 mm; (2) = 200 mkm; (3) = 3 mm; (4) = 100 mkm; (5,7,9,11) = 3 mm; (6) = 200 mkm; (8,12) = 100 mkm; (10) = 50 mkm.
According to histological data, for example, the primary morphogenic callus obtained from the wheat embryo, in the initial stages of development, is represented by a system of meristematic cells of similar size [33] (Figure 1(2)). However, these histological features do not correspond to all such calli. Thus, the calli that have originated from the lateral root primordia of a number of plants are initially already heterogeneous (in [34]). Apparently, the histological status of a primary morphogenic callus may differ depending on the explant nature and the in vitro culturation conditions.
Calli intensively increase “critical mass” with repeated mitotic divisions of their constituent cells (Figure 1(3)). Furthermore, there is a gradual occurrence of both cells’ heterogeneity in the shapes, sizes, structures and histological zonalities of calli. In the thicknesses of calli (less often on the surface), so-called morphogenetic centers are distinguished. Such centers are represented by two zones of cells. There are the central zone of densely located meristematic cells (“meristematic center” [6][29], “meristemoid” [35], “meristematic node” [36]) and the peripheral zone of loosely located parenchymal cells that have lost meristematic activity [29][37][38] (Figure 1(4)).
It can be assumed that the presence of a morphogenetic center is an obligatory histological feature of primary morphogenic calli, since all future in vitro morphogenesis pathways will somehow be connected with the central meristematic zones of such centers. For example, the formation of meristems (among the authors, promeristems) is regarded as one of the four phases of de novo shoot regeneration in callus [11].
One of the decisive factors in this case is the specialized intercellular interactions of densely located meristematic cells. Such close intercellular contacts are necessary both for the functioning of cells and for the coordination of their activities in the central zone of the morphogenetic center. The opinion was expressed that future morphogenesis pathways would depend on whether a group of cells could establish and maintain coordinated behavior as an integrated unit using symplastic interactions [39]. Researchers agree with this opinion. Indeed, the tight cell–cell symplastic interactions through plasmodesmata in the central meristematic zone of a morphogenetic center differ from those in the surrounding parenchymal peripheral zone, where plasmodesmata are significantly reduced. Symplastic transport provides a reliable exchange of hormones and nutrients between the cells in the central morphogenetic center zone, which makes it possible to consider this zone an integrated structure.
The literature has presented publications devoted to the similarity of the meristem structures in primary in vitro morphogenic calli and in planta root primordia. For example, in Arabidopsis thaliana, the structure of the callus tissue obtained on an in vitro induction medium is similar to the structure of the apical meristem of the root of this plant [26]. Interestingly, in Arabidopsis thaliana, similarities were also revealed in the molecular mechanisms of in vitro callus formation and de novo root regeneration: in particular, the participation of the WOX11 gene in both processes [9].
The similarities in the histological features of primary morphogenic calli and root primordia encouraged us to turn to the functions of quiescent centers in root apical meristems in planta. This question was raised, for example, in work [26]. It is known that the quiescent center, consisting of a pool of rarely dividing stem cells (also called pluripotent cells [27]), together with the often-dividing surrounding cells of initials, will form a niche of stem cells; initials give rise to root tissues [40][41]. The key role of auxin in the activation of quiescent centers was revealed in experiments using auxin-responsive reporters or direct endogenous auxin measurements; other hormones are also of great importance, especially cytokinins, which control auxin transport and cell division in quiescent centers (in [42]). Researchers assume that some meristematic cells (possibly stem cells) of morphogenetic centers are activated in primary morphogenic calli under the actions of endogenous auxins and cytokinins. This assumption was confirmed by comparing the data of a histological analysis of wheat calli with the results of an immunohistochemical study of them. It has been established that endogenous auxins and cytokinins are localized in the meristematic cells of actively developing morphogenetic centers in calli [29].
In general, histological prerequisites for the future realization of morphogenesis pathways are created in primary morphogenic calli in the initial stages of in vitro culturation.
References
- de Almeida, M.; Graner, E.M.; Brondani, G.E.; de Oliveira, L.S.; Artioli, F.A.; de Almeida, L.V.; Leone, G.F.; Baccarin, F.J.B.; de Oliveira, A.P.; Cordeiro, G.M.; et al. Plant morphogenesis: Theorical bases. Adv. Forest. Sci. 2015, 2, 13–22.
- Gordon-Kamm, B.; Sardesai, N.; Arling, M.; Lowe, K.; Hoerster, G.; Betts, S.; Jones, T. Using Morphogenic Genes to Improve and Regeneration of Transgenic Plants. Plants 2019, 8, 38.
