
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Denis Mihaela Panaitescu | -- | 3732 | 2023-06-21 15:32:56 | | | |
| 2 | Lindsay Dong | + 1 word(s) | 3733 | 2023-06-25 03:08:01 | | |
Video Upload Options
Nanocellulose can be obtained from low-cost sources and has been extensively studied in the last decades due to its biodegradability, biocompatibility, low weight, large specific surface area, and good mechanical and optical properties. The nanocellulose properties palette can be greatly expanded by incorporating different metals, metal oxides or carbon nanomaterials, with the formation of multifunctional hybrids. Nanocellulose–nanocarbon hybrids are emerging nanomaterials that can respond to many current challenges in areas such as water purification, energy storage and conversion, or biomedicine for drug delivery, tissue engineering, antitumor and antimicrobial therapies, and many others. Nanocellulose/nanodiamonds hybrids combine the bio-based origin, biodegradability, good dispersion in water, and non-toxicity of nanocellulose with the high thermal conductivity, excellent mechanical resistance, and great structural stability of nanodiamonds.
1. Introduction
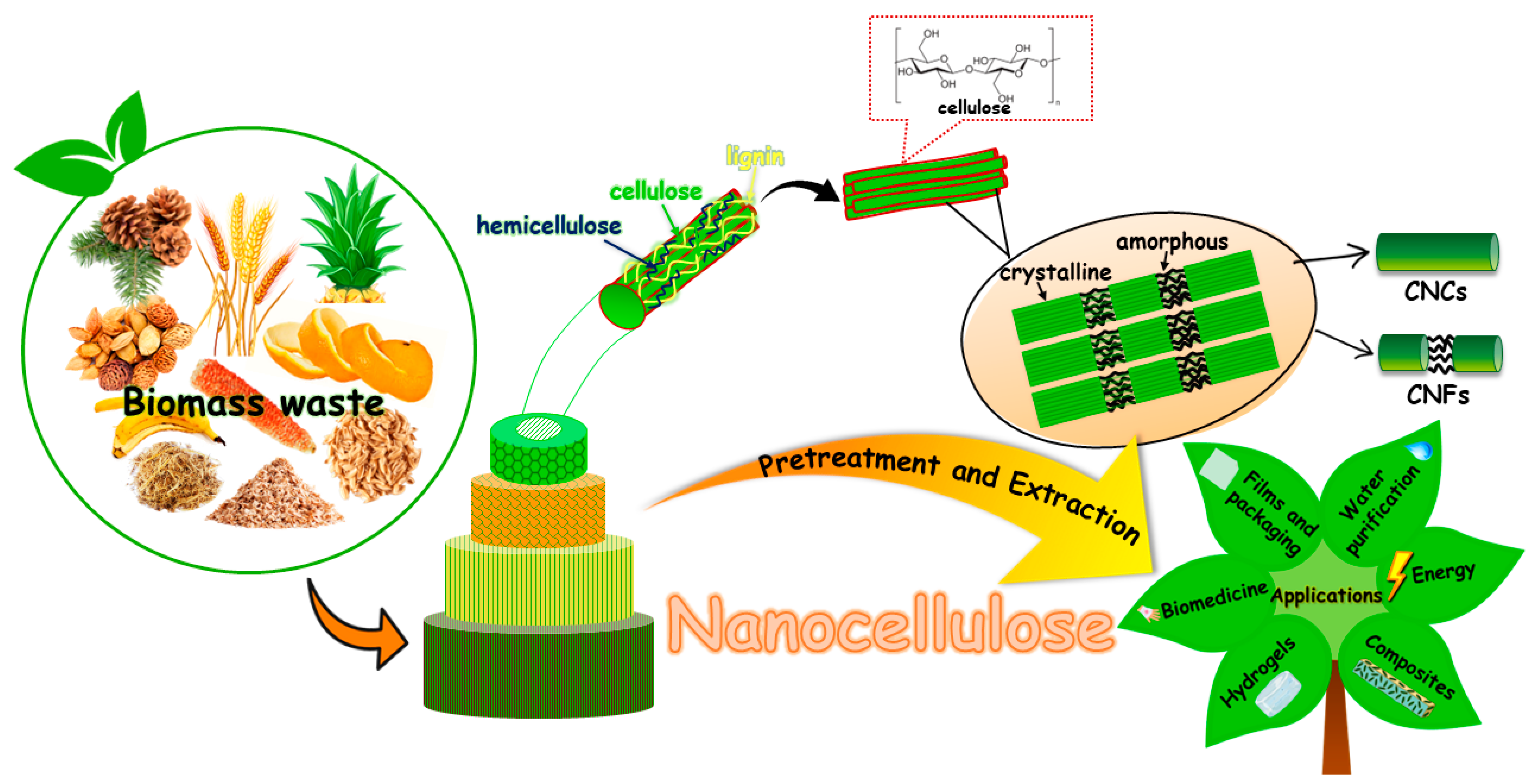
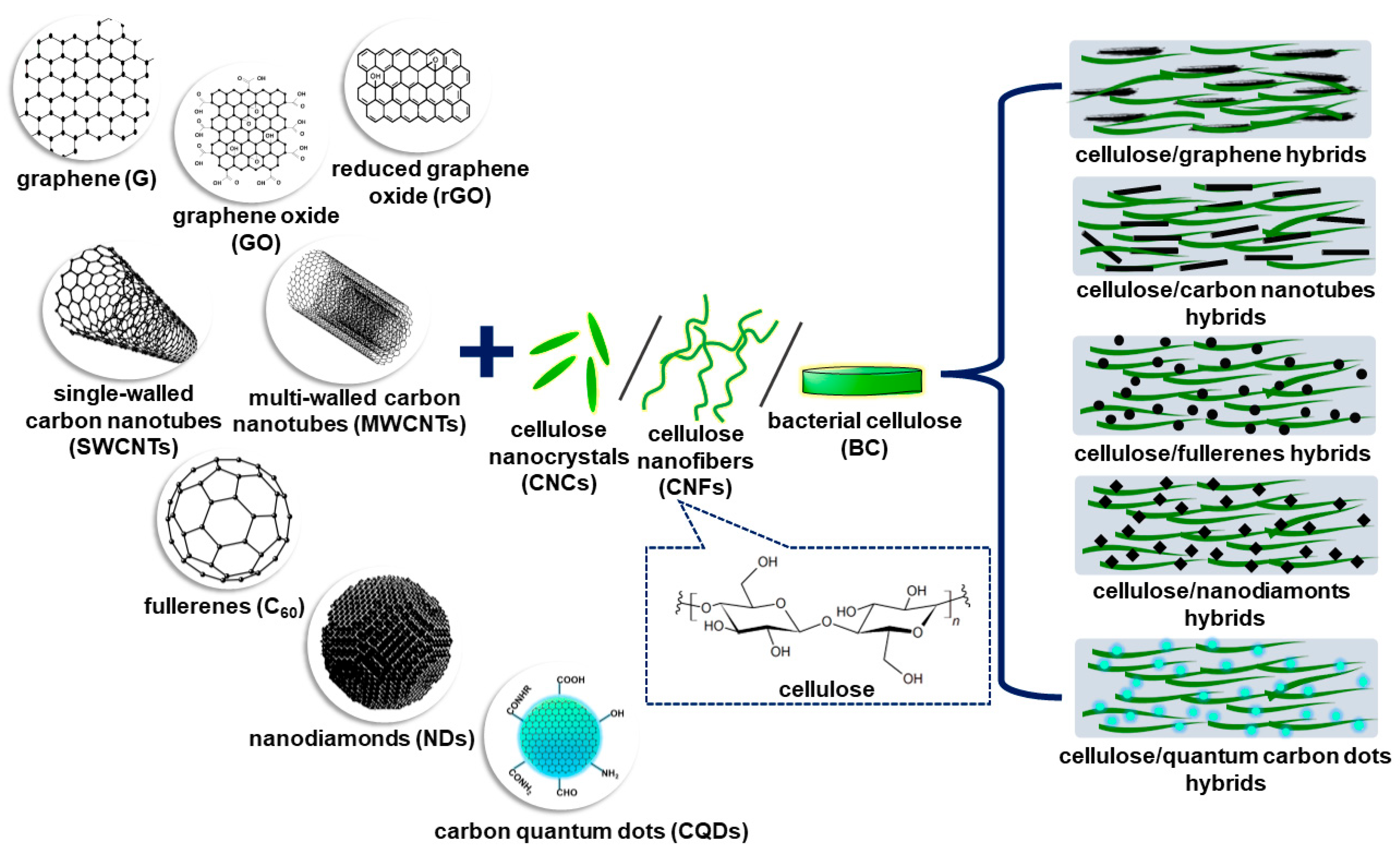
2. Nanocellulose–Nanodiamonds Hybrids
2.1. Nanocellulose
2.2. Nanodiamonds
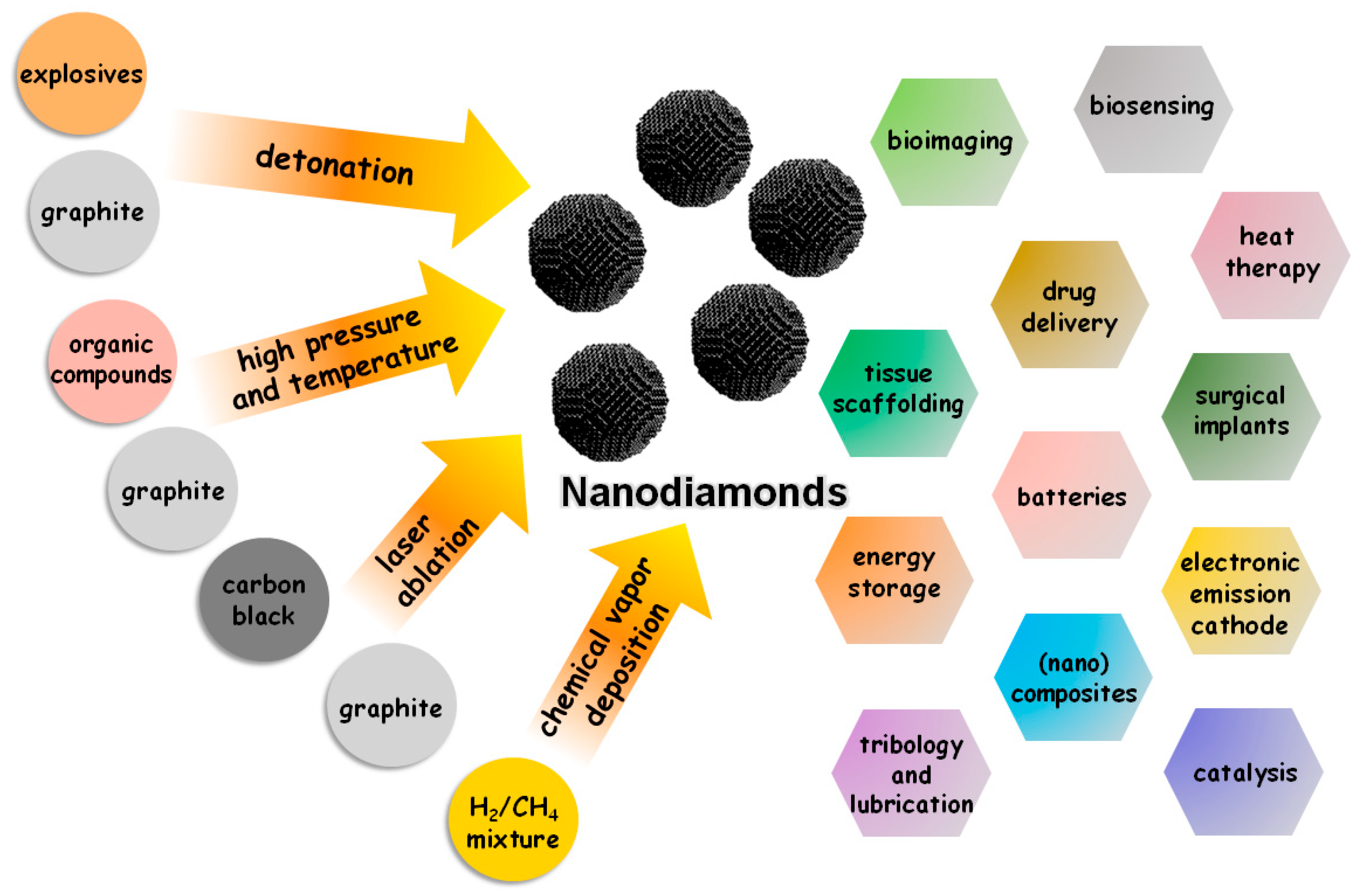
2.3. Preparation of Nanocellulose–Nanodiamonds Hybrids
2.4. Main Characteristics of Nanocellulose–Nanodiamonds Hybrids
2.4.1. Thermal Conductivity
2.4.2. Mechanical Properties
2.4.3. Optical Properties
2.5. Applications of Nanocellulose–Nanodiamond Hybrids
References
- Zinge, C.; Kandasubramanian, B. Nanocellulose based biodegradable polymers. Eur. Polym. J. 2020, 133, 109758.
- Magalhães, M.I.; Almeida, A.P.C. Nature-Inspired Cellulose-Based Active Materials: From 2D to 4D. Appl. Biosci. 2023, 2, 94–114.
