
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lilia Sabantina | -- | 3119 | 2023-06-20 10:41:58 | | | |
| 2 | Peter Tang | -1 word(s) | 3118 | 2023-06-20 21:32:28 | | |
Video Upload Options
The number of cancer patients is rapidly increasing worldwide. Among the leading causes of human death, cancer can be regarded as one of the major threats to humans. Although many new cancer treatment procedures such as chemotherapy, radiotherapy, and surgical methods are being developed and used for testing purposes, results show limited efficiency and high toxicity, even if they have the potential to damage cancer cells in the process. In contrast, magnetic hyperthermia is a field that originated from the use of magnetic nanomaterials, which, due to their magnetic properties and other characteristics, are used in many clinical trials as one of the solutions for cancer treatment. Magnetic nanomaterials can increase the temperature of nanoparticles located in tumor tissue by applying an alternating magnetic field.
1. Introduction
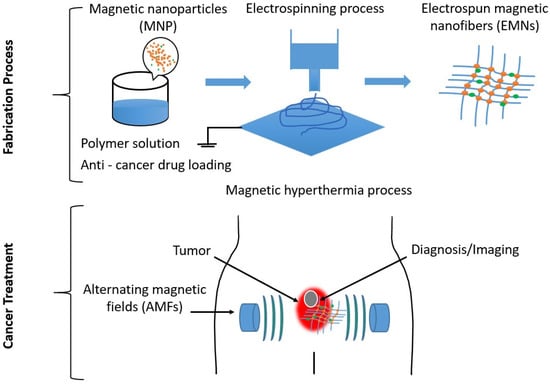
2. Fabrication Technique for Electrospun Magnetic Nanofiber Mats
3. Magnetic Hyperthermia Process and Materials
3.1. Hyperthermia
3.2. Magnetic Hyperthermia
3.3. Magnetic Hyperthermia Involving Magnetic Nanomaterials
3.4. Mechanism of Thermal Energy Generation Using Magnetic Nanomaterials
3.5. Magnetic Hyperthermia with Electrospun Magnetic Nanofiber Mats
4. Drug Delivery and Electrospun Magnetic Nanofiber Mats for Cancer Treatment
5. Diagnostics Technology and Electrospun Magnetic Nanofiber Mats and Magnetic Nanomaterials for Cancer Treatment
References
- International Agency for Research on Cancer, WHO. Breast Cancer, Latest Global Cancer Data: Cancer Burden Rises to 19.3 Million New Cases and 10.0 Million Cancer Deaths in 2020. 2020. Available online: https://www.iarc.who.int/faq/latest-global-cancer-data-2020-qa/ (accessed on 20 March 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2021, 71, 209–249.
- Brero, F.; Albino, M.; Antoccia, A.; Arosio, P.; Avolio, M.; Berardinelli, F.; Bettega, D.; Calzolari, P.; Ciocca, M.; Corti, M.; et al. Hadron Therapy, Magnetic Nanoparticles and Hyperthermia: A Promising Combined Tool for Pancreatic Cancer Treatment. Nanomaterials 2020, 10, 1919.
- Osial, M.; Pregowska, A. The Application of Artificial Intelligence in Magnetic Hyperthermia Based Research. Future Internet 2022, 14, 356.
- Nishikawa, A.; Suzuki, Y.; Kaneko, M.; Ito, A. Combination of magnetic hyperthermia and immunomodulators to drive complete tumor regression of poorly immunogenic melanoma. Cancer Immunol. Immunother. 2022, 1–12.
- Stone, R.; Willi, T.; Rosen, Y.; Mefford, O.; Alexis, F. Targeted magnetic hyperthermia. Ther. Deliv. 2011, 2, 815–838.
- Jeon, M.J.; Ahn, C.H.; Kim, H.; Chung, I.J.; Jung, S.; Kim, H.-J.; Youn, J.K.; Kim, Y. The intratumoral administration of ferucarbotran conjugated with doxorubicin improved therapeutic effect by magnetic hyperthermia combined with pharmacotherapy in a hepatocellular carcinoma model. J. Exp. Clin. Cancer Res. 2014, 33, 57.
- Costa, L.A.A.; Mateus, M.; Borges, J.P.; Silva, J.C.; Barreiros, S.; Soares, P.I.P. Superparamagnetic Iron Oxide Nanozymes for Synergistic Cancer Treatment. Mater. Proc. 2022, 8, 3.
- Orozco-Henao, J.M.; Coral, D.F.; Muraca, D.; Moscoso-Londoño, O.; Mendoza Zélis, P.; van Raap, M.B.F.; Sharma, S.K.; Pirota, K.R.; Knobel, M. Effects of Nanostructure and Dipolar Interactions on Magnetohyperthermia in Iron Oxide Nanoparticles. J. Phys. Chem. C 2016, 120, 12796–12809.
- Santana, G.L.; Crovace, M.C.; Mazón, E.E.; de Oliveira, A.J.A.; Pavan, T.Z.; Zanotto, E.D. Smart Bone Graft Composite for Cancer Therapy Using Magnetic Hyperthermia. Materials 2022, 15, 3187.
- Mokhosi, S.R.; Mdlalose, W.; Nhlapo, A.; Singh, M. Advances in the Synthesis and Application of Magnetic Ferrite Nanoparticles for Cancer Therapy. Pharmaceutics 2022, 14, 937.
- Caizer, I.S.; Caizer, C. Superparamagnetic Hyperthermia Study with Cobalt Ferrite Nanoparticles Covered with γ-Cyclodextrins by Computer Simulation for Application in Alternative Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 4350.
- Garanina, A.S.; Nikitin, A.A.; Abakumova, T.O.; Semkina, A.S.; Prelovskaya, A.O.; Naumenko, V.A.; Erofeev, A.S.; Gorelkin, P.V.; Majouga, A.G.; Abakumov, M.A.; et al. Cobalt Ferrite Nanoparticles for Tumor Therapy: Effective Heating versus Possible Toxicity. Nanomaterials 2022, 12, 38.
- Veres, T.; Voniatis, C.; Molnár, K.; Nesztor, D.; Fehér, D.; Ferencz, A.; Gresits, I.; Thuróczy, G.; Márkus, B.G.; Simon, F.; et al. An Implantable Magneto-Responsive Poly(aspartamide) Based Electrospun Scaffold for Hyperthermia Treatment. Nanomaterials 2022, 12, 1476.
- Minuti, A.E.; Stoian, G.; Herea, D.-D.; Radu, E.; Lupu, N.; Chiriac, H. Fe-Cr-Nb-B Ferrofluid for Biomedical Applications. Nanomaterials 2022, 12, 1488.
- Qu, Y.; Wang, Z.; Sun, M.; Zhao, T.; Zhu, X.; Deng, X.; Zhang, M.; Xu, Y.; Liu, H. A Theranostic Nanocomplex Combining with Magnetic Hyperthermia for Enhanced Accumulation and Efficacy of pH-Triggering Polymeric Cisplatin (IV) Prodrugs. Pharmaceuticals 2022, 15, 480.
- Proenca, M.P. Multifunctional Magnetic Nanowires and Nanotubes. Nanomaterials 2022, 12, 1308.
- Yu, J.; Cao, C.; Fang, F.; Pan, Y. Enhanced Magnetic Hyperthermia of Magnetoferritin through Synthesis at Elevated Temperature. Int. J. Mol. Sci. 2022, 23, 4012.
- Tsamos, D.; Krestou, A.; Papagiannaki, M.; Maropoulos, S. An Overview of the Production of Magnetic Core-Shell Nanoparticles and Their Biomedical Applications. Metals 2022, 12, 605.
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209.
- Perecin, C.J.; Gratens, X.P.M.; Chitta, V.A.; Leo, P.; de Oliveira, A.M.; Yoshioka, S.A.; Cerize, N.N.P. Synthesis and Characterization of Magnetic Composite Theragnostics by Nano Spray Drying. Materials 2022, 15, 1755.
