
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fulga Tanasa | -- | 3147 | 2023-06-19 11:07:16 | | | |
| 2 | Conner Chen | -4 word(s) | 3143 | 2023-06-21 04:48:45 | | |
Video Upload Options
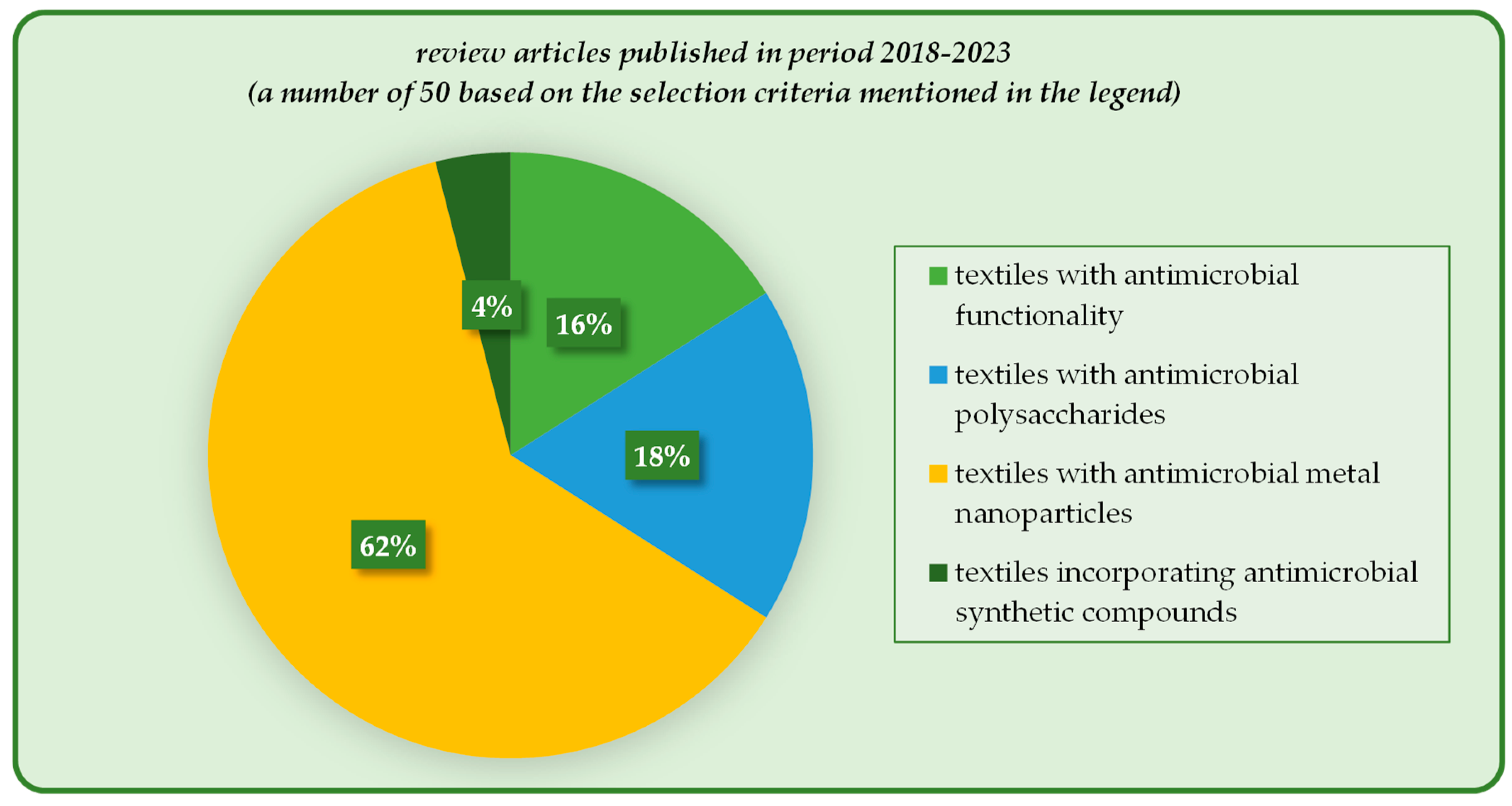
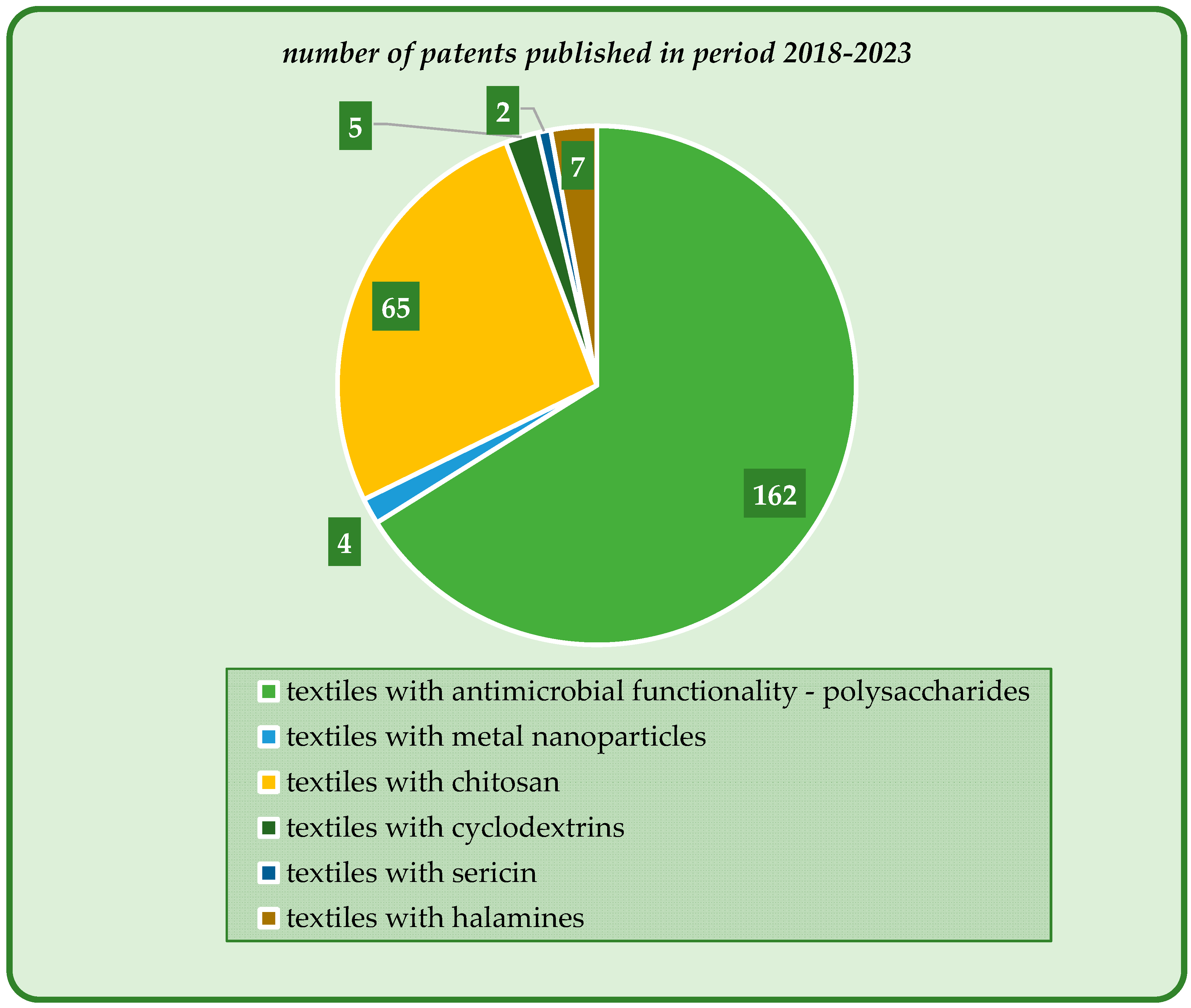
Textiles with antimicrobial functionality have been intensively and extensively investigated in the recent decades, mostly because they are present in everyday life in various applications: medicine and healthcare, sportswear, clothing and footwear, furniture and upholstery, air and water purification systems, food packaging etc. Their ability to kill or limit the growth of the microbial population in a certain context defines their activity against bacteria, fungi, and viruses, and even against the initial formation of the biofilm prior to microorganisms’ proliferation. Various classes of antimicrobials have been employed for these highly specialized textiles, namely, organic synthetic reagents and polymers, metals and metal oxides (micro- and nanoparticles), and natural and naturally derived compounds, and their activity and range of applications are critically assessed.