- Gaarslev, N.; Swinnen, G.; Soyk, S. Meristem transitions and plant architecture—Learning from domestication for crop breeding. Plant Physiol. 2021, 187, 1045–1056.
- Claßen-Bockhoff, R.; De Craene, L.P.R.; Becker, A. Editorial: From Meristems to Floral Diversity: Developmental Options and Constraints. Front. Ecol. Evol. Sec. Evol. Dev. Biol. 2021, 9, 7954.
- Altman, A. Plant tissue culture and biotechnology: Perspectives in the history and prospects of the International Association of Plant Biotechnology (IAPB). In Vitro Cell. Dev. Biol. Plant. 2019, 55, 590–594.
- Ghiorghiță, G. A Journey into of the Universe of In Vitro Cultures of Plants. Callogenesis. Environ. Natur. Resour. Res. 2019, 9, 45–60.
- Bednarek, P.T.; Orlowska, R. Plant tissue culture environment as a switch-key of (epi)genetic changes. Plant Cell Tissue Organ Cult. 2020, 140, 245–257.
- Ibanez, S.; Carneros, E.; Testillano, P.S.; Perez-Perez, J.M. Advances in Plant Regeneration: Shake, Rattle and Roll. Plants 2020, 9, 897.
- Wan, Q.; Zhai, N.; Xie, D.; Liu, W.; Xu, L. WOX11: The founder of plant organ regeneration. Cell Regen. 2023, 12, 1.
- Sugiyama, M. Historical review of research on plant cell dedifferentiation. J. Plant Res. 2015, 128, 349–359.
- Shin, J.; Bae, S.; Seo, P.J. De novo shoot organogenesis during plant regeneration. J. Exp. Bot. 2020, 71, 63–72.
- Shin, S.Y.; Choi, Y.; Kim, S.-G.; Park, S.-J.; Moon, K.-B.; Kim, H.-S.; Jeon, J.H.; Cho, H.S.; Lee, H.-J. Submergence promotes auxin-induced callus formation through ethylene-mediated post-transcriptional control of auxin receptors. Mol. Plant 2022, 15, 1947–1961.
- Kadri, A.; De March, G.G.; Guerineau, F.; Cosson, V.; Ratet, P. WUSCHEL Overexpression Promotes Callogenesis and Somatic Embryogenesis in Medicago truncatula Gaertn. Plants 2021, 10, 715.
- Suo, J.; Zhou, C.; Zeng, Z.; Li, X.; Bian, H.; Wang, J.; Zhu, M.; Han, N. Identification of regulatory factors promoting embryogenic callus formation in barley through transcriptome analysis. BMC Plant Biol. 2021, 21, 145.
- Lee, K.; Kim, J.H.; Park, O.S.; Jung, Y.J.; Seo, P. Ectopic expression of WOX5 promoters cytokinin signaling and de novo shoot regeneration. Plant Cell Rep. 2022, 41, 2415–2422.
- Bidabadi, S.S.; Jain, S.M. Cellular, Molecular, and Physiological Aspects of In Vitro Plant Regeneration. Plants 2020, 9, 702.
- Xu, C.; Hu, Y. The molecular regulation of cell pluripotency in plants. aBIOTECH 2020, 1, 169–177.
- Twaij, B.M.; Jazar, Z.H.; Hasan, M.N. Trends in the Use of Tissue Culture, Applications and Future Aspects. Int. J. Plant Biol. 2020, 11, 8385.
- Yegorova, N.A. Biotechnology of Essential Oil Plants: Creation of New Forms and Micropropogation In Vitro; Publishing House “Autograph”: Simpheropol, Russia, 2021; pp. 11–272. ISBN 978-5-6045452-9-8. (In Russian)
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissue cultures in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–131.
- Kruglova, N.N.; Titova, G.E.; Seldimirova, O.A. Callusogenesis as an in vitro Morphogenesis Pathway in Cereals. Russ. J. Dev. Biol. 2018, 49, 245–259.
- Yu, Y.; Qin, W.; Li, Y.; Zhang, C.; Wang, Y.; Yang, Z.; Ge, X.; Li, F. Red light promotes cotton embryogenic callus formation by influencing endogenous hormones, polyamines and antioxidative enzyme activities. Plant Growth Regul. 2019, 87, 187–199.
- Krikorian, A.D.; Berquam, D.L. Plant Cell and Tissue Cultures: The Role of Haberlandt. In Plant Tissue Culture; Laimer, M., Rücker, W., Eds.; Springer: Vienna, Austria, 2003; pp. 25–26.
- Feher, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536.
