Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Diletta Di Mitri | -- | 2282 | 2023-06-20 11:17:16 | | | |
| 2 | Sirius Huang | Meta information modification | 2282 | 2023-06-21 03:44:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Portale, F.; Di Mitri, D. Natural Killer Cells in Cancer: Mechanisms of Dysfunction. Encyclopedia. Available online: https://encyclopedia.pub/entry/45851 (accessed on 07 February 2026).
Portale F, Di Mitri D. Natural Killer Cells in Cancer: Mechanisms of Dysfunction. Encyclopedia. Available at: https://encyclopedia.pub/entry/45851. Accessed February 07, 2026.
Portale, Federica, Diletta Di Mitri. "Natural Killer Cells in Cancer: Mechanisms of Dysfunction" Encyclopedia, https://encyclopedia.pub/entry/45851 (accessed February 07, 2026).
Portale, F., & Di Mitri, D. (2023, June 20). Natural Killer Cells in Cancer: Mechanisms of Dysfunction. In Encyclopedia. https://encyclopedia.pub/entry/45851
Portale, Federica and Diletta Di Mitri. "Natural Killer Cells in Cancer: Mechanisms of Dysfunction." Encyclopedia. Web. 20 June, 2023.
Copy Citation
Natural killer cells (NK) are innate lymphocytes endowed with the ability to recognize and kill cancer cells. Consequently, adoptive transfer of autologous or allogeneic NK cells represents a novel opportunity in cancer treatment that is under clinical investigation. However, cancer renders NK cells dysfunctional, thus restraining the efficacy of cell-based therapies.
NK cells
tumor microenvironment
immunotherapy
1. Introduction
Natural killer cells were first described in the late 1960s as cells capable of killing cancer cells without restriction by HLA molecules [1]. The concept of NK cells as a tool for cancer therapy was formally proved when the infusion of NK cells was first employed in the treatment of leukemia patients, with promising efficacy [2]. Nowadays, we are conscious that NK cells are key in anti-tumor immunity, given their potent cytotoxicity against cancer cells. Therapeutic approaches that take advantage of the anti-tumor activities of NK cells, such as the adoptive transfer of genetically modified NK cells and the modulation of checkpoint molecules, turned out to be promising immunotherapeutic strategies to eliminate cancer. Unfortunately, exposure to tumor cells and