1. Introduction
1.1. General Background
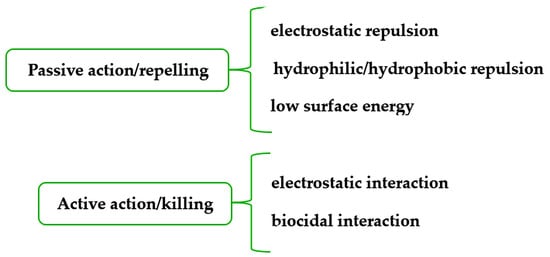
- -
-
biostats, biocides (antibacterial, antifungal, antiviral), barriers, and antibiofilm;
- -
-
textiles with bound or leaching antimicrobial finishing;
- -
-
textiles made of natural (cotton, wool, silk, linen) or synthetic fibers (PP, PE, PES) or blends (cotton/elastane, cotton/PES, wool/acrylic);
- -
-
textiles able to release compounds with biologic activity;
- -
-
wearable and washing resistant.
1.2. Processing Techniques
2. Synthetic Antimicrobial Agents for Textile Finishing

| Antimicrobial Agent | Properties and Applications | Antimicrobial Mechanism | Ref. |
|---|---|---|---|
| Quaternary ammonium compounds Polymeric materials having onium salts (quaternary ammonium and/or phosphonium salts) Quaternary ammonium polyethylenimine |
- Healthcare, household products, surface preservation, food industry, pharmaceutical/cosmetic (preservation) - Highly effective as antimicrobial agents in orthodontic cements to introduce antibacterial activity toward S. mutants and L. casei |
The long, lipophilic alkyl chain of the quaternary ammonium compounds perforates cell membranes, and produces the release of cytoplasmic components, autolysis and cell death of the microbial strain | [52][55][56][57][58][59] |
| Halogenated phenols Triclosan |
- Antiseptic, disinfectant, fungicide, pesticide, antimicrobial, antiseptic, preservative - Antimicrobial activity against many types of Gram-positive and Gram-negative non- spore-forming bacteria, some fungi - Clinical settings, consumer products (cosmetics, cleaning products, paint, plastic materials, toys) - Durable antifungal finishing of cotton fabrics |
Inhibits the active site of enoyl-acyl carrier protein reductase enzyme, which is essential to the fatty acids synthesis of bacteria and the building of the cell membrane | [10][58][60][61] |
| Chlorhexidine Hexametaphosphate salt of chlorhexidine (as nanoparticles) Polyhexamethylene biguanide (PHMB) |
- Preoperative skin cleansing preparations, hand disinfectants, and oral mouth rinses - Efficient antimicrobial agent against gram-negative and -positive bacteria and yeasts. - Biomedical materials and consumer products - Antimicrobial efficacy against MRSA and P. aeruginosa, in both planktonic and biofilm growth conditions - Healthcare uniforms - Nonspecific antimicrobial properties and remained efficient (>99% against S. aureus and K. pneumoniae) after use for 5 months |
Chlorhexidine inhibits membrane-bound ATPase, based on cell membrane disruption and leakage of intracellular constituents, a rapid process with most damage occurring within 20 s of exposure The positively charged biguanidines bind to negatively charged phosphate group of the bacterial cell wall or virus envelope, breaking the membrane integrity, which leads to cell lysis and subsequent cell death |
[25][62][63][64] |
| N-halamines | - Antimicrobial activity against a broad spectrum of microorganisms, rechargeability, nontoxicity to humans - Medical devices, water purification, hospitals, antibacterial modification of cotton fabrics - Antimicrobial activity against aerosolized bacteria - Air filtration technology - Biocidal properties against S. aureus and E. coli - Food packaging and biomedical applications |
The direct transfer of oxidative halogens to a cell after contact resulting in oxidation of the amino acids in the cell membrane and inactivation the microorganism | [46][49][65][66][67][68] |
