The bioactive compounds in fruits, vegetables, herbs, and spices are very vulnerable and can be easily degraded by different factors, including enzymes, thermal treatment, pH, oxidation, light, and/or hydrolysis. Some of the main examples of degradation reactions include: oxidation and hydrolysis of vitamin C, oxidation of phenols, flavonoids, glycosides and hydrolysis of esters. Therefore, actions taken for preventing such degradation are critically important not only for producers, but also for consumers, for whom the presence of these compounds is desirable for health-related requirements. In particular, the degradation of bioactive compounds during thermal treatment (e.g., blanching, pasteurization, sterilization and/or drying) represents a severe problem that must be tackled in the food industry.
1. Degradation Mechanism of Vitamin C
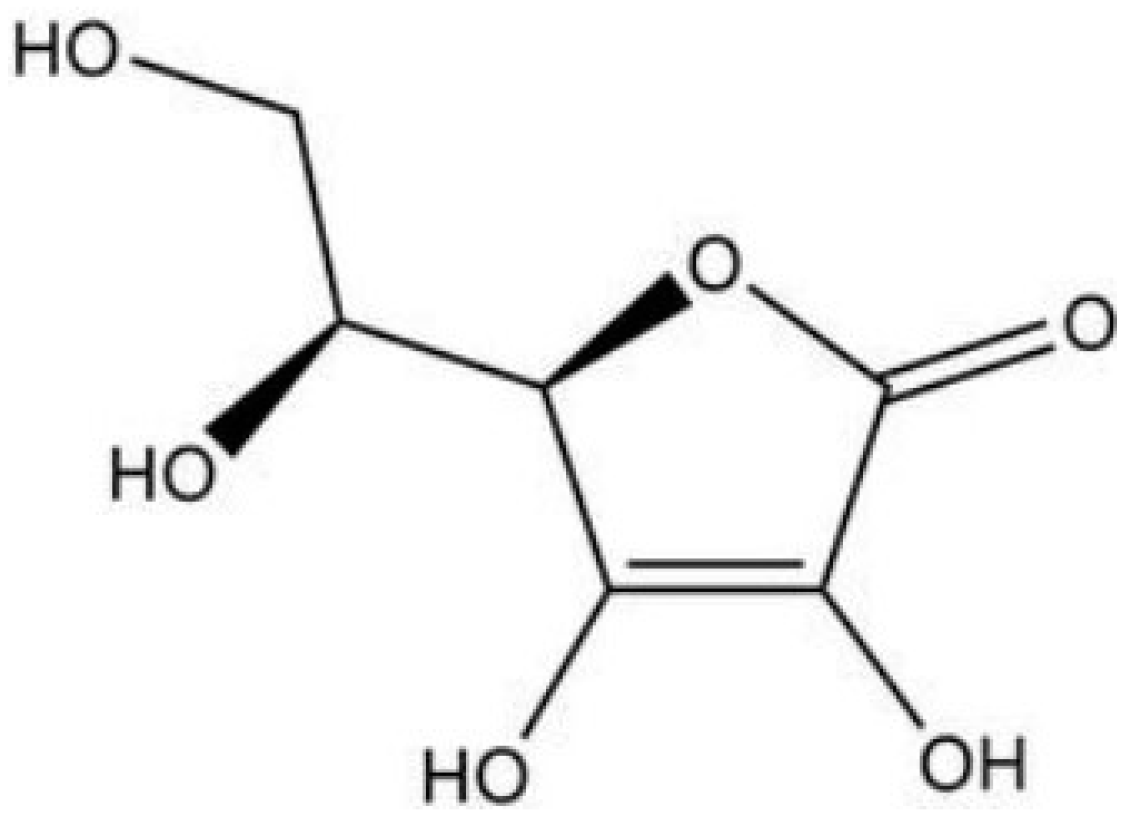
The spatial structure of vitamin C (i.e., L-ascorbic acid) is shown in
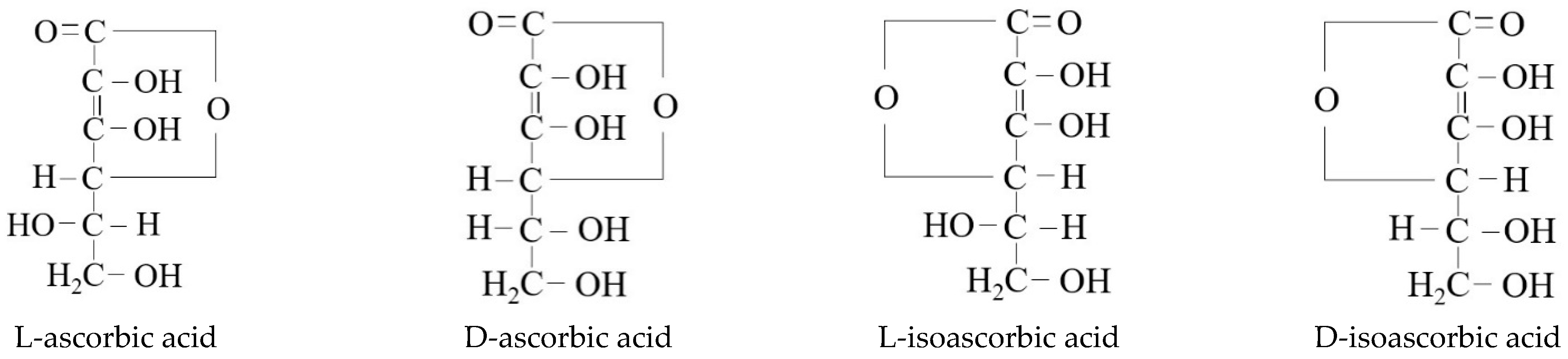