- Ribeiro, B.C.; Alvarez, C.A.R.; Alves, B.C.; Rodrigues, J.M.; Queiroz, M.J.R.P.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Coutinho, P.J.G.; et al. Development of Thermo- and pH-Sensitive Liposomal Magnetic Carriers for New Potential Antitumor Thienopyridine Derivatives. Materials 2022, 15, 1737.
- Ferdows, M.; Alam, J.; Murtaza, G.; Tzirtzilakis, E.E.; Sun, S. Biomagnetic Flow with CoFe2O4 Magnetic Particles through an Unsteady Stretching/Shrinking Cylinder. Magnetochemistry 2022, 8, 27.
- Alkahtani, M.; Zharkov, D.K.; Leontyev, A.V.; Shmelev, A.G.; Nikiforov, V.G.; Hemmer, P.R. Lightly Boron-Doped Nanodiamonds for Quantum Sensing Applications. Nanomaterials 2022, 12, 601.
- Pefanis, G.; Maniotis, N.; Tsiapla, A.-R.; Makridis, A.; Samaras, T.; Angelakeris, M. Numerical Simulation of Temperature Variations during the Application of Safety Protocols in Magnetic Particle Hyperthermia. Nanomaterials 2022, 12, 554.
- Maffei, M.E. Magnetic Fields and Cancer: Epidemiology, Cellular Biology, and Theranostics. Int. J. Mol. Sci. 2022, 23, 1339.
- Araujo, R.T.; Neta, M.S.B.; Coaquira, J.A.H.; Chaves, S.B.; Machado, F. A New Design for Magnetic Poly(vinyl pivalate) for Biomedical Applications: Synthesis, Characterization, and Evaluation of Cytotoxicity in Fibroblasts, Keratinocytes, and Human Melanoma Cells. Colloids Interfaces 2022, 6, 7.
- Caizer, C.; Caizer-Gaitan, I.S.; Watz, C.G.; Dehelean, C.A.; Bratu, T.; Soica, C. High Efficacy on the Death of Breast Cancer Cells Using SPMHT with Magnetite Cyclodextrins Nanobioconjugates. Pharmaceutics 2023, 15, 1145.
- Simões, B.T.; Almeida, F.V.; Borges, J.P.; Soares, P.I.P. Extracellular Hyperthermia for the Treatment of Advanced Cutaneous Melanoma. Materals Proc. 2022, 8, 56.
- García, J.; Gutiérrez, R.; González, A.S.; Jiménez-Ramirez, A.I.; Álvarez, Y.; Vega, V.; Reith, H.; Leistner, K.; Luna, C.; Nielsch, K.; et al. Exchange Bias Effect of (NiO,Ni(OH)2) Core/Shell Nanowires Synthesized by Electrochemical Deposition in Nanoporous Alumina Membranes. Int. J. Mol. Sci. 2023, 24, 7036.
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204.
- Healy, S.; Bakuzis, A.F.; Goodwill, P.W.; Attaluri, A.; Bulte, J.W.M.; Ivkov, R. Clinical magnetic hyperthermia requires integrated magnetic particle imaging. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1779.
- Narayanaswamy, V.; Al-Omari, I.A.; Kamzin, A.S.; Issa, B.; Obaidat, I.M. Tailoring Interfacial Exchange Anisotropy in Hard–Soft Core-Shell Ferrite Nanoparticles for Magnetic Hyperthermia Applications. Nanomaterials 2022, 12, 262.
- Jiao, W.; Zhang, T.; Peng, M.; Yi, J.; He, Y.; Fan, H. Design of Magnetic Nanoplatforms for Cancer Theranostics. Biosensors 2022, 12, 38.
- Álvarez, E.; Estévez, M.; Gallo-Cordova, A.; González, B.; Castillo, R.R.; Morales, M.D.P.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Superparamagnetic Iron Oxide Nanoparticles Decorated Mesoporous Silica Nanosystem for Combined Antibiofilm Therapy. Pharmaceutics 2022, 14, 163.
- Tran, H.-V.; Ngo, N.M.; Medhi, R.; Srinoi, P.; Liu, T.; Rittikulsittichai, S.; Lee, T.R. Multifunctional Iron Oxide Magnetic Nanoparticles for Biomedical Applications: A Review. Materials 2022, 15, 503.
- Blachowicz, T.; Hutten, A.; Ehrmann, A. Electromagnetic Interference Shielding with Electrospun Nanofiber Mats-A Review of Production, Physical Properties and Performance. Fibers 2022, 10, 47.
- Blachowicz, T.; Ehrmann, A. Most recent developments in electrospun magnetic nanofibers: A review. J. Eng. Fibers Fabr. 2020, 15, 1558925019900843.
- Storck, J.L.; Grothe, T.; Tuvshinbayar, K.; Diestelhorst, E.; Wehlage, D.; Brockhagen, B.; Wortmann, M.; Frese, N.; Ehrmann, A. Stabilization and Incipient Carbonization of Electrospun Polyacrylonitrile Nanofibers Fixated on Aluminum Substrates. Fibers 2020, 8, 55.
- Döpke, C.; Grothe, T.; Steblinski, P.; Klöcker, M.; Sabantina, L.; Kosmalska, D.; Blachowicz, T.; Ehrmann, A. Magnetic Nanofiber Mats for Data Storage and Transfer. Nanomaterials 2019, 9, 92.
- Blachowicz, T.; Grzybowski, J.; Steblinski, P.; Ehrmann, A. Neuro-Inspired Signal Processing in Ferromagnetic Nanofibers. Biomimetics 2021, 6, 32.
- Mu, Q.; Zhang, Q.; Yu, W.; Su, M.; Cai, Z.; Cui, K.; Ye, Y.; Liu, X.; Ding, L.; Chen, B.; et al. Robust Multiscale-Oriented Thermoresponsive Fibrous Hydrogels with Rapid Self-Recovery and Ultrafast Response Underwater. ACS Appl. Mater. Interfaces 2020, 12, 33152–33162.
- Mu, Q.; Zhang, Q.; Gao, L.; Chu, Z.; Cai, Z.; Zhang, X.; Wang, K.; Wei, Y. Structural Evolution and Formation Mechanism of the Soft Colloidal Arrays in the Core of PAAm Nanofibers by Electrospun Packing. Langmuir 2017, 33, 10291.
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Moulefera, I.; Pljonkin, A.; Elleuch, K.; Sabantina, L. Magnetic Carbon Nanofiber Mats for Prospective Single Photon Avalanche Diode (SPAD) Sensing Applications. Sensors 2022, 21, 7873.
- Fokin, N.; Grothe, T.; Mamun, A.; Trabelsi, M.; Klöcker, M.; Sabantina, L.; Döpke, C.; Blachowicz, T.; Hütten, A.; Ehrmann, A. Magnetic properties of electrospun magnetic nanofiber mats after stabilization and carbonization. Materials 2020, 13, 1552.
- Blachowicz, T.; Grzybowski, J.; Ehrmann, A. Micromagnetic Simulations of Nanoparticles with Varying Amount of Agglomeration. Macromol. Symp. 2020, 402, 2100381.
- Hellert, C.; Wortmann, M.; Frese, N.; Grötsch, G.; Cornelißen, C.; Ehrmann, A. Adhesion of Electrospun Poly(acrylonitrile) Nanofibers on Conductive and Isolating Foil Substrates. Coatings 2021, 11, 249.
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Ehrmann, A. Increased Mechanical Properties of Carbon Nanofiber Mats for Possible Medical Applications. Fibers 2019, 7, 98.
- Shabatina, T.I.; Vernaya, O.I.; Shimanovskiy, N.L.; Melnikov, M.Y. Metal and Metal Oxides Nanoparticles and Nanosystems in Anticancer and Antiviral Theragnostic Agents. Pharmaceutics 2023, 15, 1181.
- Kozior, T.; Mamun, A.; Trabelsi, M.; Wortmann, M.; Lilia, S.; Ehrmann, A. Electrospinning on 3D Printed Polymers for Mechanically Stabilized Filter Composites. Polymers 2019, 11, 2034.