| 5,5-dimethylhydantoin | Cotton fabric with regenerable antibacterial properties against S. aureus | Coating dimethylhydantoin on cotton fabric (by pad–dry–plasma–cure process) followed by chlorination inhibits the bacteria | [69] |
| Cinnamic acid derivatives | Pharmacological, antifungal, and antibacterial action | Plasma membrane disruption, nucleic acid and protein damage, and the induction of intracellular reactive oxygen species | [70][71][72] |
| Polyaniline and its derivatives | - Bacteria-resistant surfaces against S. aureus and E. coli - Wall and room-door coatings in hospitals |
Different oxidation states of polyaniline and presence of functional groups | [73] |
| Polypyrrole (nanoparticles) | Antimicrobial treatment against S. aureus and E. coli of polyester fabrics | The positive charges (=NH+) in the polypyrrole backbone that are created by dopant compounds | [74][75] |
| Polythiophenes | Antimicrobial compounds able to kill bacteria selectively by damaging negatively charged cell envelopes | Cationic charges with capacity to create huge amounts of singlet oxygen that interact with organism | [76] |
References
- Novi, V.T.; Gonzalez, A.; Brockgreitens, J.; Abbas, A. Highly Efficient and Durable Antimicrobial Nanocomposite Textiles. Sci. Rep. 2022, 12, 17332.
- Roychoudhury, S.; Das, A.; Sengupta, P.; Dutta, S.; Roychoudhury, S.; Choudhury, A.P.; Fuzayel Ahmed, A.B.; Bhattacharjee, S.; Slama, P. Viral Pandemics of the Last Four Decades: Pathophysiology, Health Impacts and Perspectives. Int. J. Environ. Res. Public Health 2020, 17, 9411.
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736.
- Antimicrobial Textiles Market, Global Industry Size Forecast. Available online: https://www.marketsandmarkets.com/Market-Reports/antimicrobial-textile-market-254286152.html (accessed on 21 March 2023).
- Halepoto, H.; Gong, T.; Memon, H. A Bibliometric Analysis of Antibacterial Textiles. Sustainability 2022, 14, 11424.
- Miraftab, M. High Performance Medical Textiles: An Overview; Woodhead Publishing Limited: Sawston, UK, 2014; ISBN 9780857099075.
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498.
- Jiang, C.; Dejarnette, S.; Chen, W.; Scholle, F.; Wang, Q.; Ghiladi, R.A. Color-Variable Dual-Dyed Photodynamic Antimicrobial Polyethylene Terephthalate (PET)/Cotton Blended Fabrics. Photochem. Photobiol. Sci. 2023, 1–18.
- Antunes, J.; Matos, K.; Carvalho, S.; Cavaleiro, A.; Cruz, S.M.A.; Ferreira, F. Carbon-Based Coatings in Medical Textiles Surface Functionalisation: An Overview. Processes 2021, 9, 1997.
- Zanoaga, M.; Tanasa, F. Antimicrobial Reagents as Functional Finishing for Textiles Intended for Biomedical Applications. I. Synthetic Organic Compounds. Chem. J. Mold. 2017, 9, 14–32.
- Tanasa, F.; Zanoaga, M. Antimicrobial Reagents as Functional Finishing for Textiles Intended for Biomedical Applications. II. Metals and Metallic Compounds: Silver. In IFMBE Proceedings; Springer: Berlin/Heidelberg, Germany, 2016; Volume 55, pp. 305–308.
- Zanoaga, M.; Tanasa, F. Antimicrobial Reagents as Functional Finishing for Textiles Intended for Biomedical Applications. III. Other Metals and Metallic Compounds. In IFMBE Proceedings; Springer: Berlin/Heidelberg, Germany, 2016; Volume 55, pp. 309–314.
- Riaz, S.; Ashraf, M. Recent Advances in Development of Antimicrobial Textiles. In Advances in Functional Finishing of Textiles; Springer: Singapore, 2020; pp. 129–168.
