Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Meysam Tayebi | -- | 1920 | 2023-06-21 11:22:49 | | | |
| 2 | Dean Liu | Meta information modification | 1920 | 2023-06-25 03:52:34 | | | | |
| 3 | Dean Liu | Meta information modification | 1920 | 2023-06-28 09:49:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Masoumi, Z.; Tayebi, M.; Tayebi, M.; Masoumi Lari, S.A.; Sewwandi, N.; Seo, B.; Lim, C.; Kim, H.; Kyung, D. Electrocatalytic Reactions for Converting CO2 to Value-Added Products. Encyclopedia. Available online: https://encyclopedia.pub/entry/45922 (accessed on 08 February 2026).
Masoumi Z, Tayebi M, Tayebi M, Masoumi Lari SA, Sewwandi N, Seo B, et al. Electrocatalytic Reactions for Converting CO2 to Value-Added Products. Encyclopedia. Available at: https://encyclopedia.pub/entry/45922. Accessed February 08, 2026.
Masoumi, Zohreh, Meysam Tayebi, Mahdi Tayebi, S. Ahmad Masoumi Lari, Nethmi Sewwandi, Bongkuk Seo, Choong-Sun Lim, Hyeon-Gook Kim, Daeseung Kyung. "Electrocatalytic Reactions for Converting CO2 to Value-Added Products" Encyclopedia, https://encyclopedia.pub/entry/45922 (accessed February 08, 2026).
Masoumi, Z., Tayebi, M., Tayebi, M., Masoumi Lari, S.A., Sewwandi, N., Seo, B., Lim, C., Kim, H., & Kyung, D. (2023, June 21). Electrocatalytic Reactions for Converting CO2 to Value-Added Products. In Encyclopedia. https://encyclopedia.pub/entry/45922
Masoumi, Zohreh, et al. "Electrocatalytic Reactions for Converting CO2 to Value-Added Products." Encyclopedia. Web. 21 June, 2023.
Copy Citation
Carbon dioxide (CO2) emissions are an important environmental issue that causes greenhouse and climate change effects on the earth. Nowadays, CO2 has various conversion methods to be a potential carbon resource, such as photocatalytic, electrocatalytic, and photo-electrocatalytic. CO2 conversion into value-added products has many advantages, including facile control of the reaction rate by adjusting the applied voltage and minimal environmental pollution. The development of efficient electrocatalysts and improving their viability with appropriate reactor designs is essential for the commercialization of this environmentally friendly method.
electrochemical reaction
CO2 conversion
reduction reaction
1. Introduction
Since the industrial revolution of the 19th century, fossil fuels such as petroleum, natural gas, and coal have been used as the main source of energy to power economies and civilizations [1]. There is a need to reduce CO2 emissions because the burning of these fossil fuels has resulted in excessive CO2 emissions into the atmosphere, which have had significant negative effects on the environment and pose an immediate threat to human societies [2][3][4]. The swift transformation of the need of energy and chemical industries from fossil fuels to renewable energy resources, for example, solar and wind, can be identified as one of the solutions to achieve the closed-looped configurations on the carbon footprint [5][6][7].
Nonetheless, several artificial solutions to limit or reduce CO2 emissions have been created, such as technological innovation to increase coal burning efficiency in boilers (reducing coal consumption) and carbon capture and sequestration (CCS) [8][9][10] though CCS is a costly and an energy-consuming technology. In fact, dangerous CO2 leakage is a major concern that inhibits the commercialized large-scale deployment of CCS. As a result, fixation of CO2 remains a significant concern on a global scale [11][12][13].
Hence, currently, the best strategy is to use atmospheric CO2 as a renewable feedstock to create a few chemical products with added value, such as light olefins, urea, formic acid, methanol, syngas, and (poly)carbonate [14]. A technique such as this will reduce the atmospheric CO2 levels while producing fuels and industrial chemicals, reducing the reliance on traditional fossil fuels [15][16]. Therefore, several CO2 reduction strategies, such as photochemical, electrochemical, thermochemical, and biochemical procedures, have been developed and extensively researched [17][18].