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I. Nanostructured biocomposites from aliphatic polyesters and bacterial cellulose. Ind. Crops Prod. 2016, 93, 251–266.
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546.
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crops Prod. 2016, 93, 26–38.
- Frone, A.N.; Chiulan, I.; Panaitescu, D.M.; Nicolae, C.A.; Ghiurea, M.; Galan, A.-M. Isolation of cellulose nanocrystals from plum seed shells, structural and morphological characterization. Mater. Lett. 2017, 194, 160–163.
- Méndez-Loranca, E.; Vidal-Ruiz, A.M.; Martínez-González, O.; Huerta-Aguilar, C.A.; Gutierrez-Uribe, J.A. Beyond cellulose extraction: Recovery of phytochemicals and contaminants to revalorize agricultural waste. Bioresour. Technol. 2023, 21, 101339.
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 2020, 27, 575–593.
- Chen, L.; Yu, H.; Dirican, M.; Fang, D.; Tian, Y.; Yan, C.; Xie, J.; Jia, D.; Liu, H.; Wang, J.; et al. Highly Transparent and Colorless Nanocellulose/Polyimide Substrates with Enhanced Thermal and Mechanical Properties for Flexible OLED Displays. Adv. Mater. Interfaces 2020, 7, 2000928.
- Usurelu, C.D.; Badila, S.; Frone, A.N.; Panaitescu, D.M. Poly(3-hydroxybutyrate) Nanocomposites with Cellulose Nanocrystals. Polymers 2022, 14, 1974.
- Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr. For. Rep. 2019, 5, 76–89.
- Qian, H.; Liu, J.; Wang, X.; Pei, W.; Fu, C.; Ma, M.; Huang, C. The state-of-the-art application of functional bacterial cellulose-based materials in biomedical fields. Carbohydr. Polym. 2023, 300, 120252.
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Casarica, A.; Nicolae, C.A.; Ghiurea, M.; Trusca, R.; Damian, C.M. Structural and morphological characterization of bacterial cellulose nano-reinforcements prepared by mechanical route. Mater. Des. 2016, 110, 790–801.
