
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maura Rojas | -- | 3276 | 2023-06-18 16:16:37 | | | |
| 2 | Lindsay Dong | Meta information modification | 3276 | 2023-06-19 03:33:43 | | |
Video Upload Options
Trypanosomiases are a group of tropical diseases that have devastating health and socio-economic effects worldwide. In humans, these diseases are caused by the pathogenic kinetoplastids Trypanosoma brucei, causing African trypanosomiasis or sleeping sickness, and Trypanosoma cruzi, causing American trypanosomiasis or Chagas disease. Antimicrobial peptides (AMPs) are small peptides synthesized by both prokaryotes and (unicellular and multicellular) eukaryotes, where they fulfill functions related to competition strategy with other organisms and immune defense. These AMPs can bind and induce perturbation in cell membranes, leading to permeation of molecules, alteration of morphology, disruption of cellular homeostasis, and activation of cell death. These peptides have activity against various pathogenic microorganisms, including parasitic protists.
1. Introduction
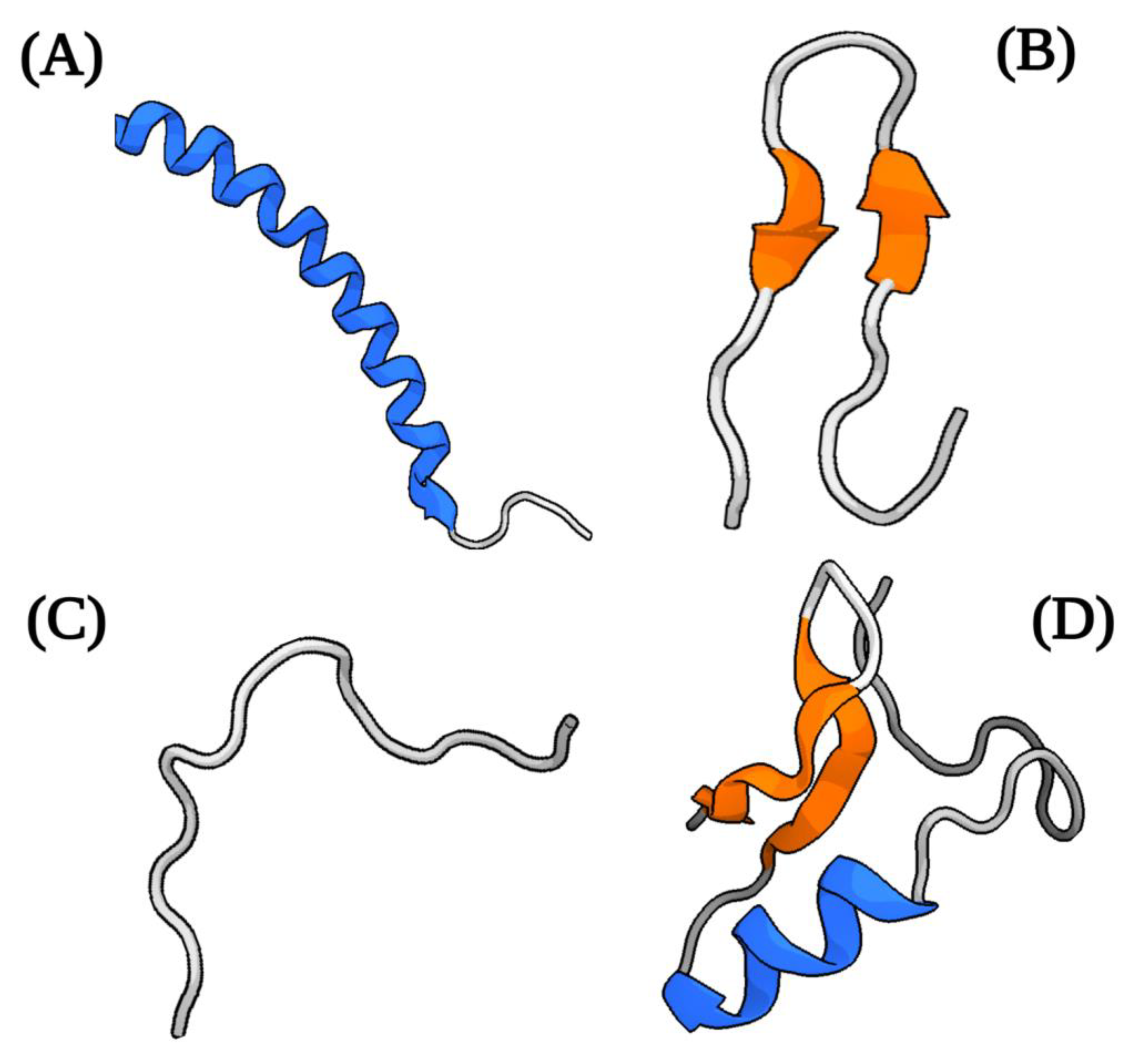
2. Antimicrobial Peptides (AMPs)
3. Current Treatment of Trypanosomiases
4. AMPs with Antiparasitic Activity
5. Antimicrobial Peptides against Kinetoplastids Causing Neglected Tropical Diseases
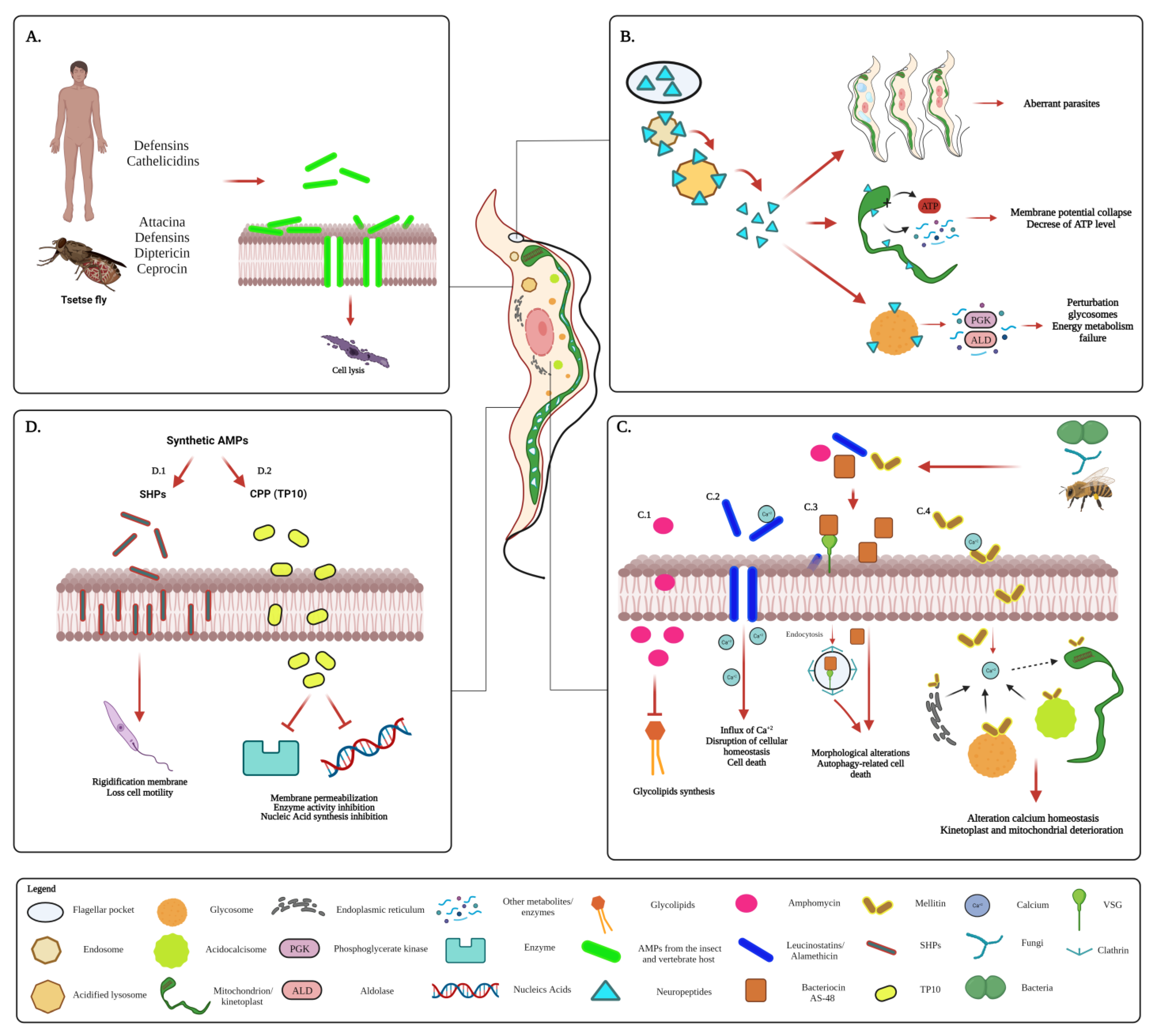
5.1. AMPs against T. brucei
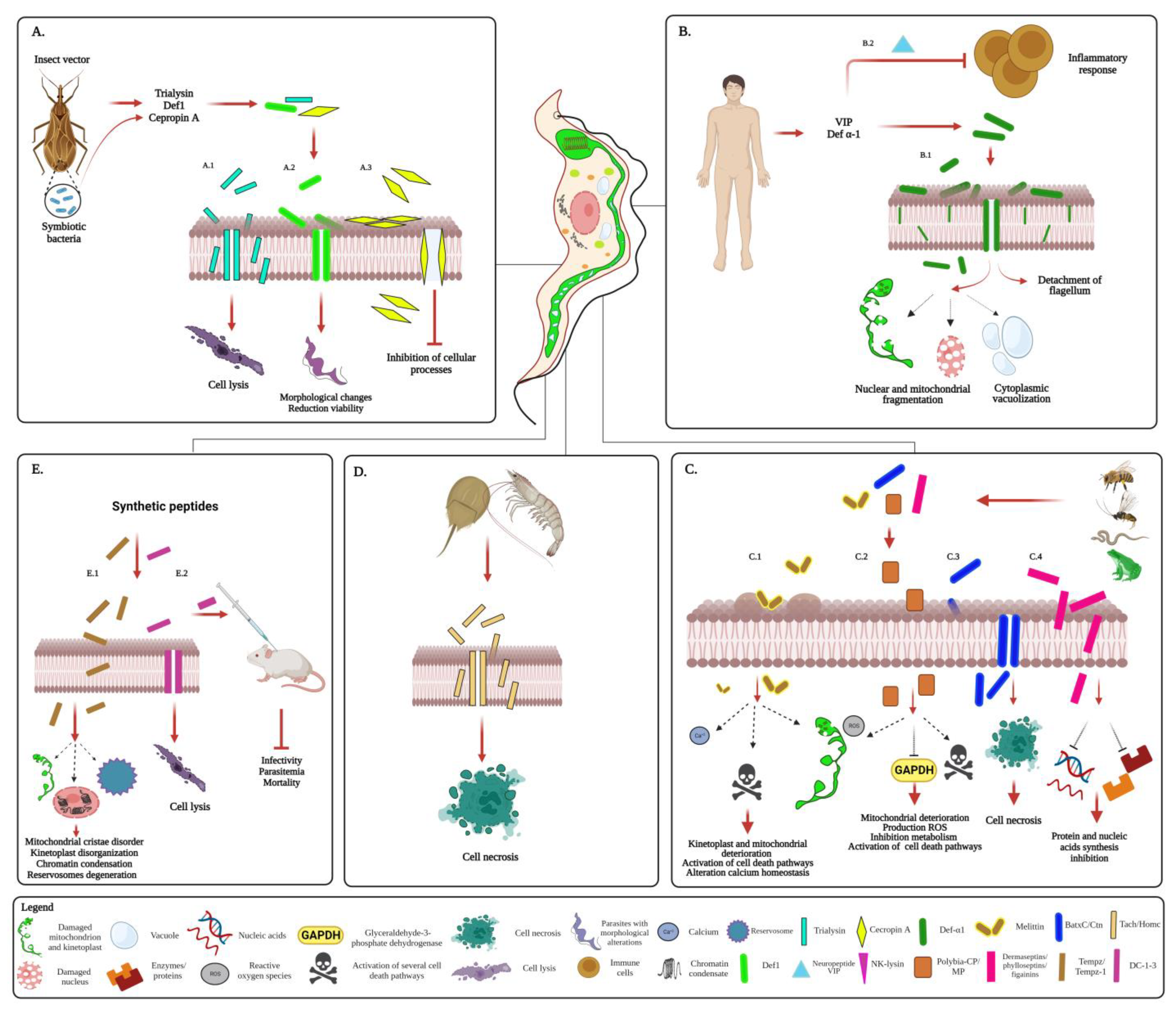
5.2. AMPs against T. cruzi
6. Conclusions
References
- d’Avila-Levy, C.M.; Boucinha, C.; Kostygov, A.; Santos, H.L.C.; Morelli, K.A.; Grybchuk-Ieremenko, A.; Duval, L.; Votýpka, J.; Yurchenko, V.; Grellier, P.; et al. Exploring the Environmental Diversity of Kinetoplastid Flagellates in the High-Throughput DNA Sequencing Era. Memórias Inst. Oswaldo Cruz 2015, 110, 956–965.
