
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chrysoula Kosmeri | -- | 1184 | 2023-06-21 11:44:37 | | | |
| 2 | Conner Chen | Meta information modification | 1184 | 2023-06-25 05:35:19 | | |
Video Upload Options
Data regarding the nutritional management of preterm small for gestational age (SGA) infants are scarce. In the recent report of ESPGHAN, the recommended energy for very preterm infants during hospitalization has been increased, yet this may not fit the needs of all preterm infants. It is important to distinguish fetal growth-restricted (FGR) infants from constitutional SGA infants, as well as preterm SGA from preterm AGA infants, since they may have different nutritional needs. Preterm FGR infants, and specifically infants < 29 weeks’ gestation, accumulate nutrient deficits due to intrauterine malnutrition, prematurity, morbidities, delayed initiation of feeding, and feeding intolerance. Therefore, these infants may need more aggressive nutrition for optimal catch-up growth and neurologic development.
1. Introduction
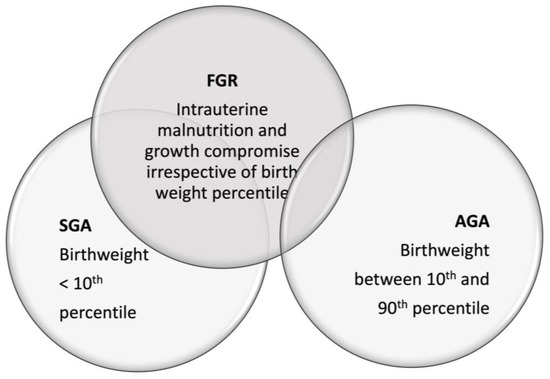
2. The Distinction between Constitutional SGA and FGR Infants
References
- Supplementary Digital Content no.1. ESPGHAN Committee of Nutrition (CoN) Position Paper on Enteral Nutrition for Preterm Infants: Introduction, Methods and Limitations. Available online: http://links.lww.com/MPG/C974 (accessed on 6 April 2023).
- Fleig, L.; Hagan, J.; Lee, M.L.; Abrams, S.A.; Hawthorne, K.M.; Hair, A.B. Growth outcomes of small for gestational age preterm infants before and after implementation of an exclusive human milk-based diet. J. Perinatol. 2021, 41, 1859–1864.
- Shah, M.D.; Shah, S.R. Nutrient deficiencies in the premature infant. Pediatr. Clin. N. Am. 2009, 56, 1069–1083.
- Dorling, J.; Kempley, S.; Leaf, A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F359–F363.
- Chan, P.Y.; Morris, J.M.; Leslie, G.I.; Kelly, P.J.; Gallery, E.D. The long-term effects of prematurity and intrauterine growth restriction on cardiovascular, renal, and metabolic function. Int. J. Pediatr. 2010, 2010, 280402.
- Martín-Calvo, N.; Goni, L.; Tur, J.A.; Martínez, J.A. Low birth weight and small for gestational age are associated with complications of childhood and adolescence obesity: Systematic review and meta-analysis. Obes. Rev. 2022, 23 (Suppl. S1), e13380.
- Damhuis, S.E.; Ganzevoort, W.; Gordijn, S.J. Abnormal Fetal Growth: Small for Gestational Age, Fetal Growth Restriction, Large for Gestational Age: Definitions and Epidemiology. Obstet. Gynecol. Clin. N. Am. 2021, 48, 267–279.
- Maulik, D. Fetal growth restriction: The etiology. Clin. Obstet. Gynecol. 2006, 49, 228–235.
- Kesavan, K.; Devaskar, S.U. Intrauterine Growth Restriction: Postnatal Monitoring and Outcomes. Pediatr. Clin. N. Am. 2019, 66, 403–423.
- WHO. Expert Committee on Physical Status: The Use Interpretation of Anthropometry & World Health Organization. In Physical Status: The Use of and Interpretation of Anthropometry, Report of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1995.
- Clayton, P.E.; Cianfarani, S.; Czernichow, P.; Johannsson, G.; Rapaport, R.; Rogol, A. Management of the child born small for gestational age through to adulthood: A consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J. Clin. Endocrinol. Metab. 2007, 92, 804–810.
- Lees, C.C.; Romero, R.; Stampalija, T.; Dall’Asta, A.; DeVore, G.A.; Prefumo, F.; Frusca, T.; Visser, G.H.A.; Hobbins, J.C.; Baschat, A.A.; et al. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: An evidence-based approach. Am. J. Obstet. Gynecol. 2022, 226, 366–378.
- Lee, A.C.; Kozuki, N.; Cousens, S.; Stevens, G.A.; Blencowe, H.; Silveira, M.F.; Sania, A.; Rosen, H.E.; Schmiegelow, C.; Adair, L.S.; et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: Analysis of CHERG datasets. BMJ 2017, 358, j3677.