- Eichhorn, S.J.; Etale, A.; Wang, J.; Berglund, L.A.; Li, Y.; Cai, Y.; Chen, C.; Cranston, E.D.; Johns, M.A.; Fang, Z.; et al. Current international research into cellulose as a functional nanomaterial for advanced applications. J. Mater. Sci. 2022, 57, 5697–5767.
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994.
- Pradeep, H.K.; Patel, D.H.; Onkarappa, H.S.; Pratiksha, C.C.; Prasanna, G.D. Role of nanocellulose in industrial and pharmaceutical sectors—A review. Int. J. Biol. Macromol. 2022, 207, 1038–1047.
- Spagnuolo, L.; D’Orsi, R.; Operamolla, A. Nanocellulose for Paper and Textile Coating: The Importance of Surface Chemistry. ChemPlusChem 2022, 87, e202200204.
- Gómez, C.; Serpa, A.; Velásquez-Cock, J.; Gañán, P.; Castro, C.; Vélez, L.; Zuluaga, R. Vegetable nanocellulose in food science: A review. Food Hydrocoll. 2016, 57, 178–186.
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57.
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent trends and applications in the food industry. Food Hydrocoll. 2022, 127, 107484.
- Luo, Q.; Shen, H.; Zhou, G.; Xu, X. A mini-review on the dielectric properties of cellulose and nanocellulose-based materials as electronic components. Carbohydr. Polym. 2023, 303, 120449.
- Yang, G.; Kong, H.; Chen, Y.; Liu, B.; Zhu, D.; Guo, L.; Wei, G. Recent advances in the hybridization of cellulose and carbon nanomaterials: Interactions, structural design, functional tailoring, and applications. Carbohydr. Polym. 2022, 279, 118947.
- Valencia, L.; Handa, R.; Monti, S.; Jasso-Salcedo, A.B.; Georgouvelas, D.; Magana, I.; Dıaz de Leon, R.; Velikov, K.P.; Mathew, A.J.; Kumar, S. On the mineralization of nanocellulose to produce functional hybrid materials. J. Mater. Chem. A 2022, 10, 9248–9276.
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392.
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine. Nanomaterials 2020, 10, 196.
- Trache, D.; Thakur, V.K. Nanocellulose and Nanocarbons Based Hybrid Materials: Synthesis, Characterization and Applications. Nanomaterials 2020, 10, 1800.
- Nauman Javed, R.M.; Al-Othman, A.; Tawalbeh, M.; Olabi, A.G. Recent developments in graphene and graphene oxide materials for polymer electrolyte membrane fuel cells applications. Renew. Sustain. Energy Rev. 2022, 168, 112836.
- Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and graphene oxide for bio-sensing: General properties and the effects of graphene ripples. Acta Biomater. 2021, 131, 62–79.
- Lin, P.-C.; Wu, J.-Y.; Liu, W.-R. Green and facile synthesis of few-layer graphene via liquid exfoliation process for Lithium-ion batteries. Sci. Rep. 2018, 8, 9766.
- Wang, Y.; Yang, J.; Song, Y.; Yang, Q.; Xiong, C.; Shi, Z. Porous and three-dimensional carbon aerogels from nanocellulose/pristine graphene for high-performance supercapacitor electrodes. Diam. Relat. Mater. 2023, 132, 109626.
- Panda, P.K.; Dash, P.; Yang, J.M.; Chang, Y.H. Development of chitosan, graphene oxide, and cerium oxide composite blended films: Structural, physical, and functional properties. Cellulose 2022, 29, 2399–2411.
- Mohan, V.B.; Jakisch, L.; Jayaraman, K.; Bhattacharyya, D. Role of chemical functional groups on thermal and electrical properties of various graphene oxide derivatives: A comparative X-ray photoelectron spectroscopy analysis. Mater. Res. Express 2018, 5, 035604.