- Stuart, K.; Brun, R.; Croft, S.; Fairlamb, A.; Gürtler, R.E.; McKerrow, J.; Reed, S.; Tarleton, R. Kinetoplastids: Related Protozoan Pathogens, Different Diseases. J. Clin. Investig. 2008, 118, 1301–1310.
- Crowe, L.P.; Morris, M.T. Glycosome Heterogeneity in Kinetoplastids. Biochem. Soc. Trans. 2021, 49, 29–39.
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I.P. An Overview on Target-Based Drug Design against Kinetoplastid Protozoan Infections: Human African Trypanosomiasis, Chagas Disease and Leishmaniases. Molecules 2021, 26, 4629.
- Filardy, A.A.; Guimarães-Pinto, K.; Nunes, M.P.; Zukeram, K.; Fliess, L.; Pereira, L.; Oliveira Nascimento, D.; Conde, L.; Morrot, A. Human Kinetoplastid Protozoan Infections: Where Are We Going Next? Front. Immunol. 2018, 9, 1493.
- Trypanosomiasis, Human African (Sleeping Sickness). Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 3 December 2022).
- Rao, S.P.S.; Barrett, M.P.; Dranoff, G.; Faraday, C.J.; Gimpelewicz, C.R.; Hailu, A.; Jones, C.L.; Kelly, J.M.; Lazdins-Helds, J.K.; Mäser, P.; et al. Drug Discovery for Kinetoplastid Diseases: Future Directions. ACS Infect. Dis. 2019, 5, 152–157.
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan Persister-like Cells and Drug Treatment Failure. Nat. Rev. Microbiol. 2019, 17, 607–620.
- Ward, A.I.; Olmo, F.; Atherton, R.L.; Taylor, M.C.; Kelly, J.M. Trypanosoma Cruzi Amastigotes That Persist in the Colon during Chronic Stage Murine Infections Have a Reduced Replication Rate. Open Biol. 2020, 10, 200261.
- Crilly, N.P.; Mugnier, M.R. Thinking Outside the Blood: Perspectives on Tissue-Resident Trypanosoma Brucei. PLoS Pathog. 2021, 17, e1009866.