Figure 1, characterized by being easily soluble in water, less soluble in ethanol, and insoluble in organic solvents such as chloroform, benzene, ether, petroleum ether, grease, etc. There are three isomers of vitamin C (i.e., D-ascorbic acid, L-isoascorbic acid, and D-isoascorbic acid) that can be mutually converted into ascorbic acid (vitamin C) under certain conditions. The main difference in chemical structure between L-ascorbic acid and its isomers is shown in
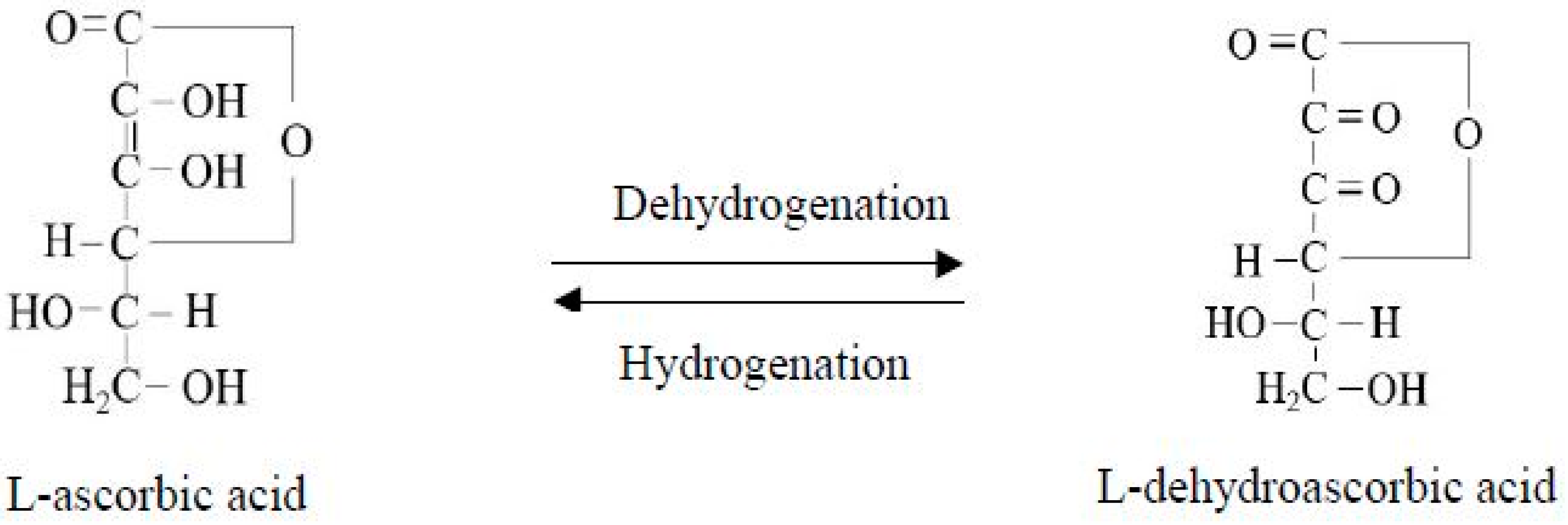
Figure 2. During the drying process under the influence of temperature, oxygen, water vapor and other factors, vitamin C undergoes oxidation and hydrolysis reactions. Under certain conditions, L-ascorbic acid can be oxidized to produce L-dehydroascorbic acid. The L-dehydroascorbic acid can also be reduced in the plant and converted back to L-ascorbic acid as shown in
Figure 3. This change is reversible, such that L-dehydroascorbic acid still has biological activity. However, when L-dehydroascorbic acid is further oxidized and hydrolyzed to form 2,3-Diketo-L-gulonic acid, its activity disappears, causing vitamin C to lose its effectiveness, and this process is irreversible
[1]. The oxidation reaction of vitamin C is as follows (this change is reversible):
Figure 1. The spatial structure of vitamin C.