- Khalid, A.; Yi, W.; Yoo, S.; Abbas, S.; Si, J.; Hou, X.; Hou, J. Single-chirality of single-walled carbon nanotubes (SWCNTs) through chromatography and its potential biological applications. New J. Chem. 2023, 47, 992.
- Ramezani, M.; Dehghani, A.; Sherif, M.M. Carbon nanotube reinforced cementitious composites: A comprehensive review. Constr. Build. Mater. 2022, 315, 125100.
- Thomas, B.; Raj, M.C.; Athira, C.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625.
- Smirnov, V.V.; Manevitch, L.I. Carbon Nanotubes in Arrays: Competition of van-der-Waals and Elastic Forces. Dokl. Phys. 2019, 64, 218–221.
- Milano, F.; Guascito, M.R.; Semeraro, P.; Sawalha, S.; Da Ros, T.; Operamolla, A.; Giotta, L.; Prato, M.; Valli, L. Nanocellulose/Fullerene Hybrid Films Assembled at the Air/Water Interface as Promising Functional Materials for Photo-Electrocatalysis. Polymers 2021, 13, 243.
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500.
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88.
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766.
- Singhania, R.R.; Patel, A.K.; Tsai, M.-L.; Chen, C.-W.; Dong, C.D. Genetic modification for enhancing bacterial cellulose production and its applications. Bioengineered 2021, 12, 6793–6807.
- Lei, W.; Jin, D.; Liu, H.; Tong, Z.; Zhang, H. An Overview of Bacterial Cellulose in Flexible Electrochemical Energy Storage. ChemSusChem 2020, 13, 3731–3753.
- Oprea, M.; Panaitescu, D.M. Nanocellulose Hybrids with Metal Oxides Nanoparticles for Biomedical Applications. Molecules 2020, 25, 4045.
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23.
- Zhai, W.; Srikanth, N.; Kong, L.B.; Zhou, K. Carbon nanomaterials in tribology. Carbon 2017, 119, 150–171.
- Vejpravová, J. Mixed sp2–sp3 Nanocarbon Materials: A Status Quo Review. Nanomaterials 2021, 11, 2469.
- Shenderova, O.; Koscheev, A.; Zaripov, N.; Petrov, I.; Skryabin, Y.; Detkov, P.; Turner, S.; Van Tendeloo, G. Surface Chemistry and Properties of Ozone-Purified Detonation Nanodiamonds. J. Phys. Chem. C 2011, 115, 9827–9837.
- Morimune-Moriya, S.; Salajkova, M.; Zhou, Q.; Nishino, T.; Berglund, L.A. Reinforcement Effects from Nanodiamond in Cellulose Nanofibril Films. Biomacromolecules 2018, 19, 2423–2431.
- Qin, J.-X.; Yang, X.-G.; Lv, C.-F.; Li, Y.-Z.; Liu, K.-K.; Zang, J.-H.; Yang, X.; Dong, L.; Shan, C.-X. Nanodiamonds: Synthesis, properties, and applications in nanomedicine. Mater. Des. 2021, 210, 110091.
- Gong, P.; Li, L.; Fu, G.-E.; Shu, S.; Li, M.; Wang, Y.; Qin, Y.; Kong, X.; Chen, H.; Jiao, C.; et al. Highly flexible cellulose nanofiber/single-crystal nanodiamond flake heat spreader films for heat dissipation. J. Mater. Chem. C 2022, 10, 12070.
- Zhang, X.; Wei, S.; Lei, C.; Wei, J.; Lu, B.; Ding, Y.; Zhu, C. Application of printed nanocrystalline diamond film for electron emission cathode. Appl. Surf. Sci. 2011, 257, 5185–5189.