Among these technologies, lowering CO2 emissions using renewable power is especially tempting due to its enormous potential, simple reaction units, controlled selectivity, and modest efficiency for practical industrial applications [19]. Furthermore, it is possible to think of electrocatalytic carbon dioxide reduction (ECR) as a useful method for storing the renewable energy discussed above in chemical forms [20][21][22][23][24]. ECR paired with renewable energy techniques as electricity sources are widely employed in the energy sectors and chemicals, and it may offer a promising route to create considerable amounts of chemicals and carbon-neutral fuels [25][26]. Electrochemical CO2 conversion offers various benefits over other methods: (i) using renewable energy sources such as solar, wind, geothermal, and tidal; (ii) the mechanism is simpler and precise in terms of administering as it only requires the monitoring of reaction temperatures and the potential of electrodes; (iii) having scalable, compact and highly efficient on demand transmutation systems; (iv) hydrocarbons can be formed from water, carbon dioxide, and renewable electricity [27][28].
The main question is how to build a high-performance CO2 conversion system that has all the desired qualities at the same time [29]. The main component of a high-performance CO2 conversion system is a system that has higher operational current density and produces better faradaic and energy efficiency for CO2R [30][31]. Many research efforts in ECR have been directed to the search for better electrocatalyst materials, because appropriate electrocatalysts have a better active site that ideally leads to the synthesis of desirable products at high rates and low overpotentials [32][33][34][35].
Metals, metal oxides, two-dimensional materials, and functional microorganisms have all been investigated as CO2 reduction electrocatalyst materials. Metals’ catalytic durability, selectivity, and activity could be improved by controlling their crystal faceting, morphology, and size [36]. There are activities for electrocatalytic CO2 reduction in metal oxides such as Co3O4 [37][38], CuO [39][40], ZnO [41][42], and TiO2 [43][44]. Contrary to pure metal catalysts, most CO2 reduction process intermediates are expected to bind via their oxygen atoms and those of metal oxides. This criterion implies that metal oxides have higher oxygenate selectivity than pure metal catalysts [45][46].
Two-dimensional (2D) materials with nanosheets can exhibit unique features and great performance in catalytic processes when used as catalysts. Two-dimensional electrocatalysts decrease the energy barrier for CO2 activation, improve electrical conductivity, and have a high surface-active site density, which makes them promising for highly efficient CO2 conversion [47][48]. Because, as compared to ordinary bulk materials, they have a significantly higher percentage of bare surface atoms and higher specific surface areas, they might provide an abundance of active sites, enhancing catalytic processes [49][50]. It should be noted that highly exposed surface atoms might escape and create defect structures, resulting in lower coordination numbers of surface atoms, which are attractive locations for reactant or intermediate adsorption. Similarly, nanosheet edge atoms with low coordination numbers can display unique catalytic characteristics. As a result, 2D structures can boost reactant chemisorption and improve catalytic efficiency [51].
Bio-catalysis, which incorporates microbes and enzymes, has received a great deal of interest because the value-added products can be produced under mild circumstances with remarkable selectivity and without any undesirable byproducts [52][53]. Given previous research in bio-inspired molecular structure design, expanded and dynamic connections through the materials, biological, and chemical science domains will synergistically promote catalyst development [49][50]. Microbial electrosynthesis (MES) utilizes self-replicating bacteria as a catalyst at room temperature and pressure, which enables a more economical and ecologically benign process than traditional chemical catalyst-based conversion. To metabolize CO2, bacteria in MES exchange electrons directly or indirectly using electron shuttle molecules [54]. To recycle anthropogenic CO2, electroactive microorganisms are employed in MES as a biocatalyst on suitable electrode materials [55].
2. Concepts of Electrochemical CO2 Reduction Reaction
Concepts of Electrochemical CO2 Reduction Reaction
The electrochemical conversion of CO2, a linear stable molecule with a powerful C–O bond (750 kJ mol−1), is challenging. Multi-electron/proton transfer processes, a large variety of possible reaction intermediates, and an ECR in an aqueous electrolyte are all part of the extremely complicated process of ECR [56][57].