- Su, Y.H.; Tang, L.P.; Zhao, X.Y.; Zhang, X.S. Plant cell totipotency: Insights into cellular reprogramming. J. Integr. Plant Biol. 2020, 63, 12972.
- Zhai, N.; Xu, L. Pluripotency acquisition in the middle cell layer of callus is required for organ regeneration. Nat. Plants 2021, 7, 1453–1460.
- Müller-Xing, R.; Xing, Q. The plant stem-cell niche and pluripotency: 15 years of an epigenetic perspective. Front. Plant Sci. 2022, 13, 1018559.
- Istiaq, A.; Ohta, K. Ribosome-Induced Cellular Multipotency, an Emerging Avenue in Cell Fate Reversal. Cells 2021, 10, 2276.
- Seldimirova, O.A.; Kudoyarova, G.R.; Kruglova, N.N.; Zaytsev, D.Y.; Veselov, S.Y. Changes in distribution of cytokinins and auxins in cell during callus induction and organogenesis in vitro in immature embryo culture of wheat. In Vitro Cell Dev. Biol.-Plant 2016, 52, 251–264.
- Seldimirova, O.A.; Kudoyarova, G.R.; Kruglova, N.N.; Galin, I.R.; Veselov, D.S. Somatic Embryogenesis in Wheat and Barley callus in vitro Is determined by the level of Indoleacetic and Abscisic Acids. Russ. J. Dev. Biol. 2019, 50, 124–135.
- Awan, M.F.; Iqbal, M.S.; Sharif, M.N.; Tabassum, B.; Tariq, M.; Murtaza, S.; Ali, S.; Raza, A.; Bukhari, S.A.R.; Nasir, I.A. Evaluation of genotypic and hormone mediated callus induction and regeneration in sugarcane (Saccharum officinarum L.). Int. J. Bot. Stud. 2019, 4, 70–76.
- Asadi-Aghbolaghi, M.; Dedicova, B.; Ranade, S.S.; Le, K.-C.; Sharifzadeh, F.; Omidi, M.; Egertsdotter, U. Protocol development for somatic embryogenesis, SSR markers and genetic modification of Stipagrostis pennata (Trin.) De Winter. Plant Methods 2021, 17, 70.
- Kruglova, N.N.; Titova, G.E.; Seldimirova, O.A.; Zinatullina, A.E. Cytophysiological features of the Cereal-based Experimental System “Embryo In Vivo–Callus In Vitro”. Russ. J. Dev. Biol. 2021, 52, 199–214.
- Sugimoto, K.; Gordon, S.P.; Meyerowith, E.M. Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011, 21, 212–2018.
- Hu, J.B.; Liu, J.; Yan, H.B.; Xie, C.H. Histological observations of morphogenesis in petiole derived callus of Amorphophallus rivieri Durieu in vitro. Plant Cell Rep. 2005, 24, 642–648.
- Artigas Ramirez, M.D.; Fernández da Silva, R. Morpho-anatomical characterization of secondary somatic embryogenesis in Azadirachta indica (Meliaceae). Acta Bot. Mex. 2018, 122, 7–20.
- Ijaz, B.; Sudiro, C.; Hyder, M.Z.; Malik, S.I.; Farrakh, S.; Schiavo, F.; Yasmin, T. Histo-morphological analysis of rice callus cultures reveals differential regeneration response with varying media combinations. In Vitro Cell. Dev. Biol. Plant. 2019, 55, 569–580.
- Lopez-Ruiz, B.A.; Juarez-Gonzalez, V.T.; Sandoval-Zapotitla, E.; Dinkova, T.D. Development-Related miRNA Expression and Target Regulation during Staggered In Vitro Plant Regeneration of Tuxpeno VS-535 Maize Cultivar. Int. J. Mol. Sci. 2019, 20, 2079.
- Marzec, M.; Kurczynska, E. Importance of symplasmic communication in cell differentiation. Plant Sign. Behav. 2014, 9, e27931.
- Dubrovsky, J.G.; Vissenberg, K. The quiescent centre and root apical meristem: Organization and function. J. Exp. Bot. 2021, 72, 6673–6678.
- Strotmann, V.I.; Stahl, Y. At the root of quiescence: Function and regulation of the quiescent center. J. Exp. Bot. 2021, 72, 6716–6726.
- Yamoune, A.; Cuyacot, A.R.; Zdarska, M.; Hejatko, J. Hormonal orchestration of root apical meristem formation and maintenance in Arabidopsis. J. Exp. Bot. 2021, 72, 6768–6788.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
559
Revisions:
2 times
(View History)
Update Date:
26 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




