
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jessica Da Silva | -- | 1358 | 2023-06-21 18:35:43 | | | |
| 2 | Conner Chen | Meta information modification | 1358 | 2023-06-25 05:38:29 | | |
Video Upload Options
Diabetic Foot Ulcers (DFUs) are deep tissue lesions on the lower extremities, mainly associated with sustained hyperglycemia, peripheral neuropathy, and peripheral arterial disease (PAD). Globally, a lower limb is amputated every 20 to 30 s, with DFU being responsible for 85 to 95% of cases. Furthermore, individuals with DFUs typically display an increased risk of mortality, more than the double risk of those with DM without a DFU.
1. Pathophysiology of Diabetic Foot Ulcers
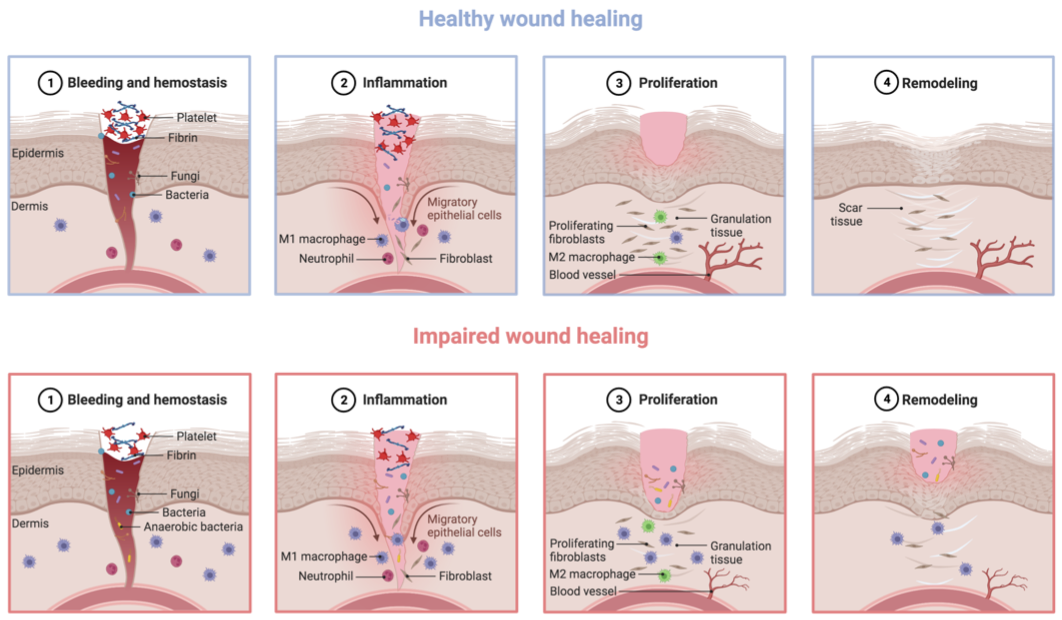
2. Impaired Healing in Diabetic Foot Ulcers
3. Management of Diabetic Foot Ulcer
4. Biomaterials as a Promising Therapeutic Platform for Wound Dressings
A therapeutic strategy with rising potential to handle the challenging macro and micro wound environment of individuals with DM involves the use of biomaterials as wound dressings. Biomaterials have long been related to unique versatility, biocompatibility, biodegradability, and hydrophilicity, characteristics that make them ideal candidates for therapeutic applications [43][44][45]. Furthermore, biomaterials have also been explored for their innate properties for wound healing [43][44][45][46]. An ideal wound dressing for the management of DFUs should present several key features. Firstly, it must demonstrate excellent biocompatibility and biodegradability to ensure tissue healing. Secondly, it should create a moist and warm environment conducive to tissue regeneration. Thirdly, the dressing should prevent polymicrobial infections to ensure proper wound healing. Finally, it should exhibit adequate porosity that enables gas exchange, and stimulate cell migration, proliferation, and neovascularization, as depicted in Figure 2 [43][46].