- Sánchez-Valdéz, F.J.; Padilla, A.; Wang, W.; Orr, D.; Tarleton, R.L. Spontaneous Dormancy Protects Trypanosoma Cruzi during Extended Drug Exposure. eLife 2018, 7, e34039.
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The Antimicrobial Peptides and Their Potential Clinical Applications. Am. J. Transl. Res. 2019, 11, 3919–3931.
- Amino, R.; Martins, R.M.; Procopio, J.; Hirata, I.Y.; Juliano, M.A.; Schenkman, S. Trialysin, a Novel Pore-Forming Protein from Saliva of Hematophagous Insects Activated by Limited Proteolysis. J. Biol. Chem. 2002, 277, 6207–6213.
- Deslouches, B.; Di, Y.P. Antimicrobial Peptides: A Potential Therapeutic Option for Surgical Site Infections. Clin. Surg. 2017, 2, 1740.
- Díaz-Garrido, P.; Cárdenas-Guerra, R.E.; Martínez, I.; Poggio, S.; Rodríguez-Hernández, K.; Rivera-Santiago, L.; Ortega-López, J.; Sánchez-Esquivel, S.; Espinoza, B. Differential Activity on Trypanosomatid Parasites of a Novel Recombinant Defensin Type 1 from the Insect Triatoma (Meccus) Pallidipennis. Insect. Biochem. Mol. Biol. 2021, 139, 103673.
- Papagianni, M. Ribosomally Synthesized Peptides with Antimicrobial Properties: Biosynthesis, Structure, Function, and Applications. Biotechnol. Adv. 2003, 21, 465–499.
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123-20.
- Finking, R.; Marahiel, M.A. Biosynthesis of Nonribosomal Peptides1. Annu. Rev. Microbiol. 2004, 58, 453–488.
- Marahiel, M.A.; Stachelhaus, T.; Mootz, H.D. Modular Peptide Synthetases Involved in Nonribosomal Peptide Synthesis. Chem. Rev. 1997, 97, 2651–2674.
- Ueki, N.; Someya, K.; Matsuo, Y.; Wakamatsu, K.; Mukai, H. Cryptides: Functional Cryptic Peptides Hidden in Protein Structures. Biopolymers 2007, 88, 190–198.
- Park, C.B.; Yi, K.-S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure–Activity Analysis of Buforin II, a Histone H2A-Derived Antimicrobial Peptide: The Proline Hinge Is Responsible for the Cell-Penetrating Ability of Buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250.
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779.
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 453.
- Ma, R.; Wong, S.W.; Ge, L.; Shaw, C.; Siu, S.W.; Kwok, H.F. In Vitro and MD Simulation Study to Explore Physicochemical Parameters for Antibacterial Peptide to Become Potent Anticancer Peptide. Mol. Ther. Oncolytics 2019, 16, 7–19.
- Koehbach, J.; Craik, D.J. The Vast Structural Diversity of Antimicrobial Peptides. Trends Pharmacol. Sci. 2019, 40, 517–528.
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial Peptides (AMPs): A Promising Class of Antimicrobial Compounds. J. Appl. Microbiol. 2022, 132, 1573–1596.
- Lascano, F.; García Bournissen, F.; Altcheh, J. Review of Pharmacological Options for the Treatment of Chagas Disease. Br. J. Clin. Pharmacol. 2022, 88, 383–402.
- Venturelli, A.; Tagliazucchi, L.; Lima, C.; Venuti, F.; Malpezzi, G.; Magoulas, G.E.; Santarem, N.; Calogeropoulou, T.; Cordeiro-da-Silva, A.; Costi, M.P. Current Treatments to Control African Trypanosomiasis and One Health Perspective. Microorganisms 2022, 10, 1298.