to the components of the tumor microenvironment (TME) impairs NK-cell effector functions and makes them dysfunctional. Dysfunctional NK cells are defined by a limited release of effector cytokines and a decreased ability to kill malignant cells. Multiple mechanisms are implicated in such dysfunction, spanning from inhibition of recruitment to the tumor bed, activation of inhibitory processes, blowing up of activation signals, and deregulation of metabolism (Figure 1).
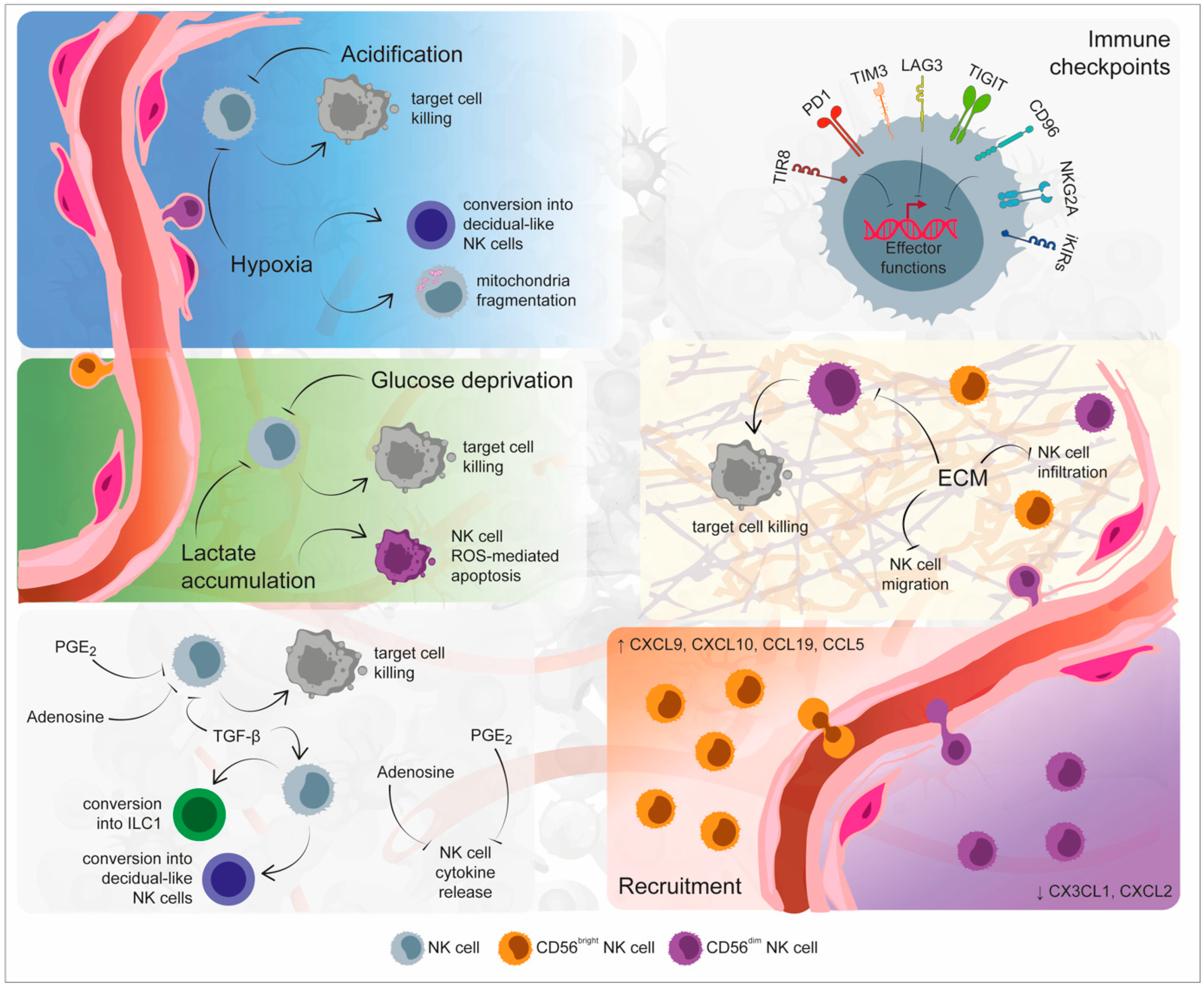
Figure 1. Mechanisms of Natural Killer (NK) cell dysfunction within the tumor microenvironment. The specific environmental conditions that mark the tumor site are responsible for NK cell exhaustion and dysfunction. Indeed, hypoxia, low pH, glucose deprivation, and lactate accumulation could determine both the inhibition of NK cell cytolytic activity and NK cell apoptosis. Soluble factors released by immune, tumor, and stromal cells, including transforming growth factor (TGF)-β, adenosine, and prostaglandin E2 (PGE2), not only inhibit NK cell functionality, but they additionally determine their conversion toward a non-cytolytic phenotype. An additional inhibitory mechanism exploited by the tumor microenvironment is represented by the imbalance in immune checkpoint molecules, responsible for NK cell exhausted status. The recruitment and infiltration of NK cells within the tumor site are inhibited by the alteration of extracellular matrix stiffness as well as by the imbalanced presence of chemokines. Created partly with BioRender.com.
2. Inhibition of Recruitment
NK cells express a heterogeneous repertoire of chemoattractant receptors that are distinct for each cell subset, and thus differentially regulate the recruitment of each population to the tumor bed. CD56bright NK cells are known to express CCR2, CCR5, CCR7, and CXCR3, while CD56dim NK cells specifically express CXCR1, CXCR2, CX3CR1, S1P5, and Chemerin Receptor 23 (ChemR23) [3]. The abundance of NK cells in tumors correlates with a good prognosis in most cancers; however, NK cell infiltration inside the tumor tissue is generally limited, thus suggesting that cancer cells engage in strategies that target chemoattraction to limit the recruitment of NK cells and promote escape from killing [4].