- Ostadhossein, F.; Mahmoudi, N.; Morales-Cid, G.; Tamjid, E.; Navas-Martos, F.J.; Soriano-Cuadrado, B.; Paniza, J.M.L.; Simchi, A. Development of Chitosan/Bacterial Cellulose Composite Films Containing Nanodiamonds as a Potential Flexible Platform for Wound Dressing. Materials 2015, 8, 6401–6418.
- Luo, X.; Zhang, H.; Cao, Z.; Cai, N.; Xue, Y.; Yu, F. A simple route to develop transparent doxorubicin-loaded nanodiamonds/cellulose nanocomposite membranes as potential wound dressings. Carbohydr. Polym. 2016, 143, 231–238.
- Mahdavi, M.; Mahmoudi, N.; RezaieAnaran, F.; Simchi, A. Electrospinning of Nanodiamond-Modified Polysaccharide Nanofibers with Physico-Mechanical Properties Close to Natural Skins. Mar. Drugs 2016, 14, 128.
- Kato, T.; Matsumoto, T.; Hongo, C.; Nishino, T. Mechanical and thermal properties of cellulose nanofiber composites with nanodiamond as nanocarbon filler. Nanocomposites 2018, 4, 127–136.
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85.
- Zhang, Y.; Wang, W.; Zhang, F.; Huang, L.; Dai, K.; Li, C.; Liu, D.; Sun, Y.; Ren, D.; Wu, J.; et al. Micro-diamond assisted bidirectional tuning of thermal conductivity in multifunctional graphene nanoplatelets/nanofibrillated cellulose films. Carbon 2022, 189, 265–275.
- Song, N.; Cui, S.; Hou, X.; Ding, P.; Shi, L. Significant Enhancement of Thermal Conductivity in Nanofibrillated Cellulose Films with Low Mass Fraction of Nanodiamond. ACS Appl. Mater. Interfaces 2017, 9, 40766–40773.
- Zhu, H.; Parvinian, S.; Preston, C.; Vaaland, O.; Ruan, Z.; Hu, L. Transparent nanopaper with tailored optical properties. Nanoscale 2013, 5, 3787–3792.
- Li, L.; Li, M.; Zhang, Z.; Qin, Y.; Shui, X.; Xia, J.; Xiong, S.; Wang, B.; Zhang, Z.; Wei, Z.; et al. Robust composite film with high thermal conductivity and excellent mechanical properties by constructing a long-range ordered sandwich structure. J. Mater. Chem. A 2022, 10, 9922–9931.
- Jiao, E.; Wu, K.; Liu, Y.; Zhang, H.; Zheng, H.; Xu, C.; Shi, J.; Lu, M. Nacre-like robust cellulose nanofibers/MXene films with high thermal conductivity and improved electrical insulation by nanodiamond. J. Mater. Sci. 2022, 57, 2584–2596.
- Chen, Q.; Mao, K.; Yao, Y.; Huang, X.; Zhang, Z. Nanodiamond/cellulose nanocrystals composite-based acoustic humidity sensor. Sens. Actuators B Chem. 2022, 373, 132748.
- Li, Z.; Wang, J.; Xu, Y.; Shen, M.; Duan, C.; Dai, L.; Ni, Y. Green and sustainable cellulose-derived humidity sensors: A review. Carbohydr. Polym. 2021, 270, 118385.
- Vega-Figueroa, K.; Santillán, J.; García, C.; González-Feliciano, J.A.; Bello, S.A.; Rodríguez, Y.G.; Ortiz-Quiles, E.; Nicolau, E. Assessing the suitability of cellulose-nanodiamond composite as a multifunctional biointerface material for bone tissue regeneration. ACS Biomater. Sci. Eng. 2017, 3, 960–968.
- Das, D.; Dey, R.; Das, S.; Hussain, S.; Ghosh, A.K.; Pal, A.K. Nano-Ag/DLC/Cellulose Free-Standing Films towards Anti-bacterial and Bio-compatible Futuristic Bandage Applications. J. Polym. Environ. 2020, 28, 284–294.