Electrochemical reduction has been researched in aqueous solutions with various metal cathodes, as well as in several organic solvents. Although the successfully documented six-electron and eight-electron conversions to methanol and methane exist, the commonly discussed reduction products are carbon monoxide, acetic acid, and formic acid [58][59][60]. The main ECR products’ half electrochemical thermodynamic reactions are shown in Table 1, ethanol (CH3CH2OH), ethylene (C2H4), formic acid (HCOOH), methanol (CH3OH), methane (CH4), carbon monoxide (CO), and acetate (CH3COOH), with reporting of their standard redox potentials at acid and base electrolytes [61][62].
Table 1. Standard redox potentials (VRHE) for ECR generation processes in acid and base.

In an ECR process, CO2 molecules adsorb on the catalyst surface and interact with the atoms there to produce *CO2, which is then followed by many progressive transfers of electrons and/or protons toward different end products. For instance, methane is thought to originate via the pathways given below (Scheme 1) [63]:

Scheme 1. Pathway for the electrochemical conversion of methane from CO2.
A multistep reaction process, electrochemical CO2 reduction typically involves a different number of electron reaction pathways. The reaction frequently happens at the electrolyte–electrode interface for heterogeneous catalysts used in CO2 reduction, where the electrode is typically a solid electrocatalyst and the electrolyte is typically an aqueous solution saturated with CO2 through bubbling.
Water is transformed to oxygen and CO2 is reduced to the CO2 anion radical at the anode in a single-electron ECR (CO2−). The first step of converting CO2 to reduced carbon species is difficult because the reaction rate is very slow. The single-electron CO2 reduction to CO2− with a pH of 7 exhibits an unfavorable and energetic reaction, with a thermodynamic potential of roughly −1.90 V vs. SHE. Furthermore, the formation of the CO2 intermediate is essential to the formation of the 2e− reduction products and the initial process can be considered the rate-limiting step [64].
Several electron/proton transfer processes are involved in the electrochemical CO2RR, and CO2 can be reduced into a collection of gaseous and liquid products by diverse pathways, including hydrocarbons (CH4 and C2H4), alcohols (CH3OH and C2H5OH), carbon monoxide (CO), and formic acid (HCOOH) [65]. This depends on the electrolytic conditions and the electrocatalysts used (e.g., applied potential, electrolyte, etc.) [28][37][66]. Without a catalyst, it is challenging to complete the first stage of CO2 activation, which produces the intermediate CO2− radical. However, with the aid of an electrocatalyst, the CO2− radical can be stabilized via a chemical link created between CO2 and the electrocatalyst, leading to less negative redox potential. Moreover, proton-coupled electron transfer is advantageous at the likely range of 0.20 to 0.60 V vs. SHE. The end products are influenced by the electrocatalyst and electrolyte selections as well as the quantities of electrons and protons transferred [51]. Therefore, the activation routes of some typical products in CO2RR are briefly shown in Figure 1 [64].
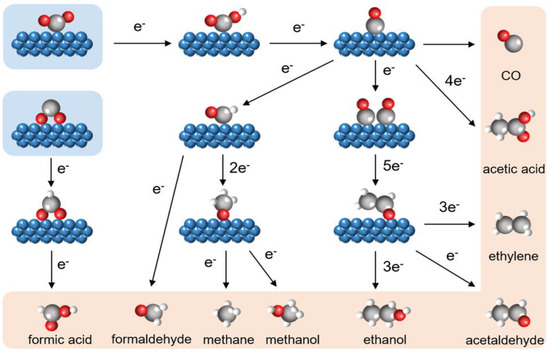
Figure 1. An overview of CO2RR’s reaction pathways leading to various products [64].
Molecule reactants may react with various CO2RR intermediates at any phase since CO2RR contains several reaction steps and intermediates, which greatly broadens the range of possible products. Consequently, potential products can be selectively derived through the adjustment of the adsorption and desorption capability of electrocatalysts to distinct reaction intermediates from coupled CO2RR [64].