- Ibrahim, A.; Laquerre, J.-É.; Forcier, P.; Deregnaucourt, V.; Decaens, J.; Vermeersch, O.; Ibrahim, A.; Laquerre, J.-É.; Forcier, P.; Deregnaucourt, V.; et al. Antimicrobial Agents for Textiles: Types, Mechanisms and Analysis Standards. In Textiles for Functional Applications; IntechOpen: London, UK, 2021.
- Jin, L.; Zhou, F.; Wu, S.; Cui, C.; Sun, S.; Li, G.; Chen, S.; Ma, J. Development of Novel Segmented-Pie Microfibers from Copper-Carbon Nanoparticles and Polyamide Composite for Antimicrobial Textiles Application. Text. Res. J. 2021, 92, 3–14.
- Suh, I.-Y.; Kim, Y.-J.; Zhao, P.; Cho, D.S.; Kang, M.; Huo, Z.-Y.; Kim, S.-W. Self-Powered Microbial Blocking Textile Driven by Triboelectric Charges. Nano Energy 2023, 110, 108343.
- Saha, J.; Mondal, M.I.H. Antimicrobial Textiles from Natural Resources: Types, Properties and Processing. In Antimicrobial Textiles from Natural Resources; Woodhead Publishing: Sawston, UK, 2021; pp. 1–43.
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial Textile: Recent Developments and Functional Perspective. Polym. Bull. 2022, 79, 5747–5771.
- Favatela, M.F.; Otarola, J.; Ayala-Peña, V.B.; Dolcini, G.; Perez, S.; Torres Nicolini, A.; Alvarez, V.A.; Lassalle, V.L. Development and Characterization of Antimicrobial Textiles from Chitosan-Based Compounds: Possible Biomaterials against SARS-CoV-2 Viruses. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1473–1486.
- Assylbekova, G.; Alotaibi, H.F.; Yegemberdiyeva, S.; Suigenbayeva, A.; Sataev, M.; Koshkarbaeva, S.; Abdurazova, P.; Sakibayeva, S.; Prokopovich, P. Sunlight Induced Synthesis of Silver Nanoparticles on Cellulose for the Preparation of Antimicrobial Textiles. J. Photochem. Photobiol. 2022, 11, 100134.
- Naebe, M.; Haque, A.N.M.A.; Haji, A. Plasma-Assisted Antimicrobial Finishing of Textiles: A Review. Engineering 2022, 12, 145–163.
- Nortjie, E.; Basitere, M.; Moyo, D.; Nyamukamba, P. Extraction Methods, Quantitative and Qualitative Phytochemical Screening of Medicinal Plants for Antimicrobial Textiles: A Review. Plants 2022, 11, 2011.
- Ribeiro, A.I.; Shvalya, V.; Cvelbar, U.; Silva, R.; Marques-Oliveira, R.; Remião, F.; Felgueiras, H.P.; Padrão, J.; Zille, A. Stabilization of Silver Nanoparticles on Polyester Fabric Using Organo-Matrices for Controlled Antimicrobial Performance. Polymers 2022, 14, 1138.
- Liu, Z.; Wang, Z.; Meng, Y.; Song, Y.; Li, L.; Yu, M.; Li, J. Electron Beam Irradiation Grafting of Metal-Organic Frameworks onto Cotton to Prepare Antimicrobial Textiles. RSC Adv. 2023, 13, 1853–1861.
- Yim, S.; Cheung, J.W.; Cheng, I.Y.; Ho, L.W.; Szeto, S.S.; Chan, P.; Lam, Y.; Kan, C. Longitudinal Study on the Antimicrobial Performance of a Polyhexamethylene Biguanide (PHMB)-Treated Textile Fabric in a Hospital Environment. Polymers 2023, 15, 1203.
- Morris, H.; Murray, R. Medical Textiles. In Textile Progress; Taylor & Francis: Abingdon, UK, 2020; Volume 52, pp. 1–127.
- Britannica. Microbiology: Definition, History, & Microorganisms. Available online: https://www.britannica.com/science/microbiology (accessed on 28 April 2023).