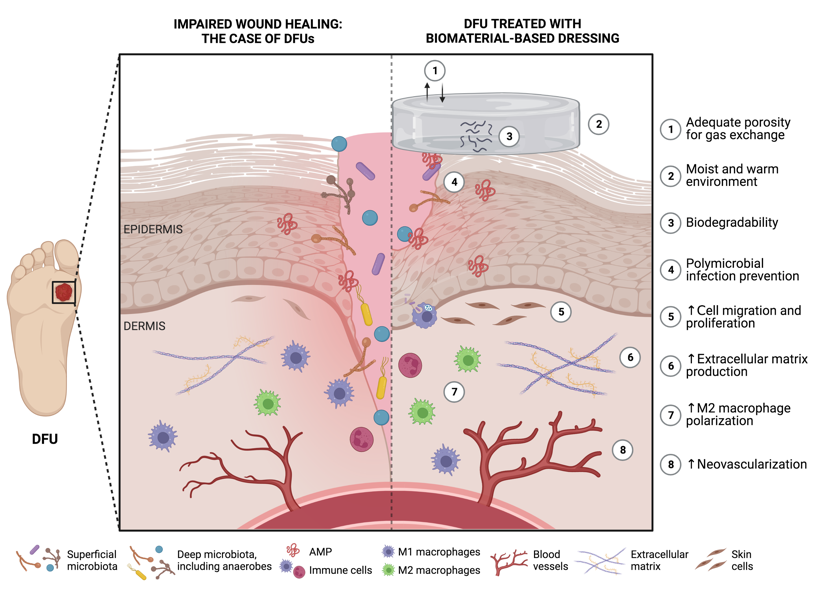
Figure 2. Potential features of functional biomaterials as ideal wound dressings for diabetic foot ulcer (DFU) healing (produced using BioRender). ↑ represents an increase.
References
- Yang, L.; Rong, G.; Wu, Q. Diabetic foot ulcer: Challenges and future. World J. Diabetes 2022, 13, 1014–1034.
- Baig, M.S.; Banu, A.; Zehravi, M.; Rana, R.; Burle, S.S.; Khan, S.L.; Islam, F.; Siddiqui, F.A.; Massoud, E.E.S.; Rahman, M.H.; et al. An Overview of Diabetic Foot Ulcers and Associated Problems with Special Emphasis on Treatments with Antimicrobials. Life 2022, 12, 1054.
- Srivastava, P.; Sondak, T.; Sivashanmugam, K.; Kim, K. A Review of Immunomodulatory Reprogramming by Probiotics in Combating Chronic and Acute Diabetic Foot Ulcers (DFUs). Pharmaceutics 2022, 14, 2436.
- Akkus, G.; Sert, M. Diabetic foot ulcers: A devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J. Diabetes 2022, 13, 1106–1121.
- Edmonds, M.; Manu, C.; Vas, P. The current burden of diabetic foot disease. J. Clin. Orthop. Trauma 2021, 17, 88–93.
- Megallaa, M.H.; Ismail, A.A.; Zeitoun, M.H.; Khalifa, M.S. Association of diabetic foot ulcers with chronic vascular diabetic complications in patients with type 2 diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1287–1292.
- Da Silva, J.; Leal, E.C.; Carvalho, E. Bioactive antimicrobial peptides as therapeutic agents for infected diabetic foot ulcers. Biomolecules 2021, 11, 1894.
- Kalan, L.R.; Meisel, J.S.; Loesche, M.A.; Horwinski, J.; Soaita, I.; Chen, X.; Uberoi, A.; Gardner, S.E.; Grice, E.A. Strain- and Species-Level Variation in the Microbiome of Diabetic Wounds Is Associated with Clinical Outcomes and Therapeutic Efficacy. Cell Host Microbe 2019, 25, 641–655.e5.
- Kareliya, H.; Bichile, L.; Bal, A.; Varaiya, A.; Bhalekar, P. Fungal Infection in Diabetic Foot a Clinicomicrobiological Study. Acta Sci. Mcrobiol. 2019, 2, 49–55.
- Kalshetti, V.T.; Wadile, R.; Bothikar, S.T.; Ambade, V.; Bhate, V.M. Study of fungal infections in diabetic foot Ulcer. Indian J. Microbiol. Res. 2017, 4, 87–89.
- Raiesi, O.; Shabandoust, H.; Dehghan, P.; Shamsaei, S.; Soleimani, A. Fungal infection in foot diabetic patients. J. Basic Res. Med. Sci. 2018, 5, 47–51.