A laboratory electrochemical H-cell consists of oxygen evolution reaction (OER) happening at the surface of an anode that generates electrons (e−) and protons (H+) or consumes hydroxyl ions (OH−); a cathode in order to reduce CO2 to produces such as HCOOH/HCOO− or CO, and make OH−; an electrolyte with the intention of transporting CO2 to the active cathode sites and conduct ions; a membrane that allows ion exchange to take apart the anode and cathode; and a bias with suitable value to move electrons from anode to cathode (Figure 2a). A few crucial steps in a CO2R process are involved in such a system, including (1) movement of products into liquid phases or bulk gases from the cathode/electrolyte interface, (2) product desorption from the electrode, (3) transfer of electrons from the cathode to intermediates, (4) adsorption of CO2 into adsorbed intermediates such as *CHO, *CO, and *COOH, (5) the surface of the cathode absorbing CO2, (6) transport of dissolved CO2 to the cathode/electrolyte interface from the bulk electrolyte, and (7) CO2 mass transfer to the bulk electrolyte from the gas phase [67].
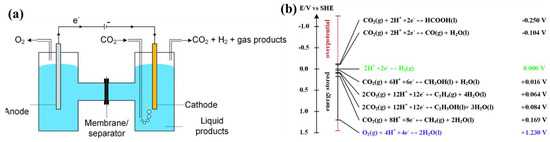
One of the most critical problems of the electrochemical CO2R technologies to function at large-scale is to obtain a great CO2 selectivity to desired value-added products to reduce product separation costs and complexity. High selectivity is difficult to achieve due to, as shown in Figure 2b, the majority of CO2R reactions’ standard potentials (Eo) and the hydrogen evolution reaction (HER) all being within a limited variety (−0.250 V to 0.169 V vs. standard hydrogen electrode) (SHE) [36].
3. Product Selectivity Parameters
The applied potential, pressure, temperature, type of electrolyte (pH, concentration, and composition), and type of electrocatalyst (crystallographic structure, chemical state, composition, and morphology) are all variables that affect selectivity, FE, and ECR performance.
In addition, the selectivity of catalysts for various products varies. The type and quantity of electrolytes also affect the catalyst’s activity and selectivity. While C2 products (such as ethanol, ethylene, and acetic acid) have primarily been observed using copper-based catalysts, C1 products (such as CO, methane, methanol, and formic acid) can develop in a variety of materials [68][69].
References
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303.
- Gattuso, J.P.; Magnan, A.; Billé, R.; Cheung, W.W.L.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting Futures for Ocean and Society from Different Anthropogenic CO2 Emissions Scenarios. Science 2015, 349, aac4722.
- Greenblatt, J.B.; Miller, D.J.; Ager, J.W.; Houle, F.A.; Sharp, I.D. The Technical and Energetic Challenges of Separating (Photo) Electrochemical Carbon Dioxide Reduction Products. Joule 2018, 2, 381–420.
- Mostafa, M.M.M.; Shawky, A.; Zaman, S.F.; Narasimharao, K.; Abdel Salam, M.; Alshehri, A.A.; Khdary, N.H.; Al-Faifi, S.; Chowdhury, A.D. Visible-Light-Driven CO2 Reduction into Methanol Utilizing Sol-Gel-Prepared CeO2-Coupled Bi2O3 Nanocomposite Heterojunctions. Catalysts 2022, 12, 1479.
- Gwóźdź, M.; Brzęczek-Szafran, A. Carbon-Based Electrocatalyst Design with Phytic Acid—A Versatile Biomass-Derived Modifier of Functional Materials. Int. J. Mol. Sci. 2022, 23, 11282.
- Mallamace, D.; Papanikolaou, G.; Perathoner, S.; Centi, G.; Lanzafame, P. Comparing Molecular Mechanisms in Solar NH3 Production and Relations with CO2 Reduction. Int. J. Mol. Sci. 2020, 22, 139.
- Khdary, N.H.; Ghanem, M.A. Metal–Organic–Silica Nanocomposites: Copper, Silver Nanoparticles–Ethylenediamine–Silica Gel and Their CO2 Adsorption Behaviour. J. Mater. Chem. 2012, 22, 12032–12038.
- Chen, Z.; Wang, X.; Liu, L. Electrochemical Reduction of Carbon Dioxide to Value-Added Products: The Electrocatalyst and Microbial Electrosynthesis. Chem. Rec. 2019, 19, 1272–1282.