- Tania, I.S.; Ali, M.; Arafat, M.T. Processing Techniques of Antimicrobial Textiles. In Antimicrobial Textiles from Natural Resources; Woodhead Publishing: Sawston, UK, 2021; pp. 189–215.
- Gao, Y.; Cranston, R. Recent Advances in Antimicrobial Treatments of Textiles. Text. Res. J. 2008, 78, 60–72.
- Sarathi, P.; Thilagavathi, G. Synthesis and characterization of titanium dioxide nano-particles and their applications to textiles for microbe resistance. J. Text. Appar. Technol. Manag. 2009, 6, 138525674.
- Mohamed, F.A.; Abd El-Megied, S.A.; Bashandy, M.S.; Ibrahim, H.M. Synthesis, Application and Antibacterial Activity of New Reactive Dyes Based on Thiazole Moiety. Pigment Resin Technol. 2018, 47, 246–254.
- Tanasă, F.; Nechifor, M.; Teacă, C.A.; Stanciu, M.C. Physical Methods for the Modification of the Natural Fibers Surfaces. In Surface Treatment Methods of Natural Fibres and their Effects on Biocomposites; Woodhead Publishing: Sawston, UK, 2022; pp. 125–146. ISBN 9780128218631.
- Yuan, X.; Yin, W.; Ke, H.; Wei, Q.; Huang, Z.; Chen, D. Properties and Application of Multi-Functional and Structurally Colored Textile Prepared by Magnetron Sputtering. J. Ind. Text. 2022, 51, 1295–1311.
- Shahidi, S.; Ghoranneviss, M. Plasma Sputtering for Fabrication of Antibacterial and Ultraviolet Protective Fabric. Cloth. Text. Res. J. 2015, 34, 37–47.
- Ma, C.; Nikiforov, A.; De Geyter, N.; Dai, X.; Morent, R.; Ostrikov, K. Future Antiviral Polymers by Plasma Processing. Prog. Polym. Sci. 2021, 118, 101410.
- Parthasarathi, V.; Thilagavathi, G. Development of Plasma Enhanced Antiviral Surgical Gown for Healthcare Workers. Fash. Text. 2015, 2, 4.
- Singh, N.; Sheikha, J. Microencapsulation and Its Application in Production of Functional Textiles. Indian J. Fibre Text. Res. 2020, 45, 495–509.
- Yip, J.; Luk, M.Y.A. Microencapsulation Technologies for Antimicrobial Textiles. In Antimicrobial Textiles; Woodhead Publishing: Sawston, UK, 2016; pp. 19–46. ISBN 9780081005859.
- Systematic, B.A.; Podgornik, B.B.; Šandri, S. Microencapsulation for Functional Textile Coatings With. Coatings 2021, 11, 1371.
- Rivero, P.J.; Goicoechea, J. Sol-Gel Technology for Antimicrobial Textiles. In Antimicrobial Textiles; Woodhead Publishing: Sawston, UK, 2016; pp. 47–72. ISBN 9780081005859.
- Abramova, A.V.; Abramov, V.O.; Bayazitov, V.M.; Voitov, Y.; Straumal, E.A.; Lermontov, S.A.; Cherdyntseva, T.A.; Braeutigam, P.; Weiße, M.; Günther, K. A Sol-Gel Method for Applying Nanosized Antibacterial Particles to the Surface of Textile Materials in an Ultrasonic Field. Ultrason. Sonochem. 2020, 60, 104788.
- Pakdel, E.; Daoud, W.A.; Wang, X. Assimilating the Photo-Induced Functions of TiO2-Based Compounds in Textiles: Emphasis on the Sol-Gel Process. Text. Res. J. 2015, 85, 1404–1428.
- Bu, Y.; Zhang, S.; Cai, Y.; Yang, Y.; Ma, S.; Huang, J.; Yang, H.; Ye, D.; Zhou, Y.; Xu, W.; et al. Fabrication of Durable Antibacterial and Superhydrophobic Textiles via in Situ Synthesis of Silver Nanoparticle on Tannic Acid-Coated Viscose Textiles. Cellulose 2019, 26, 2109–2122.