- Chellan, G.; Shivaprakash, S.; Ramaiyar, S.K.; Varma, A.K.; Varma, N.; Sukumaran, M.T.; Vasukutty, J.R.; Bal, A.; Kumar, H. Spectrum and prevalence of fungi infecting deep tissues of lower-limb wounds in patients with type 2 diabetes. J. Clin. Microbiol. 2010, 48, 2097–2102.
- Ibrahim, A.; Berkache, M.; Morency-Potvin, P.; Juneau, D.; Koenig, M.; Bourduas, K.; Freire, V. Diabetic foot infections: How to investigate more efficiently? A retrospective study in a quaternary university center. Insights Imaging 2022, 13, 88.
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706.
- Childs, D.R.; Murthy, A.S. Overview of Wound Healing and Management. Surg. Clin. N. Am. 2017, 97, 189–207.
- Petkovic, M.; Sørensen, A.E.; Leal, E.C.; Carvalho, E.; Dalgaard, L.T. Mechanistic Actions of microRNAs in Diabetic Wound Healing. Cells 2020, 9, 2228.
- Petkovic, M.; Vangmouritzen, M.; Mojsoska, B.; Jenssen, H. Immunomodulatory properties of host defence peptides in skin wound healing. Biomolecules 2021, 11, 952.
- Perez-Favila, A.; Martinez-Fierro, M.L.; Rodriguez-Lazalde, J.G.; Cid-Baez, M.A.; Zamudio-Osuna, M.D.J.; Martinez-Blanco, M.D.R.; Mollinedo-Montaño, F.E.; Rodriguez-Sanchez, I.P.; Castañeda-Miranda, R.; Garza-Veloz, I. Current therapeutic strategies in diabetic foot ulcers. Medicina 2019, 55, 714.
- Erem, C.; Hacıhasanoğlu, A.; Çelik, Ş.; Ovalı, E.; Ersöz, H.Ö.; Ukinç, K.; Deger, O.; Telatar, M. Coagulation and Fibrinolysis Parameters in Type 2 Diabetic Patients with and without Diabetic Vascular Complications. Med. Princ. Pract. 2005, 14, 22–30.
- Xiao, J.; Li, J.; Cai, L.; Chakrabarti, S.; Li, X. Cytokines and Diabetes Research. J. Diabetes Res. 2014, 2014, 920613.
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 2005, 304, 274–286.
- Lan, C.E.; Liu, I.; Fang, A.; Wen, C.; Wu, C. Hyperglycaemic conditions decrease cultured keratinocyte mobility: Implications for impaired wound healing in patients with diabetes. Br. J. Dermatol. 2008, 159, 1103–1115.
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical Vascular Endothelial Growth Factor Accelerates Diabetic Wound Healing through Increased Angiogenesis and by Mobilizing and Recruiting Bone Marrow-Derived Cells. Am. J. Pathol. 2004, 164, 1935–1947.
- Maione, A.G.; Smith, A.; Kashpur, O.; Yanez, V.; Knight, E.; Mooney, D.J.; Veves, A.; Tomic-Canic, M.; Garlick, J.A. Altered ECM deposition by diabetic foot ulcer-derived fibroblasts implicates fibronectin in chronic wound repair. Wound Repair Regen. 2016, 24, 630–643.
- Brocco, E.; Ninkovic, S.; Marin, M.; Whisstock, C.; Bruseghin, M.; Boschetti, G.; Viti, R.; Forlini, W.; Volpe, A. Diabetic foot management: Multidisciplinary approach for advanced lesion rescue. J. Cardiovasc. Surg. 2018, 59, 670–684.
- Uivaraseanu, B.; Bungau, S.; Tit, D.M.; Fratila, O.; Rus, M.; Maghiar, T.A.; Maghiar, O.; Pantis, C.; Vesa, C.M.; Zaha, D.C. Clinical, Pathological and Microbiological Evaluation of Diabetic Foot Syndrome. Medicina 2020, 56, 380.
- Ramirez-Acuña, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic foot ulcers: Current advances in antimicrobial therapies and emerging treatments. Antibiotics 2019, 8, 193.
- Apelqvist, J. Diagnostics and treatment of the diabetic foot. Endocrine 2012, 41, 384–397.
- Reardon, R.; Simring, D.; Kim, B.; Mortensen, J.; Williams, D.; Leslie, A. The diabetic foot ulcer. Aust. J. Gen. Pract. 2020, 49, 250–255.