Figure 2. The chemical structure of vitamin C and its isomers.
Figure 3. Interconversion of L-ascorbic acid and L-dehydroascorbic acid.
The reduction of L-dehydroascorbic acid to L-ascorbic acid under certain conditions is as follows:
2. Degradation Mechanisms of Phenols, Flavonoids and Glycosides
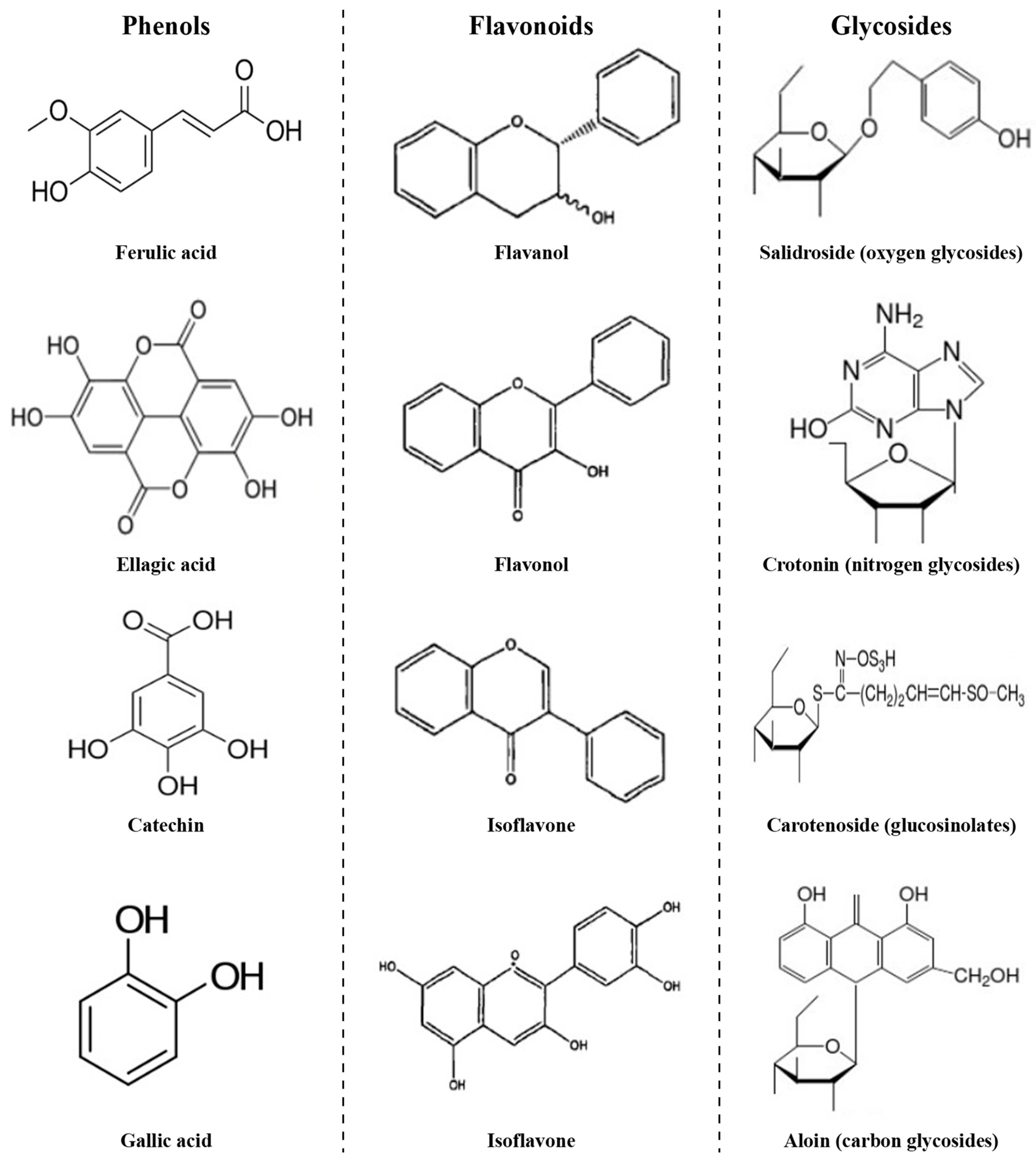
Except for flavonoids and glycosides, all other polyphenols are classed as phenols, mainly including phenolic acids (such as gallic acid, ellagic acid and ferulic acid) and tannin (such as caffetannins, labiataetannins and phlorotannins)
[2]. Meanwhile, phenolic acids are the most common phenols.