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive Review on Electrospinning Techniques as Versatile Approaches toward Antimicrobial Biopolymeric Composite Fibers. Mater. Sci. Eng. C 2019, 101, 306–322.
- Han, D.; Sherman, S.; Filocamo, S.; Steckl, A.J. Long-Term Antimicrobial Effect of Nisin Released from Electrospun Triaxial Fiber Membranes. Acta Biomater. 2017, 53, 242–249.
- Dong, A.; Wang, Y.J.; Gao, Y.; Gao, T.; Gao, G. Chemical Insights into Antibacterial N-Halamines. Chem. Rev. 2017, 117, 4806–4862.
- Zain, N.M.; Akindoyo, J.O.; Beg, M.D.H. Synthetic Antimicrobial Agent and Antimicrobial Fabrics: Progress and Challenges. IIUM Eng. J. 2018, 19, 10–29.
- Li, Z.; Chen, J.; Cao, W.; Wei, D.; Zheng, A.; Guan, Y. Permanent Antimicrobial Cotton Fabrics Obtained by Surface Treatment with Modified Guanidine. Carbohydr. Polym. 2018, 180, 192–199.
- Liu, Y.; Ren, X.; Liang, J. Antimicrobial Modification Review. BioResources 2015, 10, 1964–1985.
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current Status, Occurrence, Environmental Risks and Bioaccumulation Potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684.
- Kumar, S.B. Chlorhexidine Mouthwash—A Review. J. Pharm. Sci. Res. 2017, 9, 1450–1452.
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655.
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic Antimicrobial Polymers: Recent Advances in Molecular Design. Polym. Chem. 2018, 9, 2407–2427.
- Huang, K.S.; Yang, C.H.; Huang, S.L.; Chen, C.Y.; Lu, Y.Y.; Lin, Y.S. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578.
- Gerba, C.P. Quaternary Ammonium Biocides: Efficacy in Application. Appl. Environ. Microbiol. 2015, 81, 464–469.
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. hua Quaternary Ammonium-Based Biomedical Materials: State-of-the-Art, Toxicological Aspects and Antimicrobial Resistance. Prog. Polym. Sci. 2017, 71, 53–90.
- El-Newehy, M.H.; Meera, M.A.; Aldalbahi, A.K.; Thamer, B.M.; Mahmoud, Y.A.G.; El-Hamshary, H. Biocidal Polymers: Synthesis, Characterization and Antimicrobial Activity of Bis-Quaternary Onium Salts of Poly(Aspartate-Co-Succinimide). Polymers 2020, 13, 23.
- Foksowicz-Flaczyk, J.; Walentowska, J.; Przybylak, M.; Maciejewski, H. Multifunctional Durable Properties of Textile Materials Modified by Biocidal Agents in the Sol-Gel Process. Surf. Coat. Technol. 2016, 304, 160–166.
- Sharon, E.; Sharabi, R.; Eden, A.; Zabrovsky, A.; Ben-Gal, G.; Sharon, E.; Pietrokovski, Y.; Houri-Haddad, Y.; Beyth, N. Antibacterial Activity of Orthodontic Cement Containing Quaternary Ammonium Polyethylenimine Nanoparticles Adjacent to Orthodontic Brackets. Int. J. Environ. Res. Public Health 2018, 15, 606.
- Daoud, F.C.; Coppry, M.; Moore, N.; Rogues, A.M. Do Triclosan Sutures Modify the Microbial Diversity of Surgical Site Infections? A Systematic Review and Meta-Analysis. Microorganisms 2022, 10, 927.
- Ahmed, I.; Boulton, A.J.; Rizvi, S.; Carlos, W.; Dickenson, E.; Smith, N.A.; Reed, M. The Use of Triclosan-Coated Sutures to Prevent Surgical Site Infections: A Systematic Review and Meta-Analysis of the Literature. BMJ Open 2019, 9, e029727.