Several chemokine/receptor axes have been described in the regulation of NK cell trafficking to the tumor bed, and some have been reported to modulate NK cell anti-tumor functions. The expression by tumor-infiltrating NK cells of certain chemokine receptors including CCR2, CCR5, CCR7 and CX3CR1, and the abundance of their respective ligands in the TME has been correlated to enhanced NK cell tumor infiltration and improved cytotoxic response [5]. However, tumor cells could hijack NK cell trafficking mechanisms to shut down their functions. For example, high levels of CCL19 have been reported in the serum of stage IV melanoma patients, suggesting a mechanism by which NK cells are retained in the bloodstream to avoid their migration to LNs [6]. On the same line, low levels of the chemokines CXCL12, CXCL10, and CCL27 have been detected in the TME of endometrial carcinoma, suggesting a potential impairment of NK cell migration to the tumor bed in this context [7]. More recently, the interaction between CXCL12, released by hepatic stellate cells, and CXCR4, expressed by NK cells, has been associated with the induction of NK cell quiescence and increased breast cancer outgrowth [8].
Interestingly, CD56bright NK cells that are generally endowed with limited cytotoxic functions and immune-regulatory properties are the most abundant NK subset in many tumors, including non-small cell lung cancer (NSCLC) and breast cancer, thus suggesting that tumor cells orchestrate attraction and select which NK cell population to call. For example, transforming growth factor beta (TGF-β) in the tumor tissue promotes the deregulation of the CX3CL1–CX3CR1 axis, thus reducing NK cell infiltration in hepatocellular carcinoma and breast cancer. More generally, TGF-β signaling favors the recruitment of CD56bright NK cells at the expense of the CD56dim subset and in parallel impairs NK cell activation, thus representing an interesting target to improve NK-cell-mediated therapies [9][10][11]. On the same line, increased levels of CCL19, CXCL9, and CXCL10 and decreased levels of CXCL2 in lung tumors foster the recruitment of CD16− NK cells, at the expense of the more cytotoxic CD16+ NK cells [12][13].
Interestingly, if it is generally accepted that NK cells circulate and patrol the body to search for danger, it is also established that NK cells reside in peripheral tissues and that distinct subsets show preferential organ localization [14]. Such organ-specific homing should be taken in consideration, as distinct tumors and metastatic lesions may respond differently to therapies aimed at improving NK cell recruitment. Another crucial point to consider is the spatial localization of NK cells within the tumor tissue. Indeed, evidence indicates that the abundance of NK cells in certain cancer contexts is independent from the amount and assortment of chemokines available. In addition, NK cells are often more abundant in the adjacent tissue than inside the tumor lesion [15]. Together, these data suggest that the stiffness and organization of the extracellular matrix (ECM) may impact the capability of NK cells to cross the stromal barrier around cancer [16][17]. More investigation is needed to clarify the role played by ECM on NK cells’ infiltration across cancers and may provide strategies to increase NK cell abundance in the tumor bed.
3. Modulation by Soluble Factors
Once in the tumor, infiltrating NK cells are exposed to a variety of strategies engaged by cancer cells and components of TME to establish immunosuppression. TGF-β is one of the most abundant cytokines in the TME in various cancers, and is widely known to dampen immune surveillance. TGF-β recognition by NK cells results in the inhibition of cytokine secretion and granules release, and in the modulation of cell metabolism through the mammalian Target of Rapamycin (mTOR) pathway [18]. In addition, TGF-β produced by cancer cells and by a component of the TME has been reported to inhibit NK cell anti-tumor activity via downregulation of the activating receptors, including NKG2D, NKp30, and NKp44 [19][20]. Among other soluble factors, Prostaglandin E2 (PGE2) has been reported to impair the recruitment and activation of NK cells in preclinical models. Genetic deletion of EP2 and EP4 receptors for PGE2 facilitates the early intra-tumoral accumulation of interferon gamma (IFN-γ)-producing NK cells and an IFN-γ-dependent re-education of the TME against cancer [21]. On the same line, PGE2 released by CAFs in hepatocellular carcinoma brings NK cells to dysfunction [22]. The influence of IL-10, a known inducer of immunosuppression that is abundant in the TME, is more complex. While in vitro results suggest that IL-10 exposure lowers the release of cytotoxic factors by NK cells and hampers activation, in vivo evidence in murine models showed that IL-10 enhances NK cell activation by itself or in combination with additional secreted factors, such as IL-18 and IL-2, and inhibits metastasis formation when infused in cancer models [23][24][25][26]. An interesting soluble factor enriched in the tumor bed is represented by adenosine, a purine nucleoside that recognizes specific receptors expressed on T cells, as well as NK cells and restrains cell activation [27]. Adenosine is produced upon the degradation of adenosine triphosphate (ATP) by ectonucleotidase CD38, CD39, and CD73, expressed by cancer cells and by components of the immune microenvironment, including T cells and macrophages. The investigation of the crosstalk between CD39-expressing immune subsets and NK cells promises new discoveries that may further clarify the mechanisms of NK cell dysfunction in cancer. Importantly, the variety and abundance of soluble molecules in the tumor is influenced by the cellular composition of TME, as most factors are released by both tumor cells and stromal subsets. The investigation of the crosstalk between NK cells and components of the TME is essential in order to distinguish the mechanisms that underlie NK cell dysfunction in cancers and is discussed below.