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An Overview of CO2 Capture Technologies. Energy Environ. Sci. 2010, 3, 1645–1669.
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The Role of CO2 Capture and Utilization in Mitigating Climate Change. Nat. Clim. Chang. 2017, 7, 243–249.
- Dong Zhu, D.; Long Liu, J.; Zhang Qiao, S.; Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452.
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon Capture and Storage Update. Energy Environ. Sci. 2013, 7, 130–189.
- Campeau, A.; Bishop, K.; Amvrosiadi, N.; Billett, M.F.; Garnett, M.H.; Laudon, H.; Öquist, M.G.; Wallin, M.B. Current Forest Carbon Fixation Fuels Stream CO2 Emissions. Nat. Commun. 2019, 10, 1876.
- Lamparelli, D.H.; Grimaldi, I.; Martínez-Carrión, A.; Bravo, F.; Kleij, A.W. Supercritical CO2 as an Efficient Medium for Macromolecular Carbonate Synthesis through Ring-Opening Co- and Teroligomerization. ACS Sustain. Chem. Eng. 2023, 11, 8193–8198.
- Kumar, B.; Brian, J.P.; Atla, V.; Kumari, S.; Bertram, K.A.; White, R.T.; Spurgeon, J.M. New Trends in the Development of Heterogeneous Catalysts for Electrochemical CO2 Reduction. Catal. Today 2016, 270, 19–30.
- Hu, J.; Al-Salihy, A.; Zhang, B.; Li, S.; Xu, P. Mastering the D-Band Center of Iron-Series Metal-Based Electrocatalysts for Enhanced Electrocatalytic Water Splitting. Int. J. Mol. Sci. 2022, 23, 15405.
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The Teraton Challenge. A Review of Fixation and Transformation of Carbon Dioxide. Energy Environ. Sci. 2010, 3, 43–81.
- Del Vecchio, A.; Caillé, F.; Chevalier, A.; Loreau, O.; Horkka, K.; Halldin, C.; Schou, M.; Camus, N.; Kessler, P.; Kuhnast, B.; et al. Late-Stage Isotopic Carbon Labeling of Pharmaceutically Relevant Cyclic Ureas Directly from CO2. Angew. Chem. Int. Ed. 2018, 57, 9744–9748.
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672.
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Razzaq, S.; Wang, Z.; Shagdar, E.; Zhao, J. A Techno-Economic Study of Commercial Electrochemical CO2 Reduction into Diesel Fuel and Formic Acid. J. Electrochem. Sci. Technol. 2022, 13, 148–158.
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519.
- Pei, Y.; Zhong, H.; Jin, F. A Brief Review of Electrocatalytic Reduction of CO2—Materials, Reaction Conditions, and Devices. Energy Sci. Eng. 2021, 9, 1012–1032.
- Ding, M.; Chen, Z.; Liu, C.; Wang, Y.; Li, C.; Li, X.; Zheng, T.; Jiang, Q.; Xia, C. Electrochemical CO2 Reduction: Progress and Opportunity with Alloying Copper. Mater. Rep. Energy 2023, 3, 100175.
- Sun, Z.; Ma, T.; Tao, H.; Fan, Q.; Han, B. Fundamentals and Challenges of Electrochemical CO2 Reduction Using Two-Dimensional Materials. Chem 2017, 3, 560–587.
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504.
- Dufek, E.J.; Lister, T.E.; Stone, S.G.; McIlwain, M.E. Operation of a Pressurized System for Continuous Reduction of CO2. J. Electrochem. Soc. 2012, 159, F514–F517.
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A Review of High Temperature Co-Electrolysis of H2O and CO2 to Produce Sustainable Fuels Using Solid Oxide Electrolysis Cells (SOECs): Advanced Materials and Technology. Chem. Soc. Rev. 2017, 46, 1427–1463.
- Gabardo, C.M.; Seifitokaldani, A.; Edwards, J.P.; Dinh, C.T.; Burdyny, T.; Kibria, M.G.; O’Brien, C.P.; Sargent, E.H.; Sinton, D. Combined High Alkalinity and Pressurization Enable Efficient CO2 Electroreduction to CO. Energy Environ. Sci. 2018, 11, 2531–2539.