- de Onis, M.; Blössner, M.; Villar, J. Levels and patterns of intrauterine growth retardation in developing countries. Eur. J. Clin. Nutr. 1998, 52 (Suppl. S1), S5–S15.
- He, H.; Miao, H.; Liang, Z.; Zhang, Y.; Jiang, W.; Deng, Z.; Tang, J.; Liu, G.; Luo, X. Prevalence of small for gestational age infants in 21 cities in China, 2014–2019. Sci. Rep. 2021, 11, 7500.
- Bernstein, I.M.; Horbar, J.D.; Badger, G.J.; Ohlsson, A.; Golan, A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am. J. Obstet. Gynecol. 2000, 182, 198–206.
- Lemons, J.A.; Bauer, C.R.; Oh, W.; Korones, S.B.; Papile, L.A.; Stoll, B.J.; Verter, J.; Temprosa, M.; Wright, L.L.; Ehrenkranz, R.A.; et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001, 107, E1.
- Fang, S. Management of preterm infants with intrauterine growth restriction. Early Hum. Dev. 2005, 81, 889–900.
- Ananth, C.V.; Vintzileos, A.M. Distinguishing pathological from constitutional small for gestational age births in population-based studies. Early Hum. Dev. 2009, 85, 653–658.
- Clausson, B.; Gardosi, J.; Francis, A.; Cnattingius, S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG 2001, 108, 830–834.
- Mongelli, M.; Figueras, F.; Francis, A.; Gardosi, J. A customized birthweight centile calculator developed for an Australian population. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 128–131.
- Figueras, F.; Figueras, J.; Meler, E.; Eixarch, E.; Coll, O.; Gratacos, E.; Gardosi, J.; Carbonell, X. Customised birthweight standards accurately predict perinatal morbidity. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F277–F280.
- Gardosi, J.; Chang, A.; Kalyan, B.; Sahota, D.; Symonds, E.M. Customised antenatal growth charts. Lancet 1992, 339, 283–287.
- Carberry, A.E.; Gordon, A.; Bond, D.M.; Hyett, J.; Raynes-Greenow, C.H.; Jeffery, H.E. Customised versus population-based growth charts as a screening tool for detecting small for gestational age infants in low-risk pregnant women. Cochrane Database Syst. Rev. 2014, 2014, Cd008549.
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339.
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417.
- Karlberg, J.; Albertsson-Wikland, K. Growth in full-term small-for-gestational-age infants: From birth to final height. Pediatr. Res. 1995, 38, 733–739.
- Recio Linares, A.; Bezanilla López, C.; Barasoain Millán, A.; Domínguez Uribe-Echevarría, M.; García Rodríguez, C.; Torrejón López, M.; Pérez Fernández, E.; Botija Arcos, G.; Barrio Merino, A. Longitudinal study of the newborn small for gestational age. Growth recovery and conditioning factors. Nutr. Hosp. 2022, 39, 520–529.
- Campisi, S.C.; Carbone, S.E.; Zlotkin, S. Catch-Up Growth in Full-Term Small for Gestational Age Infants: A Systematic Review. Adv. Nutr. 2019, 10, 104–111.
- Castanys-Muñoz, E.; Kennedy, K.; Castañeda-Gutiérrez, E.; Forsyth, S.; Godfrey, K.M.; Koletzko, B.; Ozanne, S.E.; Rueda, R.; Schoemaker, M.; van der Beek, E.M.; et al. Systematic review indicates postnatal growth in term infants born small-for-gestational-age being associated with later neurocognitive and metabolic outcomes. Acta Paediatr. 2017, 106, 1230–1238.
- Singhal, A.; Cole, T.J.; Fewtrell, M.; Kennedy, K.; Stephenson, T.; Elias-Jones, A.; Lucas, A. Promotion of faster weight gain in infants born small for gestational age: Is there an adverse effect on later blood pressure? Circulation 2007, 115, 213–220.
- Singhal, A.; Kennedy, K.; Lanigan, J.; Fewtrell, M.; Cole, T.J.; Stephenson, T.; Elias-Jones, A.; Weaver, L.T.; Ibhanesebhor, S.; MacDonald, P.D.; et al. Nutrition in infancy and long-term risk of obesity: Evidence from 2 randomized controlled trials. Am. J. Clin. Nutr. 2010, 92, 1133–1144.
- Santiago, A.C.T.; Cunha, L.; Vieira, N.S.A.; Oliveira Moreira, L.M.; Oliveira, P.R.; Lyra, P.P.R.; Alves, C.A.D. Breastfeeding in children born small for gestational age and future nutritional and metabolic outcomes: A systematic review. J. Pediatr. 2019, 95, 264–274.