- Kozior, T.; Trabelsi, M.; Mamun, A.; Sabantina, L.; Ehrmann, A. Stabilization of Electrospun Nanofiber Mats Used for Filters by 3D Printing. Polymers 2019, 11, 1618.
- Carvalho, A.; Gallo, J.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Hilliou, L.; Ferreira, P.M.T.; Bañobre-López, M.; Martins, J.A. Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications. Nanomaterials 2019, 9, 541.
- Peiravi, M.; Eslami, H.; Ansari, M.; Zare-Zardini, H. Magnetic hyperthermia: Potentials and limitations. J. Indian Chem. Soc. 2022, 99, 100269.
- Ji, Y.; Winter, L.; Navarro, L.; Ku, M.-C.; Periquito, J.S.; Pham, M.; Hoffmann, W.; Theune, L.E.; Calderón, M.; Niendorf, T. Controlled Release of Therapeutics from Thermoresponsive Nanogels: A Thermal Magnetic Resonance Feasibility Study. Cancers 2020, 12, 1380.
- Ganapathe, L.S.; Kazmi, J.; Mohamed, M.A.; Berhanuddin, D.D. Molarity Effects of Fe and NaOH on Synthesis and Characterisation of Magnetite (Fe3O4) Nanoparticles for Potential Application in Magnetic Hyperthermia Therapy. Magnetochemistry 2022, 8, 161.
- Salvador, M.; Marqués-Fernández, J.L.; Martínez-García, J.C.; Fiorani, D.; Arosio, P.; Avolio, M.; Brero, F.; Balanean, F.; Guerrini, A.; Sangregorio, C.; et al. Double-Layer Fatty Acid Nanoparticles as a Multiplatform for Diagnostics and Therapy. Nanomaterials 2022, 12, 205.
- Chan, M.-H.; Li, C.-H.; Chang, Y.-C.; Hsiao, M. Iron-Based Ceramic Composite Nanomaterials for Magnetic Fluid Hyperthermia and Drug Delivery. Pharmaceutics 2022, 14, 2584.
- Caizer, C. Computational Study Regarding CoxFe3−xO4 Ferrite Nanoparticles with Tunable Magnetic Properties in Superparamagnetic Hyperthermia for Effective Alternative Cancer Therapy. Nanomaterials 2021, 11, 3294.
- Nazarova, A.; Kozlovskiy, A.L.; Rusakov, V.S.; Egizbek, K.B.; Fadeev, M.S.; Prmantayeva, B.A.; Chudoba, D.; Zdorovets, M.V.; Kadyrzhanov, K.K. Study of the Applicability of Magnetic Iron-Containing Nanoparticles in Hyperthermia and Determination of Their Resistance to Degradation Processes. Crystals 2022, 12, 1816.
- Andrade, R.G.D.; Ferreira, D.; Veloso, S.R.S.; Santos-Pereira, C.; Castanheira, E.M.S.; Côrte-Real, M.; Rodrigues, L.R. Synthesis and Cytotoxicity Assessment of Citrate-Coated Calcium and Manganese Ferrite Nanoparticles for Magnetic Hyperthermia. Pharmaceutics 2022, 14, 2694.
- Cotin, G.; Kiefer, C.; Perton, F.; Ihiawakrim, D.; Blanco-Andujar, C.; Moldovan, S.; Lefevre, C.; Ersen, O.; Pichon, B.; Mertz, D.; et al. Unravelling the Thermal Decomposition Parameters for The Synthesis of Anisotropic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 881.
- Sadat, M.E.; Bud’ko, S.L.; Ewing, R.C.; Xu, H.; Pauletti, G.M.; Mast, D.B.; Shi, D. Effect of Dipole Interactions on Blocking Temperature and Relaxation Dynamics of Superparamagnetic Iron-Oxide (Fe3O4) Nanoparticle Systems. Materials 2023, 16, 496.
- Oltolina, F.; Peigneux, A.; Colangelo, D.; Clemente, N.; D’Urso, A.; Valente, G.; Iglesias, G.R.; Jiménez-Lopez, C.; Prat, M. Biomimetic Magnetite Nanoparticles as Targeted Drug Nanocarriers and Mediators of Hyperthermia in an Experimental Cancer Model. Cancers 2020, 12, 2564.
- Ragab, M.; Abouelregal, A.E.; AlShaibi, H.F.; Mansouri, R.A. Heat Transfer in Biological Spherical Tissues during Hyperthermia of Magnetoma. Biology 2021, 10, 1259.
- Zeinoun, M.; Domingo-Diez, J.; Rodriguez-Garcia, M.; Garcia, O.; Vasic, M.; Ramos, M.; Serrano Olmedo, J.J. Enhancing Magnetic Hyperthermia Nanoparticle Heating Efficiency with Non-Sinusoidal Alternating Magnetic Field Waveforms. Nanomaterials 2021, 11, 3240.
- McWilliams, B.T.; Wang, H.; Binns, V.J.; Curto, S.; Bossmann, S.H.; Prakash, P. Experimental Investigation of Magnetic Nanoparticle-Enhanced Microwave Hyperthermia. J. Funct. Biomater. 2017, 8, 21.
- Alromi, D.A.; Madani, S.Y.; Seifalian, A. Emerging Application of Magnetic Nanoparticles for Diagnosis and Treatment of Cancer. Polymers 2021, 13, 4146.
- Wei, D.-H.; Pan, K.-Y.; Tong, S.-K. Surface Modification and Heat Generation of FePt Nanoparticles. Materials 2017, 10, 181.
- Matos, R.J.R.; Soares, P.I.P.; Silva, J.C.; Borges, J.P. Magnetic Bioactive Glass-Based 3D Systems for Bone Cancer Therapy and Regeneration. Mater. Proc. 2022, 8, 18.
- Sheng, L.; Zhu, X.; Sun, M.; Lan, Z.; Yang, Y.; Xin, Y.; Li, Y. Tumor Microenvironment-Responsive Magnetic Nanofluid for Enhanced Tumor MRI and Tumor multi-treatments. Pharmaceuticals 2023, 16, 166.
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236.
- Caizer, C. Optimization Study on Specific Loss Power in Superparamagnetic Hyperthermia with Magnetite Nanoparticles for High Efficiency in Alternative Cancer Therapy. Nanomaterials 2021, 11, 40.
- Illés, E.; Tombácz, E.; Hegedűs, Z.; Szabó, T. Tunable Magnetic Hyperthermia Properties of Pristine and Mildly Reduced Graphene Oxide/Magnetite Nanocomposite Dispersions. Nanomaterials 2020, 10, 2426.
- Choi, S.K. Activation Strategies in Image-Guided Nanotherapeutic Delivery. J. Nanotheranostics 2020, 1, 78–104.
- Sahin, O.; Meiyazhagan, A.; Ajayan, P.M.; Krishnan, S. Immunogenicity of Externally Activated Nanoparticles for Cancer Therapy. Cancers 2020, 12, 3559.
- Khuyen, H.T.; Huong, T.T.; Van, N.D.; Huong, N.T.; Vu, N.; Lien, P.T.; Nam, P.H.; Nghia, V.X. Synthesis of Multifunctional Eu(III) Complex Doped Fe3O4/Au Nanocomposite for Dual Photo-Magnetic Hyperthermia and Fluorescence Bioimaging. Molecules 2023, 28, 749.
- Arsalani, S.; Arsalani, S.; Isikawa, M.; Guidelli, E.J.; Mazon, E.E.; Ramos, A.P.; Bakuzis, A.; Pavan, T.Z.; Baffa, O.; Carneiro, A.A.O. Hybrid Nanoparticles of Citrate-Coated Manganese Ferrite and Gold Nanorods in Magneto-Optical Imaging and Thermal Therapy. Nanomaterials 2023, 13, 434.
- Ghemes, C.; Dragos-Pinzaru, O.-G.; Tibu, M.; Lostun, M.; Lupu, N.; Chiriac, H. Tunnel Magnetoresistance-Based Sensor for Biomedical Application: Proof-of-Concept. Coatings 2023, 13, 227.