- Gong, Q.; Ding, P.; Xu, M.; Zhu, X.; Wang, M.; Deng, J.; Ma, Q.; Han, N.; Zhu, Y.; Lu, J.; et al. Structural Defects on Converted Bismuth Oxide Nanotubes Enable Highly Active Electrocatalysis of Carbon Dioxide Reduction. Nat. Commun. 2019, 10, 2807.
- Lv, J.-J.; Jouny, M.; Luc, W.; Zhu, W.; Zhu, J.-J.; Jiao, F.; Lv, J.; Jouny, M.; Luc, W.; Zhu, W.L.; et al. A Highly Porous Copper Electrocatalyst for Carbon Dioxide Reduction. Adv. Mater. 2018, 30, 1803111.
- Lamaison, S.; Wakerley, D.; Blanchard, J.; Montero, D.; Rousse, G.; Mercier, D.; Marcus, P.; Taverna, D.; Giaume, D.; Mougel, V.; et al. High-Current-Density CO2-to-CO Electroreduction on Ag-Alloyed Zn Dendrites at Elevated Pressure. Joule 2020, 4, 395–406.
- Wang, Z.L.; Choi, J.; Xu, M.; Hao, X.; Zhang, H.; Jiang, Z.; Zuo, M.; Kim, J.; Zhou, W.; Meng, X.; et al. Optimizing Electron Densities of Ni-N-C Complexes by Hybrid Coordination for Efficient Electrocatalytic CO2 Reduction. ChemSusChem 2020, 13, 929–937.
- Salehi-Khojin, A.; Jhong, H.R.M.; Rosen, B.A.; Zhu, W.; Ma, S.; Kenis, P.J.A.; Masel, R.I. Nanoparticle Silver Catalysts That Show Enhanced Activity for Carbon Dioxide Electrolysis. J. Phys. Chem. C 2013, 117, 1627–1632.
- Wang, Z.L.; Li, C.; Yamauchi, Y. Nanostructured Nonprecious Metal Catalysts for Electrochemical Reduction of Carbon Dioxide. Nano Today 2016, 11, 373–391.
- Zhu, Y.; Yang, X.; Peng, C.; Priest, C.; Mei, Y.; Wu, G.; Zhu, Y.; Peng, C.; Mei, Y.; Yang, X.; et al. Carbon-Supported Single Metal Site Catalysts for Electrochemical CO2 Reduction to CO and Beyond. Small 2021, 17, 2005148.
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A Review of Catalysts for the Electroreduction of Carbon Dioxide to Produce Low-Carbon Fuels. Chem. Soc. Rev. 2013, 43, 631–675.
- Bharath, G.; Rambabu, K.; Aubry, C.; Abu Haija, M.; Nadda, A.K.; Ponpandian, N.; Banat, F. Self-Assembled Co3O4 Nanospheres on N-Doped Reduced Graphene Oxide (Co3O4/N-RGO) Bifunctional Electrocatalysts for Cathodic Reduction of CO2 and Anodic Oxidation of Organic Pollutants. ACS Appl. Energy Mater. 2021, 4, 11408–11418.
- Zhang, S.Y.; Zhu, H.L.; Zheng, Y.Q. Surface Modification of CuO Nanoflake with Co3O4 Nanowire for Oxygen Evolution Reaction and Electrocatalytic Reduction of CO2 in Water to Syngas. Electrochim. Acta 2019, 299, 281–288.
- Zhong, X.; Liang, S.; Yang, T.; Zeng, G.; Zhong, Z.; Deng, H.; Zhang, L.; Sun, X. Sn Dopants with Synergistic Oxygen Vacancies Boost CO2 Electroreduction on CuO Nanosheets to CO at Low Overpotential. ACS Nano 2022, 16, 19210–19219.
- Zhao, Y.; Chang, X.; Malkani, A.S.; Yang, X.; Thompson, L.; Jiao, F.; Xu, B. Speciation of Cu Surfaces during the Electrochemical CO Reduction Reaction. J. Am. Chem. Soc. 2020, 142, 9735–9743.
