Lung cancer is the leading cause of cancer-related death worldwide according to the World Health Organization. Non-small cell lung cancer makes up the majority of cases. Immunotherapy with immune checkpoint inhibitors and targeted therapy with tyrosine kinase inhibitors and other molecular targeted agents significantly changed the treatment landscape and overall survival. Unfortunately, resistance to these treatments develops, and there is a need to identify additional innovative therapies that can overcome treatment resistance. Advancements in biomedical engineering and technology allowed for the development of novel agents, capable of delivering effective treatment directly to tumor cells. These agents include antibody drug conjugates and bispecific antibodies which have various targets and mechanisms of action.
1. Introduction
Lung cancer is the leading cause of cancer-related death worldwide according to the WHO
[1]. Non-small cell lung cancer (NSCLC) contributes to around 85% of lung cancer diagnoses (vs. 15% for small cell lung cancer), which is further categorized into adenocarcinoma 78% and squamous 18%
[2]. Since the early 2000s, the treatment options for NSCLC have exponentially increased after the discovery of immune checkpoint inhibitors and targetable ‘driver mutations’
[2][3]. Immune checkpoint inhibitors, pembrolizumab, atezolizumab, and cemiplimab were proven effective first-line agents in programmed cell death ligand 1 [PD-L1] positive metastatic NSCLC after the respective phase 3 trials
[4][5][6]. Combination PD-L1 and cytotoxic T-lymphocyte-associated protein 4 [CTLA-4] inhibitors, nivolumab and ipilimumab were also proven in the first line compared to standard-of-care chemotherapy regardless of PD-L1 status
[7].
The first successful targets of ‘driver mutations’ were epidermal growth factor receptor (EGFR)-activating mutations, most commonly deletions in exon 19 or point mutation L858R in exon 21, found in approximately 15% of adenocarcinomas
[2][8][9][10]. Tumors with these mutations demonstrated significant sensitivity to tyrosine kinase inhibitors (TKIs; gefitinib and erlotinib) prompting investigation in several clinical trials [OPTIMAL, EURTAC, ENSURE, etc.]. These trials confirmed the superior ORR and median PFS of these first-generation TKIs versus standard-of-care chemotherapy and established their use as first-line therapy
[11][12][13][14][15].
Genetic alterations developed, notably the T790M mutation, which conferred resistance to the EGFR first-generation agents
[16]. From the landmark FLAURA trial, third-generation TKI osimertinib demonstrated effectiveness as first-line treatment with improved median PFS and OS compared to the first-generation agents, thereby becoming the new standard of care
[17]. Additionally, numerous other driver mutations have entered the standard of care in first- and second-line treatment, notably ALK, ROS1, BRAF V600E, MET Ex14 skipping, RET, HER2, NTRK, and, most recently, KRAS G12C
[18][19][20][21][22][23][24][25][26]. Unfortunately, resistance either through target-dependent (changes in the structure of the tyrosine kinase/target preventing inhibition) or target-independent (upregulation of bypass signaling pathways) emerged
[27]. This resistance to treatment with molecular targeted inhibitors and immune checkpoint blockade prompted researchers to investigate mechanisms and pathways to circumvent it.
2. Bispecific Antibodies
The concept of antibodies with the capability of targeting and utilizing multiple substrates (Bispecific, Trispecific) which could then be further enhanced to utilize T cells, so-called Bispecific T cell Engagers (BiTE), is relatively new in the context of cancer treatment. It was not until 1984 when BiTEs were first shown to employ T cells to prevent the growth of tumor cells in vivo
[28]. Success in clinical trials was limited following the initial discovery, but molecular modifications discovered from preclinical data eventually translated into clinical results
[29]. The first successful use of bispecific antibodies that did not utilize T cells was seen in end-stage Hodgkin’s lymphoma where one patient treated with an NK cell activating CD16/CD30 bispecific antibody achieved a complete remission while another achieved a partial remission from the study group of 15 patients
[30]. Since then, advances in protein engineering and molecular techniques prompted a variety of formulations and targets but the most common remains bispecific antibodies that redirect effector immune cells [T cells] to target malignant cells [BiTE]
[31]. The quick adoption of this treatment modality was not universal across the spectrum of malignancies, but interest has peaked recently, and lung cancer is no exception.
2.1. C-Met Targeting
While the prevalence of EGFR resistance in NSCLC is increasing since the introduction and widespread use of TKIs, bispecific antibodies may provide a potential solution. EGFR mutations with insertions in exon 20, except A763_Y764insFQEA, have demonstrated very low response rates (<10%) to common EGFR TKIs (osimertinib, erlotinib, etc.), which is thought to be related to alterations that prevent TKI binding
[32][33]. Additionally, MET gene amplification leads to the upregulation of cMet, and the subsequent pathway allows cancer cells to bypass EGFR signaling for cell survival, thereby creating another avenue of resistance to our current EGFR-targeted treatments
[34]. The CHRYSALIS trial, a phase I trial dose-escalation/dose-expansion study, sought to focus on both resistance patterns with amivantamab, a dual EGFR and cMET bispecific antibody
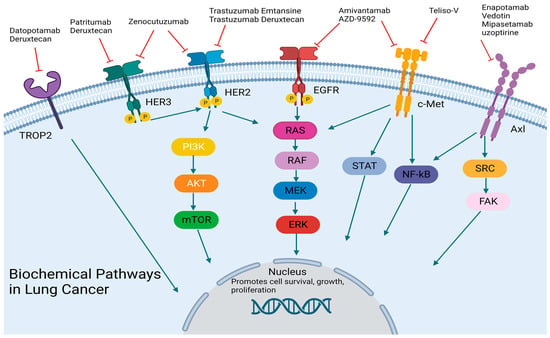
[35] (
Figure 1). The initial data were promising with a PFS of 8.3 months [95% CI, 6.5 to 10.9] and an ORR of 40% [95% CI, 29 to 51]
[34] (
Table 1). The drug appears to have tolerable side effects which include infusion reactions most commonly seen during the first cycle, 49% grade 2/3 rash, and 20% grade 2/3 paronychia
[35].