- Diab, D.E.H.; Clerc, P.; Serhan, N.; Fourmy, D.; Gigoux, V. Combined Treatments of Magnetic Intra-Lysosomal Hyperthermia with Doxorubicin Promotes Synergistic Anti-Tumoral Activity. Nanomaterials 2018, 8, 468.
- Osial, M.; Rybicka, P.; Pękała, M.; Cichowicz, G.; Cyrański, M.K.; Krysiński, P. Easy Synthesis and Characterization of Holmium-Doped SPIONs. Nanomaterials 2018, 8, 430.
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Lima, T.M.T.D.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46.
- Adam, A.; Mertz, D. Iron Silica Core-Shell Nanoparticles as Multimodal Platforms for Magnetic Resonance Imaging, Magnetic Hyperthermia, Near-Infrared Light Photothermia, and Drug Delivery. Nanomaterials 2023, 13, 1342.
- Salimi, M.; Sarkar, S.; Hashemi, M.; Saber, R. Treatment of Breast Cancer-Bearing BALB/c Mice with Magnetic Hyperthermia using Dendrimer Functionalized Iron-Oxide Nanoparticles. Nanomaterials 2020, 10, 2310.
- Ting, C.-K.; Dhawan, U.; Tseng, C.-L.; Alex Gong, C.-S.; Liu, W.-C.; Tsai, H.-D.; Chung, R.-J. Hyperthermia-Induced Controlled Local Anesthesia Administration Using Gelatin-Coated Iron–Gold Alloy Nanoparticles. Pharmaceutics 2020, 12, 1097.
- Stadler, B.J.H.; Reddy, M.; Basantkumar, R.; McGary, P.; Estrine, E.; Huang, X.; Sung, S.Y.; Tan, L.; Zou, J.; Maqableh, M.; et al. Galfenol Thin Films and Nanowires. Sensors 2018, 18, 2643.
- Yadel, C.; Michel, A.; Casale, S.; Fresnais, J. Hyperthermia Efficiency of Magnetic Nanoparticles in Dense Aggregates of Cerium Oxide/Iron Oxide Nanoparticles. Appl. Sci. 2018, 8, 1241.
- Albarqi, H.A.; Demessie, A.A.; Sabei, F.Y.; Moses, A.S.; Hansen, M.N.; Dhagat, P.; Taratula, O.R.; Taratula, O. Systemically Delivered Magnetic Hyperthermia for Prostate Cancer Treatment. Pharmaceutics 2020, 12, 1020.
- Attanayake, S.B.; Chanda, A.; Hulse, T.; Das, R.; Phan, M.-H.; Srikanth, H. Competing Magnetic Interactions and Field-Induced Metamagnetic Transition in Highly Crystalline Phase-Tunable Iron Oxide Nanorods. Nanomaterials 2023, 13, 1340.
- Tavares, F.J.T.M.; Soares, P.I.P.; Silva, J.C.; Borges, J.P. Preparation and In Vitro Characterization of Magnetic CS/PVA/HA/pSPIONs Scaffolds for Magnetic Hyperthermia and Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 1128.
- Meneses-Brassea, B.P.; Borrego, E.A.; Blazer, D.S.; Sanad, M.F.; Pourmiri, S.; Gutierrez, D.A.; Varela-Ramirez, A.; Hadjipanayis, G.C.; El-Gendy, A.A. Ni-Cu Nanoparticles and Their Feasibility for Magnetic Hyperthermia. Nanomaterials 2020, 10, 1988.
- Medina, M.A.; Oza, G.; Ángeles-Pascual, A.; González, M.M.; Antaño-López, R.; Vera, A.; Leija, L.; Reguera, E.; Arriaga, L.G.; Hernández Hernández, J.M.; et al. Synthesis, Characterization and Magnetic Hyperthermia of Monodispersed Cobalt Ferrite Nanoparticles for Cancer Therapeutics. Molecules 2020, 25, 4428.
- Simeonidis, K.; Kaprara, E.; Rivera-Gil, P.; Xu, R.; Teran, F.J.; Kokkinos, E.; Mitropoulos, A.; Maniotis, N.; Balcells, L. Hydrotalcite-Embedded Magnetite Nanoparticles for Hyperthermia-Triggered Chemotherapy. Nanomaterials 2021, 11, 1796.
- Gareev, K.G.; Grouzdev, D.S.; Kharitonskii, P.V.; Kosterov, A.; Koziaeva, V.V.; Sergienko, E.S.; Shevtsov, M.A. Magnetotactic Bacteria and Magnetosomes: Basic Properties and Applications. Magnetochemistry 2021, 7, 86.
- Caizer, C. Theoretical Study on Specific Loss Power and Heating Temperature in CoFe2O4 Nanoparticles as Possible Candidate for Alternative Cancer Therapy by Superparamagnetic Hyperthemia. Appl. Sci. 2021, 11, 5505.
- Das, R.; Masa, J.A.; Kalappattil, V.; Nemati, Z.; Rodrigo, I.; Garaio, E.; García, J.Á.; Phan, M.-H.; Srikanth, H. Iron Oxide Nanorings and Nanotubes for Magnetic Hyperthermia: The Problem of Intraparticle Interactions. Nanomaterials 2021, 11, 1380.
- de la Parte, B.H.; Irazola, M.; Pérez-Muñoz, J.; Rodrigo, I.; Iturrizaga Correcher, S.; Mar Medina, C.; Castro, K.; Etxebarria, N.; Plazaola, F.; García, J.Á.; et al. Biochemical and Metabolomic Changes after Electromagnetic Hyperthermia Exposure to Treat Colorectal Cancer Liver Implants in Rats. Nanomaterials 2021, 11, 1318.
- Ortega-Muñoz, M.; Plesselova, S.; Delgado, A.V.; Santoyo-Gonzalez, F.; Salto-Gonzalez, R.; Giron-Gonzalez, M.D.; Iglesias, G.R.; López-Jaramillo, F.J. Poly(ethylene-imine)-Functionalized Magnetite Nanoparticles Derivatized with Folic Acid: Heating and Targeting Properties. Polymers 2021, 13, 1599.
- Fatima, H.; Charinpanitkul, T.; Kim, K.-S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203.
- Moacă, E.-A.; Watz, C.-G.; Socoliuc, V.; Racoviceanu, R.; Păcurariu, C.; Ianoş, R.; Cîntă-Pînzaru, S.; Tudoran, L.B.; Nekvapil, F.; Iurciuc, S.; et al. Biocompatible Magnetic Colloidal Suspension Used as a Tool for Localized Hyperthermia in Human Breast Adenocarcinoma Cells: Physicochemical Analysis and Complex In Vitro Biological Profile. Nanomaterials 2021, 11, 1189.
- Jabalera, Y.; Sola-Leyva, A.; Carrasco-Jiménez, M.P.; Iglesias, G.R.; Jimenez-Lopez, C. Synergistic Photothermal-Chemotherapy Based on the Use of Biomimetic Magnetic Nanoparticles. Pharmaceutics 2021, 13, 625.
- Saikova, S.; Pavlikov, A.; Trofimova, T.; Mikhlin, Y.; Karpov, D.; Asanova, A.; Grigoriev, Y.; Volochaev, M.; Samoilo, A.; Zharkov, S.; et al. Hybrid Nanoparticles Based on Cobalt Ferrite and Gold: Preparation and Characterization. Metals 2021, 11, 705.
- Darwish, M.S.A.; Kim, H.; Bui, M.P.; Le, T.-A.; Lee, H.; Ryu, C.; Lee, J.Y.; Yoon, J. The Heating Efficiency and Imaging Performance of Magnesium Iron Ammonium Hydroxide Nanoparticles for Biomedical Applications. Nanomaterials 2021, 11, 1096.
- Rivera-Chaverra, M.J.; Restrepo-Parra, E.; Acosta-Medina, C.D.; Mello, A.; Ospina, R. Synthesis of Oxide Iron Nanoparticles Using Laser Ablation for Possible Hyperthermia Applications. Nanomaterials 2020, 10, 2099.