- Geioushy, R.A.; Khaled, M.M.; Alhooshani, K.; Hakeem, A.S.; Rinaldi, A. Graphene/ZnO/Cu2O Electrocatalyst for Selective Conversion of CO2 into n-Propanol. Electrochim. Acta 2017, 245, 456–462.
- Luo, W.; Zhang, Q.; Zhang, J.; Moioli, E.; Zhao, K.; Züttel, A. Electrochemical Reconstruction of ZnO for Selective Reduction of CO2 to CO. Appl. Catal. B 2020, 273, 119060.
- Windle, C.D.; Perutz, R.N. Advances in Molecular Photocatalytic and Electrocatalytic CO2 Reduction. Coord. Chem. Rev. 2012, 256, 2562–2570.
- Liu, J.Y.; Gong, X.Q.; Li, R.; Shi, H.; Cronin, S.B.; Alexandrova, A.N. (Photo) Electrocatalytic CO2 Reduction at the Defective Anatase TiO2 (101) Surface. ACS Catal. 2020, 10, 4048–4058.
- Liang, F.; Zhang, K.; Zhang, L.; Zhang, Y.; Lei, Y.; Sun, X.; Liang, F.; Zhang, K.; Zhang, Y.; Zhang, L.; et al. Recent Development of Electrocatalytic CO2 Reduction Application to Energy Conversion. Small 2021, 17, 2100323.
- Khdary, N.H.; Alayyar, A.S.; Alsarhan, L.M.; Alshihri, S.; Mokhtar, M. Metal Oxides as Catalyst/Supporter for CO2 Capture and Conversion, Review. Catalysts 2022, 12, 300.
- Li, Z.; Zhai, L.; Ge, Y.; Huang, Z.; Shi, Z.; Liu, J.; Zhai, W.; Liang, J.; Zhang, H. Wet-Chemical Synthesis of Two-Dimensional Metal Nanomaterials for Electrocatalysis. Natl. Sci. Rev. 2022, 9, nwab142.
- Cai, F.; Hu, X.; Gou, F.; Chen, Y.; Xu, Y.; Qi, C.; Ma, D.K. Ultrathin ZnIn2S4 Nanosheet Arrays Activated by Nitrogen-Doped Carbon for Electrocatalytic CO2 Reduction Reaction toward Ethanol. Appl. Surf. Sci. 2023, 611, 155696.
- Ao, C.; Feng, B.; Qian, S.; Wang, L.; Zhao, W.; Zhai, Y.; Zhang, L. Theoretical Study of Transition Metals Supported on G-C3N4 as Electrochemical Catalysts for CO2 Reduction to CH3OH and CH4. J. CO2 Util. 2020, 36, 116–123.
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The Chemistry of Two-Dimensional Layered Transition Metal Dichalcogenide Nanosheets. Nat. Chem. 2013, 5, 263–275.
- Lu, S.; Lou, F.; Yu, Z. Recent Progress in Two-Dimensional Materials for Electrocatalytic CO2 Reduction. Catalysts 2022, 12, 228.
- Luan, L.; Ji, X.; Guo, B.; Cai, J.; Dong, W.; Huang, Y.; Zhang, S. Bioelectrocatalysis for CO2 Reduction: Recent Advances and Challenges to Develop a Sustainable System for CO2 Utilization. Biotechnol. Adv. 2023, 63, 108098.
- Singh, S.; Noori, M.T.; Verma, N. Efficient Bio-Electroreduction of CO2 to Formate on a Iron Phthalocyanine-Dispersed CDC in Microbial Electrolysis System. Electrochim. Acta 2020, 338, 135887.
- Shafaat, H.S.; Yang, J.Y. Uniting Biological and Chemical Strategies for Selective CO2 Reduction. Nat. Catal. 2021, 4, 928–933.
- Lekshmi, G.S.; Bazaka, K.; Ramakrishna, S.; Kumaravel, V. Microbial Electrosynthesis: Carbonaceous Electrode Materials for CO2 Conversion. Mater. Horiz. 2023, 10, 292–312.
- Loiudice, A.; Lobaccaro, P.; Kamali, E.A.; Thao, T.; Huang, B.H.; Ager, J.W.; Buonsanti, R. Tailoring Copper Nanocrystals towards C2 Products in Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2016, 55, 5789–5792.