4. Engagement of Checkpoint Inhibitors
Similar to T lymphocytes, NK cells express a variety of checkpoint molecules that, when engaged by ligands present within the tumor, hinder cell activation and killing capability. NK cells express HLA-specific inhibitory receptors, such as KIRs, NKG2A, and Lymphocyte Activation Gene-3 (LAG-3) and non-HLA-class I-specific inhibitory receptors, including PD-1, T cell Immunoglobulin and Mucin-domain-containing molecule 3 (TIM-3), TIGIT, cluster of differentiation 112 receptor (CD112R), and CD96 [28]. PD-1 is expressed by NK cells in chronic infections, and is indicative of an exhausted state in tumor-infiltrating NK cells. Accordingly, PD-1 expression by NK cells is augmented in multiple cancers, including lymphomas, tumors of the digestive tract, ovarian cancer, breast cancer, renal adenocarcinoma, and others [29]. Interestingly, the recognition of PD-1 on NK cells by its ligands suppresses functional activation in cancer models [30]. Accordingly, the transfer of NK cells pre-incubated with a PD-1-blocking antibody sustains the recognition and killing of cancer stem cells and restrains tumor growth in a glioma model [31]. The immune cell profiling of peripheral blood in cancer patients administered with anti-PD1 immunotherapy is ongoing and will explore the impact of PD-1 engagement on NK cells, thus providing essential information on the matter (NCT02535247 and NCT01714739). Interestingly, combinatorial therapy of anti-PD-L1 and NK cell activating cytokines significantly augments the activity of NK cells against PD-L1 negative leukemia cells, thus potentially explaining the clinical response of PD-L1 negative cancers to PD-L1 inhibition [30][32]. Among other checkpoint molecules, Tim-3 has been found to be upregulated on the surface of NK cells from cancer patients affected by gastric cancer, lung cancer, renal cancer, head and neck cancer, melanoma, and multiple myeloma, among others. In murine models, Tim-3 defines exhausted NK cells with impaired cytotoxic capability, and the inhibition of Tim-3 in vitro augments effector functions and cancer killing [33][34][35]. Similar to PD-1 and Tim-3, additional HLA-independent checkpoints including CD96, CD112R, and TIGIT are increased in expression on NK cells in cancer patients and have been reported to restrain NK cell killing ability in vitro and in vivo. Interestingly, TIR8, a receptor that belongs to the IL-1 family, has recently emerged as a novel NK cell immune checkpoint in cancer and further investigation is ongoing to verify whether genetic manipulation of this receptor may be exploited for cancer therapy. In general, there is currently growing interest in the manipulation of these molecules with the aim of augmenting NK cell anti-tumor properties and improving the efficiency of NK cell-based therapies in cancer. It has, however, to be considered that most immune checkpoints are expressed by NK cells in physiology and are considered to be involved in immunotolerance, thus raising concerns on the safety of healthy tissues upon the administration of checkpoint inhibitors. Further investigation is needed on this line to address the emerging problem of excessive immune reaction and life-threatening side effects associated with the use of immunotherapies.