- Nemec, S.; Kralj, S.; Wilhelm, C.; Abou-Hassan, A.; Rols, M.-P.; Kolosnjaj-Tabi, J. Comparison of Iron Oxide Nanoparticles in Photothermia and Magnetic Hyperthermia: Effects of Clustering and Silica Encapsulation on Nanoparticles’ Heating Yield. Appl. Sci. 2020, 10, 7322.
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644.
- Schneider-Futschik, E.K.; Reyes-Ortega, F. Advantages and Disadvantages of Using Magnetic Nanoparticles for the Treatment of Complicated Ocular Disorders. Pharmaceutics 2021, 13, 1157.
- Sanad, M.F.; Meneses-Brassea, B.P.; Blazer, D.S.; Pourmiri, S.; Hadjipanayis, G.C.; El-Gendy, A.A. Superparamagnetic Fe/Au Nanoparticles and Their Feasibility for Magnetic Hyperthermia. Appl. Sci. 2021, 11, 6637.
- Xue, Y.; Lofland, S.; Hu, X. Comparative Study of Silk-Based Magnetic Materials: Effect of Magnetic Particle Types on the Protein Structure and Biomaterial Properties. Int. J. Mol. Sci. 2020, 21, 7583.
- Reichel, V.E.; Matuszak, J.; Bente, K.; Heil, T.; Kraupner, A.; Dutz, S.; Cicha, I.; Faivre, D. Magnetite-Arginine Nanoparticles as a Multifunctional Biomedical Tool. Nanomaterials 2020, 10, 2014.
- Khan, U.; Zaib, A.; Ishak, A. Magnetic Field Effect on Sisko Fluid Flow Containing Gold Nanoparticles through a Porous Curved Surface in the Presence of Radiation and Partial Slip. Mathematics 2021, 9, 921.
- Vurro, F.; Jabalera, Y.; Mannucci, S.; Glorani, G.; Sola-Leyva, A.; Gerosa, M.; Romeo, A.; Romanelli, M.G.; Malatesta, M.; Calderan, L.; et al. Improving the Cellular Uptake of Biomimetic Magnetic Nanoparticles. Nanomaterials 2021, 11, 766.
- Baino, F.; Fiume, E.; Miola, M.; Leone, F.; Onida, B.; Laviano, F.; Gerbaldo, R.; Verné, E. Fe-Doped Sol-Gel Glasses and Glass-Ceramics for Magnetic Hyperthermia. Materials 2018, 11, 173.
- Slavu, L.M.; Rinaldi, R.; Di Corato, R. Application in Nanomedicine of Manganese-Zinc Ferrite Nanoparticles. Appl. Sci. 2021, 11, 11183.
- Zhao, S.; Lee, S. Biomaterial-Modified Magnetic Nanoparticles γ-Fe2O3, Fe3O4 Used to Improve the Efficiency of Hyperthermia of Tumors in HepG2 Model. Appl. Sci. 2021, 11, 11124.
- Spoială, A.; Ilie, C.-I.; Crăciun, L.N.; Ficai, D.; Ficai, A.; Andronescu, E. Magnetite-Silica Core/Shell Nanostructures: From Surface Functionalization towards Biomedical Applications—A Review. Appl. Sci. 2021, 11, 11075.
- Cho, M.; Cervadoro, A.; Ramirez, M.R.; Stigliano, C.; Brazdeikis, A.; Colvin, V.L.; Civera, P.; Key, J.; Decuzzi, P. Assembly of Iron Oxide Nanocubes for Enhanced Cancer Hyperthermia and Magnetic Resonance Imaging. Nanomaterials 2017, 7, 72.
- Iacovita, C.; Florea, A.; Dudric, R.; Pall, E.; Moldovan, A.I.; Tetean, R.; Stiufiuc, R.; Lucaciu, C.M. Small versus Large Iron Oxide Magnetic Nanoparticles: Hyperthermia and Cell Uptake Properties. Molecules 2016, 21, 1357.
- de la Parte, B.H.; Rodrigo, I.; Gutiérrez-Basoa, J.; Iturrizaga Correcher, S.; Mar Medina, C.; Echevarría-Uraga, J.J.; Garcia, J.A.; Plazaola, F.; García-Alonso, I. Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy. Cancers 2022, 14, 3084.
- Zverev, V.; Dobroserdova, A.; Kuznetsov, A.; Ivanov, A.; Elfimova, E. Computer Simulations of Dynamic Response of Ferrofluids on an Alternating Magnetic Field with High Amplitude. Mathematics 2021, 9, 2581.
- Nikolenko, P.I.; Nizamov, T.R.; Bordyuzhin, I.G.; Abakumov, M.A.; Baranova, Y.A.; Kovalev, A.D.; Shchetinin, I.V. Structure and Magnetic Properties of SrFe12−xInxO19 Compounds for Magnetic Hyperthermia Applications. Materials 2023, 16, 347.
- Baabu, P.R.S.; Kumar, H.K.; Gumpu, M.B.; Babu, K.J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials 2023, 16, 59.
- Dabaghi, M.; Rasa, S.M.M.; Cirri, E.; Ori, A.; Neri, F.; Quaas, R.; Hilger, I. Iron Oxide Nanoparticles Carrying 5-Fluorouracil in Combination with Magnetic Hyperthermia Induce Thrombogenic Collagen Fibers, Cellular Stress, and Immune Responses in Heterotopic Human Colon Cancer in Mice. Pharmaceutics 2021, 13, 1625.
- Baki, A.; Wiekhorst, F.; Bleul, R. Advances in Magnetic Nanoparticles Engineering for Biomedical Applications—A Review. Bioengineering 2021, 8, 134.
- Lemine, O.M.; Madkhali, N.; Alshammari, M.; Algessair, S.; Gismelseed, A.; El Mir, L.; Hjiri, M.; Yousif, A.A.; El-Boubbou, K. Maghemite (γ-Fe2O3) and γ-Fe2O3-TiO2 Nanoparticles for Magnetic Hyperthermia Applications: Synthesis, Characterization and Heating Efficiency. Materials 2021, 14, 5691.
- Kahil, H.; Faramawy, A.; El-Sayed, H.; Abdel-Sattar, A. Magnetic Properties and SAR for Gadolinium-Doped Iron Oxide Nanoparticles Prepared by Hydrothermal Method. Crystals 2021, 11, 1153.
- Caizer, C.; Caizer, I.S. Study on Maximum Specific Loss Power in Fe3O4 Nanoparticles Decorated with Biocompatible Gamma-Cyclodextrins for Cancer Therapy with Superparamagnetic Hyperthermia. Int. J. Mol. Sci. 2021, 22, 10071.
- Veloso, S.R.S.; Andrade, R.G.D.; Gomes, V.; Amorim, C.O.; Amaral, V.S.; Salgueiriño, V.; Coutinho, P.J.G.; Ferreira, P.M.T.; Correa-Duarte, M.A.; Castanheira, E.M.S. Oxidative Precipitation Synthesis of Calcium-Doped Manganese Ferrite Nanoparticles for Magnetic Hyperthermia. Int. J. Mol. Sci. 2022, 23, 14145.
- Korolev, D.V.; Shulmeyster, G.A.; Istomina, M.S.; Nikiforov, A.I.; Aleksandrov, I.V.; Semenov, V.G.; Galagudza, M.M. Indocyanine Green-Containing Magnetic Liposomes for Constant Magnetic Field-Guided Targeted Delivery and Theranostics. Magnetochemistry 2022, 8, 127.
- Mittal, A.; Roy, I.; Gandhi, S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry 2022, 8, 107.
- Arkaban, H.; Ebrahimi, A.K.; Yarahmadi, A.; Zarrintaj, P.; Barani, M. Development of a multifunctional system based on acid NPs conjugated to folic acid and loaded with doxorubicin for cancer theranostics. Nanotechnology 2021, 32, 305101.
- Iacoviță, C.; Fizeșan, I.; Nitica, S.; Florea, A.; Barbu-Tudoran, L.; Dudric, R.; Pop, A.; Vedeanu, N.; Crisan, O.; Tetean, R.; et al. Silica Coating of Ferromagnetic Iron Oxide Magnetic Nanoparticles Significantly Enhances Their Hyperthermia Performances for Efficiently Inducing Cancer Cells Death In Vitro. Pharmaceutics 2021, 13, 2026.