- Mistry, H.; Varela, A.S.; Bonifacio, C.S.; Zegkinoglou, I.; Sinev, I.; Choi, Y.W.; Kisslinger, K.; Stach, E.A.; Yang, J.C.; Strasser, P.; et al. Highly Selective Plasma-Activated Copper Catalysts for Carbon Dioxide Reduction to Ethylene. Nat. Commun. 2016, 7, 12123.
- Gattrell, M.; Gupta, N.; Co, A. A Review of the Aqueous Electrochemical Reduction of CO2 to Hydrocarbons at Copper. J. Electroanal. Chem. 2006, 594, 1–19.
- Zhou, Y.; Che, F.; Liu, M.; Zou, C.; Liang, Z.; De Luna, P.; Yuan, H.; Li, J.; Wang, Z.; Xie, H.; et al. Dopant-Induced Electron Localization Drives CO2 Reduction to C2 Hydrocarbons. Nat. Chem. 2018, 10, 974–980.
- Eilert, A.; Cavalca, F.; Roberts, F.S.; Osterwalder, J.; Liu, C.; Favaro, M.; Crumlin, E.J.; Ogasawara, H.; Friebel, D.; Pettersson, L.G.M.; et al. Subsurface Oxygen in Oxide-Derived Copper Electrocatalysts for Carbon Dioxide Reduction. J. Phys. Chem. Lett. 2017, 8, 285–290.
- Albo, J.; Alvarez-Guerra, M.; Castaño, P.; Irabien, A. Towards the Electrochemical Conversion of Carbon Dioxide into Methanol. Green Chem. 2015, 17, 2304–2324.
- Babin, V.; Sallustrau, A.; Loreau, O.; Caillé, F.; Goudet, A.; Cahuzac, H.; Del Vecchio, A.; Taran, F.; Audisio, D. A General Procedure for Carbon Isotope Labeling of Linear Urea Derivatives with Carbon Dioxide. Chem. Commun. 2021, 57, 6680–6683.
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How Copper Catalyzes the Electroreduction of Carbon Dioxide into Hydrocarbon Fuels. Energy Environ. Sci. 2010, 3, 1311–1315.
- Quan, Y.; Zhu, J.; Zheng, G. Electrocatalytic Reactions for Converting CO2 to Value-Added Products. Small Sci. 2021, 1, 2100043.
- Ponsard, L.; Nicolas, E.; Tran, N.H.; Lamaison, S.; Wakerley, D.; Cantat, T.; Fontecave, M. Coupling Electrocatalytic CO2 Reduction with Thermocatalysis Enables the Formation of a Lactone Monomer. ChemSusChem 2021, 14, 2198–2204.
- Liang, S.; Huang, L.; Gao, Y.; Wang, Q.; Liu, B. Electrochemical Reduction of CO2 to CO over Transition Metal/N-Doped Carbon Catalysts: The Active Sites and Reaction Mechanism. Adv. Sci. 2021, 8, 2102886.
- Garg, S.; Li, M.; Weber, A.Z.; Ge, L.; Li, L.; Rudolph, V.; Wang, G.; Rufford, T.E. Advances and Challenges in Electrochemical CO2 Reduction Processes: An Engineering and Design Perspective Looking beyond New Catalyst Materials. J. Mater. Chem. A Mater. 2020, 8, 1511–1544.
- Nielsen, D.U.; Hu, X.M.; Daasbjerg, K.; Skrydstrup, T. Chemically and Electrochemically Catalysed Conversion of CO2 to CO with Follow-up Utilization to Value-Added Chemicals. Nat. Catal. 2018, 1, 244–254.
- Del Vecchio, A.; Talbot, A.; Caillé, F.; Chevalier, A.; Sallustrau, A.; Loreau, O.; Destro, G.; Taran, F.; Audisio, D. Carbon Isotope Labeling of Carbamates by Late-Stage , and Carbon Dioxide Incorporation. Chem. Commun. 2020, 56, 11677–11680.
More
Information
Subjects:
Electrochemistry; Engineering, Environmental; Energy & Fuels
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
571
Revisions:
3 times
(View History)
Update Date:
28 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