Figure 4 presents the chemical structure of common phenols, flavonoids, and glycosides.
Figure 4. Chemical structure of phenols (left), flavonoids (middle) and glycosides (right).
According to Figure 4, phenols, flavonoids, and glycosides have a common feature in that their molecular structure contains a benzene ring and a variable number of hydroxyl groups (–OH), which have strong reducibility. The hydroxyl groups are often oxidized to aldehyde groups, ketone groups or carboxyl groups when heated. Since the phenols, flavonoids and glycosides can be degraded with oxidation reactions, the vacuum environment is better for retaining effective ingredients content. It has been reported that vacuum drying and vacuum freeze-drying are better than other methods.
The hydroxyl group (–OH) is dehydrogenated to form aldehyde group (–CHO), and the resultant aldehyde group is further oxidized to carboxyl group.
Dehydrogenation reaction of hydroxyl group (–OH) to form ketone group (C=O), as,
Oxygenation of hydroxyl group (–OH) to carboxyl group (–COOH):
During the drying process of fruits and other materials, the degradation of the bioactive compounds is based on the above basic reactions and the degradation speed is mainly affected by temperature, oxygen and moisture content. The following are the typical degradation reactions of phenols and flavonoids (catechins and baicalein):
- (1)
-
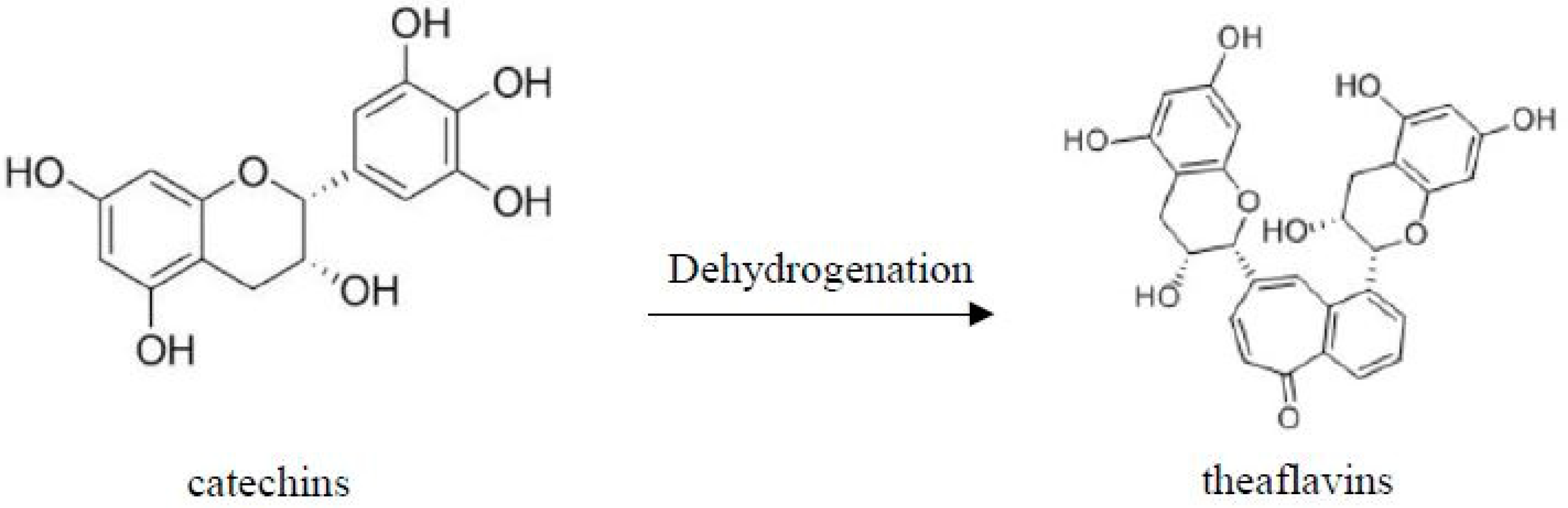
Catechins (phenols) is oxidized to theaflavins
[3]:
The molecular structure of catechins and theaflavins is shown in Figure 5.