- Häring, M.; Schiller, J.; Mayr, J.; Grijalvo, S.; Eritja, R.; Díaz, D.D. Magnetic Gel Composites for Hyperthermia Cancer Therapy. Gels 2015, 1, 135–161.
- Zamora-Mora, V.; Soares, P.I.P.; Echeverria, C.; Hernández, R.; Mijangos, C. Composite Chitosan/Agarose Ferrogels for Potential Applications in Magnetic Hyperthermia. Gels 2015, 1, 69–80.
- Andrýsková, N.; Sourivong, P.; Babincová, M.; Šimaljaková, M. Controlled Release of Tazarotene from Magnetically Responsive Nanofiber Patch: Towards More Efficient Topical Therapy of Psoriasis. Appl. Sci. 2021, 11, 11022.
- Duong, H.D.T.; Nguyen, D.T.; Kim, K.-S. Effects of Process Variables on Properties of CoFe2O4 Nanoparticles Prepared by Solvothermal Process. Nanomaterials 2021, 11, 3056.
- Persano, S.; Vicini, F.; Poggi, A.; Fernandez, J.L.C.; Rizzo, G.M.R.; Gavilán, H.; Silvestri, N.; Pellegrino, T. Elucidating the Innate Immunological Effects of Mild Magnetic Hyperthermia on U87 Human Glioblastoma Cells: An In Vitro Study. Pharmaceutics 2021, 13, 1668.
- Manescu, V.; Paltanea, G.; Antoniac, I.; Vasilescu, M. Magnetic Nanoparticles Used in Oncology. Materials 2021, 14, 5948.
- Alkhayal, A.; Fathima, A.; Alhasan, A.H.; Alsharaeh, E.H. PEG Coated Fe3O4/RGO Nano-Cube-Like Structures for Cancer Therapy via Magnetic Hyperthermia. Nanomaterials 2021, 11, 2398.
- Jivago, J.L.P.R.; Brito, J.L.M.; Capistrano, G.; Vinícius-Araújo, M.; Lima Verde, E.; Bakuzis, A.F.; Souza, P.E.N.; Azevedo, R.B.; Lucci, C.M. New Prospects in Neutering Male Animals Using Magnetic Nanoparticle Hyperthermia. Pharmaceutics 2021, 13, 1465.
- Egea-Benavente, D.; Ovejero, J.G.; Morales, M.D.P.; Barber, D.F. Understanding MNPs Behaviour in Response to AMF in Biological Milieus and the Effects at the Cellular Level: Implications for a Rational Design That Drives Magnetic Hyperthermia Therapy toward Clinical Implementation. Cancers 2021, 13, 4583.
- Han, H.; Eigentler, T.W.; Wang, S.; Kretov, E.; Winter, L.; Hoffmann, W.; Grass, E.; Niendorf, T. Design, Implementation, Evaluation and Application of a 32-Channel Radio Frequency Signal Generator for Thermal Magnetic Resonance Based Anti-Cancer Treatment. Cancers 2020, 12, 1720.
- Piehler, S.; Dähring, H.; Grandke, J.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Latorre, A.; Somoza, Á.; et al. Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives. Nanomaterials 2020, 10, 1016.
- Chung, R.-J.; Shih, H.-T. Preparation of Multifunctional Core-Shell Nanoparticles with Surface Grafting as a Potential Treatment for Magnetic Hyperthermia. Materials 2014, 7, 653–661.
- Darwish, M.S.A.; Kim, H.; Lee, H.; Ryu, C.; Young Lee, J.; Yoon, J. Engineering Core-Shell Structures of Magnetic Ferrite Nanoparticles for High Hyperthermia Performance. Nanomaterials 2020, 10, 991.
- Cardoso, B.D.; Rodrigues, A.R.O.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Castanheira, E.M.S.; Coutinho, P.J.G. Stealth Magnetoliposomes Based on Calcium-Substituted Magnesium Ferrite Nanoparticles for Curcumin Transport and Release. Int. J. Mol. Sci. 2020, 21, 3641.
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243.
- Heid, S.; Unterweger, H.; Tietze, R.; Friedrich, R.P.; Weigel, B.; Cicha, I.; Eberbeck, D.; Boccaccini, A.R.; Alexiou, C.; Lyer, S. Synthesis and Characterization of Tissue Plasminogen Activator—Functionalized Superparamagnetic Iron Oxide Nanoparticles for Targeted Fibrin Clot Dissolution. Int. J. Mol. Sci. 2017, 18, 1837.
- Aram, E.; Moeni, M.; Abedizadeh, R.; Sabour, D.; Sadeghi-Abandansari, H.; Gardy, J.; Hassanpour, A. Smart and Multi-Functional Magnetic Nanoparticles for Cancer Treatment Applications: Clinical Challenges and Future Prospects. Nanomaterials 2022, 12, 3567.
- Kulikov, O.A.; Zharkov, M.N.; Ageev, V.P.; Yakobson, D.E.; Shlyapkina, V.I.; Zaborovskiy, A.V.; Inchina, V.I.; Balykova, L.A.; Tishin, A.M.; Sukhorukov, G.B.; et al. Magnetic Hyperthermia Nanoarchitectonics via Iron Oxide Nanoparticles Stabilised by Oleic Acid: Anti-Tumour Efficiency and Safety Evaluation in Animals with Transplanted Carcinoma. Int. J. Mol. Sci. 2022, 23, 4234.
- Ferreira, L.P.; Reis, C.P.; Robalo, T.T.; Melo Jorge, M.E.; Ferreira, P.; Gonçalves, J.; Hajalilou, A.; Cruz, M.M. Assisted Synthesis of Coated Iron Oxide Nanoparticles for Magnetic Hyperthermia. Nanomaterials 2022, 12, 1870.
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvarà, P.; Metselaar, J.; Banala, S.; Straub, M.; Güvener, N.; Engelmann, U.; et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J. Nanobiotechnol. 2020, 18, 22.
- Rodrigues, R.O.; Baldi, G.; Doumett, S.; Gallo, J.; Bañobre-López, M.; Dražić, G.; Calhelha, R.C.; Ferreira, I.C.F.R.; Lima, R.; Silva, A.M.T.; et al. A Tailor-Made Protocol to Synthesize Yolk-Shell Graphene-Based Magnetic Nanoparticles for Nanomedicine. C 2018, 4, 55.
- Gonçalves, J.; Ferreira, P.; Nunes, C. Development of Magnetic Chitosan Scaffolds with Potential for Bone Regeneration and Cancer Therapy. Mater. Proc. 2022, 8, 26.
- Giovannetti, G.; Frijia, F.; Flori, A. Radiofrequency Coils for Low-Field (0.18–0.55 T) Magnetic Resonance Scanners: Experience from a Research Lab–Manufacturing Companies Cooperation. Electronics 2022, 11, 4233.
- Freis, B.; Ramirez, M.D.L.A.; Kiefer, C.; Harlepp, S.; Iacovita, C.; Henoumont, C.; Affolter-Zbaraszczuk, C.; Meyer, F.; Mertz, D.; Boos, A.; et al. Effect of the Size and Shape of Dendronized Iron Oxide Nanoparticles Bearing a Targeting Ligand on MRI, Magnetic Hyperthermia, and Photothermia Properties—From Suspension to In Vitro Studies. Pharmaceutics 2023, 15, 1104.
- Atluri, R.; Atmaramani, R.; Tharaka, G.; McCallister, T.; Peng, J.; Diercks, D.; GhoshMitra, S.; Ghosh, S. Photo-Magnetic Irradiation-Mediated Multimodal Therapy of Neuroblastoma Cells Using a Cluster of Multifunctional Nanostructures. Nanomaterials 2018, 8, 774.