5. Impact of Hypoxia and Acidification of the TME
Hypoxia is a hallmark of solid tumors and tumor-infiltrating cells undergo hypoxic stress that sustains an immunosuppressive microenvironment. When exposed to hypoxia, tumor cells as well as stromal and immune components of the TME upregulate hypoxia inducible factor 1 subunit alpha (HIF-1α), a transcription factor that in turn modulates cell metabolism, differentiation, and activation. A first report on hypoxic stress in NK cells showed that hypoxia induces an impairment of NK cell effector functions in multiple myeloma [36]. On the same line, tumor-infiltrating NK cells have been described to upregulate Hif-1α in different tumor models and the genetic deletion of Hif-1α confers to NK cells superior effector functions and a more potent tumor killing [37]. Interestingly, the constitutive expression of a high affinity CD16 receptor and internal IL-2 in NK cells makes them resistant to the deregulation of effector functions induced by hypoxia. This evidence provides additional information on the characteristics of high affinity NK cells, currently under investigation in the clinic [38]. In liver cancer patients, exposure to the hypoxic microenvironment induces an abnormal fragmentation of the mitochondria. In this context, the mechanistic target of rapamycin-GTPase dynamin-related protein 1 (mTOR-Drp1) activation caused by low oxygen availability alters the mitochondrial programming, causes a reduction in mitochondrial mass and mitochondrial membrane potential and leads NK cells to dysfunction [39]. On the same line, exposure to lactic acid produced by colorectal cancer cells induces reactive oxygen species (ROS) accumulation and mitochondrial damage in tumor-infiltrating NK cells that provokes cell dysfunction [40]. Similarly, the upregulation of lactate dehydrogenase observed in melanoma sustains the acidification of TME with the consequent downregulation of nuclear factor of activated T cells (NFAT) in T and NK cells and lower IFN-γ release [41]. Many findings have described the abundance of lactic acid as a central regulator of immunotolerance in cancer, and modulation of the tumor acidification represents a valuable approach to improve NK and T cell-based immunotherapies. It has, however, to be considered that both hypoxia and acidification of the microenvironment drive the upregulation of checkpoint molecules, such as PD-1 and PD-L1, on cancer cells and tumor-infiltrating immune subsets [42][43]. As a consequence, low oxygen and high lactic acid may render the tumor more responsive to immunotherapies based on checkpoint inhibitors, thus paradoxically becoming potential allies for immunotherapy.
References
- Lanier, L.L.; Phillips, J.H. Ontogeny of natural killer cells. Nature 1986, 319, 269–270.
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100.
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell-cancer cycle: Advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 2020, 21, 835–847.
- Castriconi, R.; Carrega, P.; Dondero, A.; Bellora, F.; Casu, B.; Regis, S.; Ferlazzo, G.; Bottino, C. Molecular Mechanisms Directing Migration and Retention of Natural Killer Cells in Human Tissues. Front. Immunol. 2018, 9, 2324.
- Yao, X.; Matosevic, S. Chemokine networks modulating natural killer cell trafficking to solid tumors. Cytokine Growth Factor. Rev. 2021, 59, 36–45.
- Cristiani, C.M.; Turdo, A.; Ventura, V.; Apuzzo, T.; Capone, M.; Madonna, G.; Mallardo, D.; Garofalo, C.; Giovannone, E.D.; Grimaldi, A.M.; et al. Accumulation of Circulating CCR7(+) Natural Killer Cells Marks Melanoma Evolution and Reveals a CCL19-Dependent Metastatic Pathway. Cancer Immunol. Res. 2019, 7, 841–852.
- Degos, C.; Heinemann, M.; Barrou, J.; Boucherit, N.; Lambaudie, E.; Savina, A.; Gorvel, L.; Olive, D. Endometrial Tumor Microenvironment Alters Human NK Cell Recruitment, and Resident NK Cell Phenotype and Function. Front. Immunol. 2019, 10, 877.
- Correia, A.L.; Guimaraes, J.C.; Auf der Maur, P.; De Silva, D.; Trefny, M.P.; Okamoto, R.; Bruno, S.; Schmidt, A.; Mertz, K.; Volkmann, K.; et al. Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature 2021, 594, 566–571.
- Bellora, F.; Castriconi, R.; Dondero, A.; Carrega, P.; Mantovani, A.; Ferlazzo, G.; Moretta, A.; Bottino, C. Human NK cells and NK receptors. Immunol. Lett. 2014, 161, 168–173.
- Castriconi, R.; Dondero, A.; Bellora, F.; Moretta, L.; Castellano, A.; Locatelli, F.; Corrias, M.V.; Moretta, A.; Bottino, C. Neuroblastoma-derived TGF-beta1 modulates the chemokine receptor repertoire of human resting NK cells. J. Immunol. 2013, 190, 5321–5328.