Figure 1. Biochemical Pathways: antibody-drug conjugates and bispecific antibodies targets. Designed using BioReader.
Table 1. Bispecific antibodies in lung cancer.
Additional investigation into amivantamab is currently underway including MARIPOSA1, a phase 3 trial evaluating the combination of amivantamab and lazertinib versus osimertinib in the first-line setting
[36]. There are also early studies in the preclinical and phase 1 settings attempting to identify additional targets and combination agents involving cMet signaling. One such example is AZD-9592, a bispecific antibody that targets both EGFR and c-Met but is also conjugated to a topoisomerase inhibitor, which allows for both cytotoxic effects and receptor signaling blockade, currently recruiting in a phase 1 trial (NCT05647122) (
Table 1).
2.2. HER2/HER3 Targeting
After human epidermal growth factor 2 (HER2) targeting was utilized in breast cancer successfully, similar attempts were made in lung cancer. Bispecific antibodies were once again implemented to target multiple facets of cell regulation. Zenocutuzumab, a bispecific antibody designed with one Fab arm that binds to HER2, allows the other Fab arm targeting human epidermal growth factor 3 (HER3) to be in a position to prevent NRG1 binding to HER3, thereby blocking activation and subsequent cell signaling
[37] (
Figure 1). This drug was first proven in pre-clinical models of lung, breast, pancreas, and ovarian cancers with neuregulin-1 (NRG1) fusions in both in vitro and in vivo settings
[38]. In the same study, a patient who had progressed on multiple lines of therapy with CD74-NRG1 NSCLC responded quickly with a partial response to treatment
[38]. Currently, there is a phase II trial evaluating this drug in both NSCLC and pancreatic cancer in patients with NRG1 fusions (NCT02912949) (
Table 1). Additional targeted therapy directed towards HER2/HER3 has entered the forefront of NSCLC treatment and will be discussed in later sections.
2.3. PD-1/CTLA-4 Targeting
Immune checkpoint inhibitors have been at the center of NSCLC treatment for the last decade. Data from clinical trials in lung cancer, as well as melanoma and renal cell carcinoma, demonstrate that dual CTLA-4 and PD-L1 inhibition improves overall survival
[7][39][40][41]. Unfortunately, there were increased rates of immune-related adverse events at the optimal effective treatment dose of CTLA-4/PD-L1 inhibitors
[42]. This left a role for novel agents that are able to target both PD-L1 and CTLA-4 while maintaining tolerable toxicity. MEDI5752, a bispecific antibody of PD-1 and CTLA4, was specifically designed to bind CTLA-4- in PD-1-positive T cells allowing a higher level of T cell proliferation
[43]. The Initial results of the phase 1b/II trial of MEDI5752 plus chemotherapy (carboplatin and pemetrexed) vs. pembrolizumab plus chemotherapy in the first line were presented at ESMO in September 2022; ORR was 50.0 [95% CI 27.2–72.8] with the study drug versus 47.6 [95% CI 25.7–70.2] in the pembrolizumab group
[44]. Notable separation in ORR was seen in the PD-L1 < 1% group with ORR of 55.6 [95% CI 21.2–86.3] with the study drug and 30.0 [95% CI 6.7–65.2] (NCT03530397) (
Table 1). Phase III trial data of this novel agent may provide additional insight into the efficacy and safety of this treatment modality. Another PD-L1 targeting agent, INBRX-105, a bispecific against PD-L1 and human 4-1BB receptor (CD137), is being investigated in a phase II study, used in conjunction with pembrolizumab in solid tumors including NSCLC (NCT03809624) (
Table 1). CD137 has been shown to enhance T cell proliferation and T cell costimulatory functions and is therefore thought to act synergistically with ICI
[45].
2.4. Small Cell Lung Cancer Targeting
Targeted therapy in small cell lung cancer (SCLC) has been disappointing in comparison to its NSCLC counterpart, but researchers have made substantial efforts to bridge this gap including an investigation into bispecific antibodies. In preclinical studies, DLL3 (delta-like ligand three) was found in high concentrations in SCLC tumor cells and was later found to be essential to tumorigenesis, prompting interest as a treatment target
[46][47]. As rovalpituzumab tesirine was unsuccessful as an antibody–drug conjugate targeting DLL3
[48], researchers evaluated bispecific molecules that target DLL3, specifically, a bispecific T cell engager [BiTE] as an alternative (AMG757)
[49]. Preclinical data on the novel agent AMG757 is promising with complete responses in both PDX models and orthotopic models due to successful T cell activation and subsequent T cell-induced tumor lysis
[49]. Additionally, no treatment-related adverse events were reported with the drug
[49]. In a phase I trial of this drug, now tarlatamab, 107 heavily pretreated patients with SCLC had an ORR of 23.4% [95% CI, 15.7 to 32.5] and median progression-free survival of 3.7 months [95% CI, 2.1 to 5.4] and 13.2 months [95% CI, 10.5 to not reached]
[50]. Notable side effects include cytokine release syndrome and CNS toxicity
[50]. This prompted a phase II trial which is currently ongoing (NCT05060016) (
Table 1).