- Vargas, G.; Cypriano, J.; Correa, T.; Leão, P.; Bazylinski, D.A.; Abreu, F. Applications of Magnetotactic Bacteria, Magnetosomes and Magnetosome Crystals in Biotechnology and Nanotechnology: Mini-Review. Molecules 2018, 23, 2438.
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815.
- Zahn, D.; Landers, J.; Buchwald, J.; Diegel, M.; Salamon, S.; Müller, R.; Köhler, M.; Ecke, G.; Wende, H.; Dutz, S. Ferrimagnetic Large Single Domain Iron Oxide Nanoparticles for Hyperthermia Applications. Nanomaterials 2022, 12, 343.
- Veloso, S.R.S.; Ferreira, P.M.T.; Martins, J.A.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetogels: Prospects and Main Challenges in Biomedical Applications. Pharmaceutics 2018, 10, 145.
- Marassi, V.; Zanoni, I.; Ortelli, S.; Giordani, S.; Reschiglian, P.; Roda, B.; Zattoni, A.; Ravagli, C.; Cappiello, L.; Baldi, G.; et al. Native Study of the Behaviour of Magnetite Nanoparticles for Hyperthermia Treatment during the Initial Moments of Intravenous Administration. Pharmaceutics 2022, 14, 2810.
- Kaczmarek, K.; Hornowski, T.; Dobosz, B.; Józefczak, A. Influence of Magnetic Nanoparticles on the Focused Ultrasound Hyperthermia. Materials 2018, 11, 1607.
- Cervantes, O.; Lopez, Z.D.R.; Casillas, N.; Knauth, P.; Checa, N.; Cholico, F.A.; Hernandez-Gutiérrez, R.; Quintero, L.H.; Paz, J.A.; Cano, M.E. A Ferrofluid with Surface Modified Nanoparticles for Magnetic Hyperthermia and High ROS Production. Molecules 2022, 27, 544.
- Ferrero, R.; Barrera, G.; Celegato, F.; Vicentini, M.; Sözeri, H.; Yıldız, N.; Atila Dinçer, C.; Coïsson, M.; Manzin, A.; Tiberto, P. Experimental and Modelling Analysis of the Hyperthermia Properties of Iron Oxide Nanocubes. Nanomaterials 2021, 11, 2179.
- Chen, H.-A.; Lu, Y.-J.; Dash, B.S.; Chao, Y.-K.; Chen, J.-P. Hyaluronic Acid-Modified Cisplatin-Encapsulated Poly(Lactic-co-Glycolic Acid) Magnetic Nanoparticles for Dual-Targeted NIR-Responsive Chemo-Photothermal Combination Cancer Therapy. Pharmaceutics 2023, 15, 290.
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic Nanoparticles for Biomedical Purposes: Modern Trends and Prospects. Magnetochemistry 2020, 6, 30.
- Coene, A.; Leliaert, J. Simultaneous Coercivity and Size Determination of Magnetic Nanoparticles. Sensors 2020, 20, 3882.
- Puiu, R.A.; Balaure, P.C.; Constantinescu, E.; Grumezescu, A.M.; Andronescu, E.; Oprea, O.-C.; Vasile, B.S.; Grumezescu, V.; Negut, I.; Nica, I.C.; et al. Anti-Cancer Nanopowders and MAPLE-Fabricated Thin Films Based on SPIONs Surface Modified with Paclitaxel Loaded β-Cyclodextrin. Pharmaceutics 2021, 13, 1356.
- Habra, K.; McArdle, S.E.B.; Morris, R.H.; Cave, G.W.V. Synthesis and Functionalisation of Superparamagnetic Nano-Rods towards the Treatment of Glioblastoma Brain Tumours. Nanomaterials 2021, 11, 2157.
- Dias, A.M.M.; Courteau, A.; Bellaye, P.-S.; Kohli, E.; Oudot, A.; Doulain, P.-E.; Petitot, C.; Walker, P.-M.; Decréau, R.; Collin, B. Superparamagnetic Iron Oxide Nanoparticles for Immunotherapy of Cancers through Macrophages and Magnetic Hyperthermia. Pharmaceutics 2022, 14, 2388.
- Cao, T.-L.; Le, T.-A.; Hadadian, Y.; Yoon, J. Theoretical Analysis for Using Pulsed Heating Power in Magnetic Hyperthermia Therapy of Breast Cancer. Int. J. Mol. Sci. 2021, 22, 8895.
- Raouf, I.; Gas, P.; Kim, H.S. Numerical Investigation of Ferrofluid Preparation during In-Vitro Culture of Cancer Therapy for Magnetic Nanoparticle Hyperthermia. Sensors 2021, 21, 5545.
- Lafuente-Gómez, N.; Milán-Rois, P.; García-Soriano, D.; Luengo, Y.; Cordani, M.; Alarcón-Iniesta, H.; Salas, G.; Somoza, Á. Smart Modification on Magnetic Nanoparticles Dramatically Enhances Their Therapeutic Properties. Cancers 2021, 13, 4095.
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 109, 083921.
- Cardoso, B.D.; Rodrigues, A.R.O.; Bañobre-López, M.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes Based on Shape Anisotropic Calcium/Magnesium Ferrite Nanoparticles as Nanocarriers for Doxorubicin. Pharmaceutics 2021, 13, 1248.
- Dhawan, U.; Tseng, C.-L.; Wang, H.-Y.; Hsu, S.-Y.; Tsai, M.-T.; Chung, R.-J. Assessing Suitability of Core/Shell Nanoparticle Geometry for Improved Theranostics in Colon Carcinoma. Nanomaterials 2021, 11, 2048.
- Fernández-Álvarez, F.; García-García, G.; Arias, J.L. A Tri-Stimuli Responsive (Maghemite/PLGA)/Chitosan Nanostructure with Promising Applications in Lung Cancer. Pharmaceutics 2021, 13, 1232.
- Ganguly, S.; Margel, S. Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery. Polymers 2021, 13, 4259.
- Hazarika, K.P.; Borah, J.P. Biocompatible Tb doped Fe3O4 nanoparticles with enhanced heating efficiency for magnetic hyperthermia application. J. Magn. Magn. Mater. 2022, 560, 169597.
- Bañobre López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400.
- Zhong, Y.; Leung, V.; Wan, L.Y.; Dutz, S.; Ko, F.K.; Häfeli, U.O. Electrospun magnetic nanofibre mats—A new bondable biomaterial using remotely activated magnetic heating. J. Magn. Magn. Mater. 2015, 380, 330–334.
- Steblinski, P.; Blachowicz, T.; Ehrmann, A. Analysis of the energy distribution of iron nano-spheres for bit-patterned media. J. Magn. Magn. Mater. 2022, 562, 169805.
- Blachowicz, T.; Grzybowski, J.; Ehrmann, A. Influence of agglomerations on magnetic properties of polymer matrices filled with magnetic nanoparticles. Mater. Today Proc. 2022, 67, 792–796.
- Sapountzi, E.; Braiek, M.; Chateaux, J.-F.; Jaffrezic-Renault, N.; Lagarde, F. Recent Advances in Electrospun Nanofiber Interfaces for Biosensing Devices. Sensors 2017, 17, 1887.
- Eslami, P.; Albino, M.; Scavone, F.; Chiellini, F.; Morelli, A.; Baldi, G.; Cappiello, L.; Doumett, S.; Lorenzi, G.; Ravagli, C.; et al. Smart Magnetic Nanocarriers for Multi-Stimuli On-Demand Drug Delivery. Nanomaterials 2022, 12, 303.
- Tehrani, M.H.H.; Soltani, M.; Moradi Kashkooli, F.; Mahmoudi, M.; Raahemifar, K. Computational Modeling of Combination of Magnetic Hyperthermia and Temperature-Sensitive Liposome for Controlled Drug Release in Solid Tumor. Pharmaceutics 2022, 14, 35.
- Comanescu, C. Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry 2022, 4, 872–930.
- Wang, Y.-J.; Lin, P.-Y.; Hsieh, S.-L.; Kirankumar, R.; Lin, H.-Y.; Li, J.-H.; Chen, Y.-T.; Wu, H.-M.; Hsieh, S. Utilizing Edible Agar as a Carrier for Dual Functional Doxorubicin-Fe3O4 Nanotherapy Drugs. Materials 2021, 14, 1824.