- Batlle, E.; Massague, J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940.
- Carrega, P.; Bonaccorsi, I.; Di Carlo, E.; Morandi, B.; Paul, P.; Rizzello, V.; Cipollone, G.; Navarra, G.; Mingari, M.C.; Moretta, L.; et al. CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J. Immunol. 2014, 192, 3805–3815.
- Laskowski, T.J.; Biederstädt, A.; Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 2022, 22, 557–575.
- Gasteiger, G.; Fan, X.; Dikiy, S.; Lee, S.Y.; Rudensky, A.Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015, 350, 981–985.
- Masetti, M.; Carriero, R.; Portale, F.; Marelli, G.; Morina, N.; Pandini, M.; Iovino, M.; Partini, B.; Erreni, M.; Ponzetta, A.; et al. Lipid-loaded tumor-associated macrophages sustain tumor growth and invasiveness in prostate cancer. J. Exp. Med. 2022, 219.
- Zhou, H.; Wang, M.; Zhang, Y.; Su, Q.; Xie, Z.; Chen, X.; Yan, R.; Li, P.; Li, T.; Qin, X.; et al. Functions and clinical significance of mechanical tumor microenvironment: Cancer cell sensing, mechanobiology and metastasis. Cancer Commun. 2022, 42, 374–400.
- Ben-Shmuel, A.; Sabag, B.; Biber, G.; Barda-Saad, M. The Role of the Cytoskeleton in Regulating the Natural Killer Cell Immune Response in Health and Disease: From Signaling Dynamics to Function. Front. Cell. Dev. Biol. 2021, 9, 609532.
- Slattery, K.; Woods, E.; Zaiatz-Bittencourt, V.; Marks, S.; Chew, S.; Conroy, M.; Goggin, C.; MacEochagain, C.; Kennedy, J.; Lucas, S.; et al. TGFβ drives NK cell metabolic dysfunction in human metastatic breast cancer. J. Immunother. Cancer 2021, 9.
- Allan, D.S.; Rybalov, B.; Awong, G.; Zuniga-Pflucker, J.C.; Kopcow, H.D.; Carlyle, J.R.; Strominger, J.L. TGF-beta affects development and differentiation of human natural killer cell subsets. Eur. J. Immunol. 2010, 40, 2289–2295.
- Viel, S.; Marcais, A.; Guimaraes, F.S.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E.; et al. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal 2016, 9, ra19.
- Bonavita, E.; Bromley, C.P.; Jonsson, G.; Pelly, V.S.; Sahoo, S.; Walwyn-Brown, K.; Mensurado, S.; Moeini, A.; Flanagan, E.; Bell, C.R.; et al. Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade. Immunity 2020, 53, 1215–1229.E8.
- Li, T.; Yang, Y.; Hua, X.; Wang, G.; Liu, W.; Jia, C.; Tai, Y.; Zhang, Q.; Chen, G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 2012, 318, 154–161.
- Wang, Z.; Guan, D.; Huo, J.; Biswas, S.K.; Huang, Y.; Yang, Y.; Xu, S.; Lam, K.P. IL-10 Enhances Human Natural Killer Cell Effector Functions via Metabolic Reprogramming Regulated by mTORC1 Signaling. Front. Immunol. 2021, 12, 619195.
- Mocellin, S.; Panelli, M.; Wang, E.; Rossi, C.R.; Pilati, P.; Nitti, D.; Lise, M.; Marincola, F.M. IL-10 stimulatory effects on human NK cells explored by gene profile analysis. Genes. Immun. 2004, 5, 621–630.
- Kundu, N.; Fulton, A.M. Interleukin-10 inhibits tumor metastasis, downregulates MHC class I, and enhances NK lysis. Cell. Immunol. 1997, 180, 55–61.
- Park, J.Y.; Lee, S.H.; Yoon, S.R.; Park, Y.J.; Jung, H.; Kim, T.D.; Choi, I. IL-15-induced IL-10 increases the cytolytic activity of human natural killer cells. Mol. Cells 2011, 32, 265–272.
- Sek, K.; Molck, C.; Stewart, G.D.; Kats, L.; Darcy, P.K.; Beavis, P.A. Targeting Adenosine Receptor Signaling in Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3837.