- Subramani, K.; Seo, H.N.; Dougherty, J.; Chaudhry, K.; Bollu, P.; Rosenthal, K.S.; Zhang, J.F. In Vitro Evaluation of Antimicrobial Activity of Chlorhexidine Hexametaphosphate Nanoparticle Coatings on Orthodontic Elastomeric Chains. Mater. Res. Express 2020, 7, 075401.
- Wood, N.J.; Jenkinson, H.F.; Davis, S.A.; Mann, S.; O’Sullivan, D.J.; Barbour, M.E. Chlorhexidine Hexametaphosphate Nanoparticles as a Novel Antimicrobial Coating for Dental Implants. J. Mater. Sci. Mater. Med. 2015, 26, 201.
- Jones, I.A.; Joshi, L.T. Biocide Use in the Antimicrobial Era: A Review. Molecules 2021, 26, 2276.
- Cheng, X.; Li, R.; Du, J.; Sheng, J.; Ma, K.; Ren, X.; Huang, T.S. Antimicrobial Activity of Hydrophobic Cotton Coated with N-Halamine. Polym. Adv. Technol. 2015, 26, 99–103.
- Demir, B.; Cerkez, I.; Worley, S.D.; Broughton, R.M.; Huang, T.S. N-Halamine-Modified Antimicrobial Polypropylene Nonwoven Fabrics for Use against Airborne Bacteria. ACS Appl. Mater. Interfaces 2015, 7, 1752–1757.
- Ren, T.; Dormitorio, T.V.; Qiao, M.; Huang, T.S.; Weese, J. N-Halamine Incorporated Antimicrobial Nonwoven Fabrics for Use against Avian Influenza Virus. Vet. Microbiol. 2018, 218, 78–83.
- Li, R.; Sheng, J.; Cheng, X.; Li, J.; Ren, X.; Huang, T.S. Biocidal Poly (Vinyl Alcohol) Films Incorporated with N-Halamine Siloxane. Compos. Commun. 2018, 10, 89–92.
- Zhou, C.E.; Kan, C.W.; Matinlinna, J.P.; Tsoi, J.K.H. Regenerable Antibacterial Cotton Fabric by Plasma Treatment with Dimethylhydantoin: Antibacterial Activity against S. Aureus. Coatings 2017, 7, 11.
- de Morais, M.C.; de Oliveira Lima, E.; Perez-Castillo, Y.; de Sousa, D.P. Synthetic Cinnamides and Cinnamates: Antimicrobial Activity, Mechanism of Action, and In Silico Study. Molecules 2023, 28, 1918.
- Imai, M.; Yokoe, H.; Tsubuki, M.; Takahashi, N. Growth Inhibition of Human Breast and Prostate Cancer Cells by Cinnamic Acid Derivatives and Their Mechanism of Action. Biol. Pharm. Bull. 2019, 42, 1134–1139.
- Cai, R.; Miao, M.; Yue, T.; Zhang, Y.; Cui, L.; Wang, Z.; Yuan, Y. Antibacterial Activity and Mechanism of Cinnamic Acid and Chlorogenic Acid against Alicyclobacillus Acidoterrestris Vegetative Cells in Apple Juice. Int. J. Food Sci. Technol. 2019, 54, 1697–1705.
- Robertson, J.; Gizdavic-Nikolaidis, M.; Swift, S. Investigation of Polyaniline and a Functionalised Derivative as Antimicrobial Additives to Create Contamination Resistant Surfaces. Materials 2018, 11, 436.
- Sanchez Ramirez, D.O.; Varesano, A.; Carletto, R.A.; Vineis, C.; Perelshtein, I.; Natan, M.; Perkas, N.; Banin, E.; Gedanken, A. Antibacterial Properties of Polypyrrole-Treated Fabrics by Ultrasound Deposition. Mater. Sci. Eng. C 2019, 102, 164–170.
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.A.; Maiti, T.K.; et al. Electroconductive Multi-Functional Polypyrrole Composites for Biomedical Applications. Appl. Mater. Today 2021, 24, 101117.
- Wang, C.Y.; Makvandi, P.; Zare, E.N.; Tay, F.R.; Niu, L. Advances in Antimicrobial Organic and Inorganic Nanocompounds in Biomedicine. Adv. Ther. 2020, 3, 2000024.