Figure 5. Formation of theaflavins from catechins through dehydrogenation.
- (2)
-
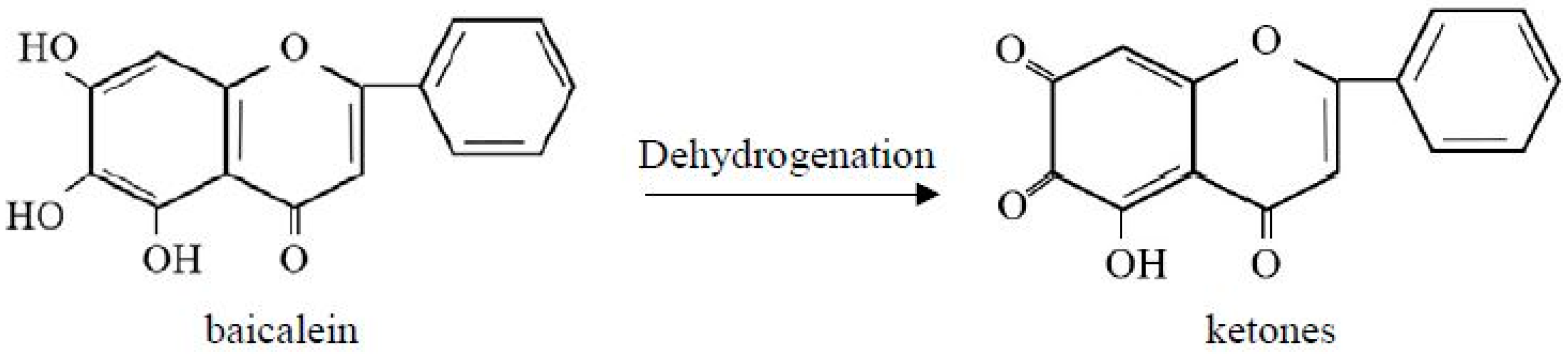
Oxidation of baicalein (flavonoids)
[4]:
The chemical structure of baicalein and its oxidation product are shown in Figure 6. When baicalein is oxidized into corresponding ketone compounds, the original yellow color changes to green, and the efficacy of the bioactive ingredients is degraded.
Figure 6. Chemical structure of baicalein and its oxidation products.
3. Degradation Mechanism of Volatile Compounds
Volatile substances provide the flavor and aroma of many economically important herbs, spices, and medicinal plants. The main volatile substances in fruits and spices are aroma volatiles, and constitute one of the important indices for the quality evaluation of products. Different fruits have different scent characteristics due to their different contents and types of volatile aromatic substances. However, not every volatile aromatic substance has the characteristic aroma components of fruits. The volatile aromatic substances constituting the fruit fragrance mainly include low-molecular-weight alcohols and esters, as well as small amounts of a low-molecular-weight, low-boiling-temperature substances such as aldehydes, ketones and terpenes. Alcohols are volatile and easily lost when heated during the drying process. Esters are generally hydrolyzed to alcohols and acids, and the hydrolyzing reaction is affected by temperature and moisture. After the volatilization of alcohols, the acidic substances remain, eventually causing the aroma components to become worthless
[5].
The hydrolysis reaction of esters can be expressed by the following general formula:
Taking the hydrolysis of ethyl acetate and ethyl formate as an example:
In addition, medicinal plants contain volatile oil, which is generally a colorless or light-yellow transparent liquid, with a special aroma and spicy burning taste. It oxidizes and deteriorates easily once in contact with air, and its volatilization is usually related to temperature, oxidation and water content during the drying process. The higher the temperature in the drying process, the faster the volatilization rate of the volatile oil, while the lower the temperature and the shorter the drying time, the more volatile oil can be retained.
In brief, the thermal degradation mechanisms of bioactive compounds discussed above are greatly affected by drying temperature. Gąsecka et al. reported that the greatest decreases in the bioactive compounds of
Leccinum scabrum was generally occurred at 70 °C. The inhibition of free radicals decreased in the following order: fresh samples > air-dried samples > samples dried at 40 °C > samples dried at 70 °C
[6]. In grape pomace dried at temperatures below 60 °C, Teles et al. hypothesized that bioactive components may be subject to oxidation by polyphenol oxidase, because temperatures of 50 °C and 40 °C were likely insufficient to inactivate this enzyme. The preservation of phenolic compounds was improved by the influence of temperature as well as a decrease in water activity, which may have also led to the decrease in enzyme activity and the occurrence of chemical reactions
[7].