- Cao, Y.; Wang, X.; Jin, T.; Tian, Y.; Dai, C.; Widarma, C.; Song, R.; Xu, F. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct. Target. Ther. 2020, 5, 250.
- Ben-Shmuel, A.; Biber, G.; Barda-Saad, M. Unleashing Natural Killer Cells in the Tumor Microenvironment-The Next Generation of Immunotherapy? Front. Immunol. 2020, 11, 275.
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668.
- Huang, B.Y.; Zhan, Y.P.; Zong, W.J.; Yu, C.J.; Li, J.F.; Qu, Y.M.; Han, S. The PD-1/B7-H1 pathway modulates the natural killer cells versus mouse glioma stem cells. PLoS ONE 2015, 10, e0134715.
- Dong, W.; Wu, X.; Ma, S.; Wang, Y.; Nalin, A.P.; Zhu, Z.; Zhang, J.; Benson, D.M.; He, K.; Caligiuri, M.A.; et al. The Mechanism of Anti-PD-L1 Antibody Efficacy against PD-L1-Negative Tumors Identifies NK Cells Expressing PD-L1 as a Cytolytic Effector. Cancer Discov. 2019, 9, 1422–1437.
- Seo, H.; Jeon, I.; Kim, B.S.; Park, M.; Bae, E.A.; Song, B.; Koh, C.H.; Shin, K.S.; Kim, I.K.; Choi, K.; et al. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat. Commun. 2017, 8, 15776.
- Da Silva, I.P.; Gallois, A.; Jimenez-Baranda, S.; Khan, S.; Anderson, A.C.; Kuchroo, V.K.; Osman, I.; Bhardwaj, N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res. 2014, 2, 410–422.
- Xu, L.; Huang, Y.; Tan, L.; Yu, W.; Chen, D.; Lu, C.; He, J.; Wu, G.; Liu, X.; Zhang, Y. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int. Immunopharmacol. 2015, 29, 635–641.
- Sarkar, S.; Germeraad, W.T.; Rouschop, K.M.; Steeghs, E.M.; van Gelder, M.; Bos, G.M.; Wieten, L. Hypoxia induced impairment of NK cell cytotoxicity against multiple myeloma can be overcome by IL-2 activation of the NK cells. PLoS ONE 2013, 8, e64835.
- Ni, J.; Wang, X.; Stojanovic, A.; Zhang, Q.; Wincher, M.; Buhler, L.; Arnold, A.; Correia, M.P.; Winkler, M.; Koch, P.S.; et al. Single-Cell RNA Sequencing of Tumor-Infiltrating NK Cells Reveals that Inhibition of Transcription Factor HIF-1alpha Unleashes NK Cell Activity. Immunity 2020, 52, 1075–1087.e1078.
- Solocinski, K.; Padget, M.R.; Fabian, K.P.; Wolfson, B.; Cecchi, F.; Hembrough, T.; Benz, S.C.; Rabizadeh, S.; Soon-Shiong, P.; Schlom, J.; et al. Overcoming hypoxia-induced functional suppression of NK cells. J. Immunother. Cancer 2020, 8, e000246.
- Zheng, X.; Qian, Y.; Fu, B.; Jiao, D.; Jiang, Y.; Chen, P.; Shen, Y.; Zhang, H.; Sun, R.; Tian, Z.; et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat. Immunol. 2019, 20, 1656–1667.
- Harmon, C.; Robinson, M.W.; Hand, F.; Almuaili, D.; Mentor, K.; Houlihan, D.D.; Hoti, E.; Lynch, L.; Geoghegan, J.; O’Farrelly, C. Lactate-Mediated Acidification of Tumor Microenvironment Induces Apoptosis of Liver-Resident NK Cells in Colorectal Liver Metastasis. Cancer Immunol. Res. 2019, 7, 335–346.
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell. Metab. 2016, 24, 657–671.
- Kumagai, S.; Koyama, S.; Itahashi, K.; Tanegashima, T.; Lin, Y.T.; Togashi, Y.; Kamada, T.; Irie, T.; Okumura, G.; Kono, H.; et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell 2022, 40, 201–218.e9.
- Labiano, S.; Palazon, A.; Melero, I. Immune response regulation in the tumor microenvironment by hypoxia. Semin. Oncol. 2015, 42, 378–386.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
715
Revisions:
2 times
(View History)
Update Date:
21 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