- Musuuza, J.; Sutherland, B.L.; Kurter, S.; Balasubramanian, P.; Bartels, C.M.; Brennan, M.B. A systematic review of multidisciplinary teams to reduce major amputations for patients with diabetic foot ulcers. J. Vasc. Surg. 2020, 71, 1433–1446.e3.
- Wang, A.; Lv, G.; Cheng, X.; Ma, X.; Wang, W.; Gui, J.; Hu, J.; Lu, M.; Chu, G.; Jin’an, C.; et al. Guidelines on multidisciplinary approaches for the prevention and management of diabetic foot disease (2020 edition). Burn. Trauma 2020, 8, tkaa017.
- Lazzarini, P.; Fernando, M.; Van Netten, J. Diabetic foot ulcers: Is remission a realistic goal? Endocrinol. Today 2019, 8, 22–26.
- Da Porto, A.; Miranda, C.; Brosolo, G.; Zanette, G.; Michelli, A.; Ros, R. Da Nutritional supplementation on wound healing in diabetic foot: What is known and what is new? World J. Diabetes 2022, 13, 940–948.
- Chuan, F.; Tang, K.; Jiang, P.; Zhou, B.; He, X. Reliability and Validity of the Perfusion, Extent, Depth, Infection and Sensation (PEDIS) Classification System and Score in Patients with Diabetic Foot Ulcer. PLoS ONE 2015, 10, e0124739.
- Biz, C.; Ruggieri, P. Minimally Invasive Surgery: Osteotomies for Diabetic Foot Disease. Foot Ankle Clin. 2023, 25, 441–460.
- Biz, C.; Belluzzi, E.; Crimì, A.; Bragazzi, N.L.; Nicoletti, P.; Mori, F.; Ruggieri, P. Minimally Invasive Metatarsal Osteotomies (MIMOs) for the Treatment of Plantar Diabetic Forefoot Ulcers (PDFUs): A Systematic Review and Meta-Analysis with Meta-Regressions. Appl. Sci. 2021, 11, 9628.
- Pombeiro, I.; Moura, J.; Pereira, M.G.; Carvalho, E. Stress-Reducing Psychological Interventions as Adjuvant Therapies for Diabetic Chronic Wounds. Curr. Diabetes Rev. 2022, 18, e060821195361.
- Pereira, M.G.; Vilaça, M.; Carvalho, E. Effectiveness of Two Stress Reduction Interventions in Patients with Chronic Diabetic Foot Ulcers (PSY-DFU): Protocol for a Longitudinal RCT with a Nested Qualitative Study Involving Family Caregivers. Int. J. Environ. Res. Public Health 2022, 19, 8556.
- Sen, P.; Demirdal, T.; Emir, B. Meta-analysis of risk factors for amputation in diabetic foot infections. Diabetes. Metab. Res. Rev. 2019, 35, e3165.
- Dörr, S.; Freier, F.; Schlecht, M.; Lobmann, R. Bacterial diversity and inflammatory response at first-time visit in younger and older individuals with diabetic foot infection (DFI). Acta Diabetol. 2021, 58, 181–189.
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375.
- Fu, X.-L.; Ding, H.; Miao, W.-W.; Mao, C.-X.; Zhan, M.-Q.; Chen, H.-L. Global recurrence rates in diabetic foot ulcers: A systematic review and meta-analysis. Diabetes. Metab. Res. Rev. 2019, 35, e3160.
- Güiza-Argüello, V.R.; Solarte-David, V.A.; Pinzón-Mora, A.V.; Ávila-Quiroga, J.E.; Becerra-Bayona, S.M. Current Advances in the Development of Hydrogel-Based Wound Dressings for Diabetic Foot Ulcer Treatment. Polymers 2022, 14, 2764.
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 724.
- Bardill, J.R.; Laughter, M.R.; Stager, M.; Liechty, K.W.; Krebs, M.D.; Zgheib, C. Topical gel-based biomaterials for the treatment of diabetic foot ulcers. Acta Biomater. 2022, 138, 73–91.
- Divyashri, G.; Badhe, R.V.; Sadanandan, B.; Vijayalakshmi, V.; Kumari, M.; Ashrit, P.; Bijukumar, D.; Mathew, M.T.; Shetty, K.; Raghu, A.V. Applications of hydrogel-based delivery systems in wound care and treatment: An up-to-date review. Polym. Adv. Technol. 2022, 33, 2025–2043.