- Lachowicz, D.; Kaczyńska, A.; Wirecka, R.; Kmita, A.; Szczerba, W.; Bodzoń-Kułakowska, A.; Sikora, M.; Karewicz, A.; Zapotoczny, S. A Hybrid System for Magnetic Hyperthermia and Drug Delivery: SPION Functionalized by Curcumin Conjugate. Materials 2018, 11, 2388.
- Cardoso, B.D.; Cardoso, V.F.; Lanceros-Méndez, S.; Castanheira, E.M.S. Solid Magnetoliposomes as Multi-Stimuli-Responsive Systems for Controlled Release of Doxorubicin: Assessment of Lipid Formulations. Biomedicines 2022, 10, 1207.
- Nieciecka, D.; Rękorajska, A.; Cichy, D.; Końska, P.; Żuk, M.; Krysiński, P. Synthesis and Characterization of Magnetic Drug Carriers Modified with Tb3+ Ions. Nanomaterials 2022, 12, 795.
- Yu, Y.; Miyako, E. Alternating-Magnetic-Field-Mediated Wireless Manipulations of a Liquid Metal for Therapeutic Bioengineering. iScience 2018, 3, 134–148.
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394.
- Kumar, C.S.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808.
- Hayashi, K.; Tokuda, A.; Nakamura, J.; Sugawara-Narutaki, A.; Ohtsuki, C. Tearable and Fillable Composite Sponges Capable of Heat Generation and Drug Release in Response to Alternating Magnetic Field. Materials 2020, 13, 3637.
- Jabalera, Y.; Oltolina, F.; Peigneux, A.; Sola-Leyva, A.; Carrasco-Jiménez, M.P.; Prat, M.; Jimenez-Lopez, C.; Iglesias, G.R. Nanoformulation Design Including MamC-Mediated Biomimetic Nanoparticles Allows the Simultaneous Application of Targeted Drug Delivery and Magnetic Hyperthermia. Polymers 2020, 12, 1832.
- Contreras-Cáceres, R.; Cabeza, L.; Perazzoli, G.; Díaz, A.; López-Romero, J.M.; Melguizo, C.; Prados, J. Electrospun Nanofibers: Recent Applications in Drug Delivery and Cancer Therapy. Nanomaterials 2019, 9, 656.
- Bazzazzadeh, A.; Dizaji, B.F.; Kianinejad, N.; Nouri, A.; Irani, M. Fabrication of poly(acrylic acid) grafted-chitosan/polyurethane/magnetic MIL-53 metal organic framework composite core-shell nanofibers for co-delivery of temozolomide and paclitaxel against glioblastoma cancer cells. Int. J. Pharm. 2020, 587, 119674.
- Brennan, G.; Bergamino, S.; Pescio, M.; Tofail, S.A.M.; Silien, C. The Effects of a Varied Gold Shell Thickness on Iron Oxide Nanoparticle Cores in Magnetic Manipulation, T1 and T2 MRI Contrasting, and Magnetic Hyperthermia. Nanomaterials 2020, 10, 2424.
- Sandre, O.; Genevois, C.; Garaio, E.; Adumeau, L.; Mornet, S.; Couillaud, F. In Vivo Imaging of Local Gene Expression Induced by Magnetic Hyperthermia. Genes 2017, 8, 61.
- Wang, L.; Lai, S.-M.; Li, C.-Z.; Yu, H.-P.; Venkatesan, P.; Lai, P.-S. D-Alpha-Tocopheryl Poly(ethylene Glycol 1000) Succinate-Coated Manganese-Zinc Ferrite Nanomaterials for a Dual-Mode Magnetic Resonance Imaging Contrast Agent and Hyperthermia Treatments. Pharmaceutics 2022, 14, 1000.
- Griaznova, O.Y.; Belyaev, I.B.; Sogomonyan, A.S.; Zelepukin, I.V.; Tikhonowski, G.V.; Popov, A.A.; Komlev, A.S.; Nikitin, P.I.; Gorin, D.A.; Kabashin, A.V.; et al. Laser Synthesized Core-Satellite Fe-Au Nanoparticles for Multimodal In Vivo Imaging and In Vitro Photothermal Therapy. Pharmaceutics 2022, 14, 994.
- Christou, E.; Pearson, J.R.; Beltrán, A.M.; Fernández-Afonso, Y.; Gutiérrez, L.; de la Fuente, J.M.; Gámez, F.; García-Martín, M.L.; Caro, C. Iron–Gold Nanoflowers: A Promising Tool for Multimodal Imaging and Hyperthermia Therapy. Pharmaceutics 2022, 14, 636.
- Halicka, K.; Cabaj, J. Electrospun Nanofibers for Sensing and Biosensing Applications—A Review. Int. J. Mol. Sci. 2021, 22, 6357.
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Farkas, K.; Földesi, I.; Szabó, Á.; Iván, B.; Tombácz, E. PEGylation of Superparamagnetic Iron Oxide Nanoparticles with Self-Organizing Polyacrylate-PEG Brushes for Contrast Enhancement in MRI Diagnosis. Nanomaterials 2018, 8, 776.
- Khizar, S.; Ahmad, N.M.; Ahmed, N.; Manzoor, S.; Hamayun, M.A.; Naseer, N.; Tenório, M.K.L.; Lebaz, N.; Elaissari, A. Aminodextran Coated CoFe2O4 Nanoparticles for Combined Magnetic Resonance Imaging and Hyperthermia. Nanomaterials 2020, 10, 2182.
- Islam, K.; Haque, M.; Kumar, A.; Hoq, A.; Hyder, F.; Hoque, S.M. Manganese Ferrite Nanoparticles (MnFe2O4): Size Dependence for Hyperthermia and Negative/Positive Contrast Enhancement in MRI. Nanomaterials 2020, 10, 2297.
- Han, X.; Li, Y.; Liu, W.; Chen, X.; Song, Z.; Wang, X.; Deng, Y.; Tang, X.; Jiang, Z. The Applications of Magnetic Particle Imaging: From Cell to Body. Diagnostics 2020, 10, 800.
- Billings, C.; Langley, M.; Warrington, G.; Mashali, F.; Johnson, J.A. Magnetic Particle Imaging: Current and Future Applications, Magnetic Nanoparticle Synthesis Methods and Safety Measures. Int. J. Mol. Sci. 2021, 22, 7651.
- Murase, K.; Mimura, A.; Banura, N.; Nishimoto, K.; Takata, H. Visualization of Magnetic Nanofibers Using Magnetic Particle Imaging. Open J. Med. Imaging 2015, 05, 56–65.
- Yu, E.Y.; Bishop, M.; Zheng, B.; Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Krishnan, K.M.; Goodwill, P.W.; Conolly, S.M. Magnetic Particle Imaging: A Novel in Vivo Imaging Platform for Cancer Detection. Nano Lett. 2017, 17, 1648–1654.
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2015, 435, 1214–1217.
- Feddersen, T.V.; Hernandez-Tamames, J.A.; Franckena, M.; van Rhoon, G.C.; Paulides, M.M. Clinical Performance and Future Potential of Magnetic Resonance Thermometry in Hyperthermia. Cancers 2021, 13, 31.
- Strbak, O.; Antal, I.; Khmara, I.; Koneracka, M.; Kubovcikova, M.; Zavisova, V.; Molcan, M.; Jurikova, A.; Hnilicova, P.; Gombos, J.; et al. Influence of Dextran Molecular Weight on the Physical Properties of Magnetic Nanoparticles for Hyperthermia and MRI Applications. Nanomaterials 2020, 10, 2468.
- Do, H.D.; Ménager, C.; Michel, A.; Seguin, J.; Korichi, T.; Dhotel, H.; Marie, C.; Doan, B.-T.; Mignet, N. Development of Theranostic Cationic Liposomes Designed for Image-Guided Delivery of Nucleic Acid. Pharmaceutics 2020, 12, 854.