- Dickie, E.A.; Giordani, F.; Gould, M.K.; Mäser, P.; Burri, C.; Mottram, J.C.; Rao, S.P.S.; Barrett, M.P. New Drugs for Human African Trypanosomiasis: A Twenty First Century Success Story. Trop. Med. Infect. Dis. 2020, 5, 29.
- Fairlamb, A.H.; Horn, D. Melarsoprol Resistance in African Trypanosomiasis. Trends Parasitol. 2018, 34, 481–492.
- Hidalgo, J.; Ortiz, J.F.; Fabara, S.P.; Eissa-Garcés, A.; Reddy, D.; Collins, K.D.; Tirupathi, R. Efficacy and Toxicity of Fexinidazole and Nifurtimox Plus Eflornithine in the Treatment of African Trypanosomiasis: A Systematic Review. Cureus 2021, 13, e16881.
- Unciti-Broceta, J.D.; Arias, J.L.; Maceira, J.; Soriano, M.; Ortiz-González, M.; Hernández-Quero, J.; Muñóz-Torres, M.; de Koning, H.P.; Magez, S.; Garcia-Salcedo, J.A. Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis. PLoS Pathog. 2015, 11, e1004942.
- Pérez-Molina, J.A.; Molina, I. Chagas Disease. Lancet 2018, 391, 82–94.
- Apt, W.; Zulantay, I. Update on the treatment of Chagas’ disease. Rev. Med. Chil. 2011, 139, 247–257.
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current Trends in the Pharmacological Management of Chagas Disease. Int. J. Parasitol. Drugs Drug Resist. 2019, 12, 7–17.
- Jackson, Y.; Wyssa, B.; Chappuis, F. Tolerance to Nifurtimox and Benznidazole in Adult Patients with Chronic Chagas’ Disease. J. Antimicrob. Chemother. 2020, 75, 690–696.
- Vázquez, C.; García-Vázquez, E.; Carrilero, B.; Simón, M.; Franco, F.; Iborra, M.A.; Gil-Gallardo, L.J.; Segovia, M. Pregnancy and Chagas Disease: Benznidazole’s Impact on Pregnancy and Newborns: A Report of Four Cases. Am. J. Trop. Med. Hyg. 2020, 102, 1075–1077.
- Edwards, M.S.; Montgomery, S.P. Chagas Disease: Implementation of Screening to Benefit Mother and Infant. Clin. Perinatol. 2021, 48, 331–342.
- Campos, M.C.O.; Leon, L.L.; Taylor, M.C.; Kelly, J.M. Benznidazole-Resistance in Trypanosoma Cruzi: Evidence That Distinct Mechanisms Can Act in Concert. Mol. Biochem. Parasitol. 2014, 193, 17–19.
- Revollo, S.; Oury, B.; Vela, A.; Tibayrenc, M.; Sereno, D. In Vitro Benznidazole and Nifurtimox Susceptibility Profile of Trypanosoma Cruzi Strains Belonging to Discrete Typing Units TcI, TcII, and TcV. Pathogens 2019, 8, 197.
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306.
- Carrillo, I.; Rabelo, R.A.N.; Barbosa, C.; Rates, M.; Fuentes-Retamal, S.; González-Herrera, F.; Guzmán-Rivera, D.; Quintero, H.; Kemmerling, U.; Castillo, C.; et al. Aspirin-Triggered Resolvin D1 Reduces Parasitic Cardiac Load by Decreasing Inflammation in a Murine Model of Early Chronic Chagas Disease. PLoS Negl. Trop. Dis. 2021, 15, e0009978.
- Giovati, L.; Ciociola, T.; Magliani, W.; Conti, S. Antimicrobial Peptides with Antiprotozoal Activity: Current State and Future Perspectives. Future Med. Chem. 2018, 10, 2569–2572.
- Pretzel, J.; Mohring, F.; Rahlfs, S.; Becker, K. Antiparasitic Peptides. Adv. Biochem. Eng. Biotechnol. 2013, 135, 157–192.
- de Moura, G.A.; de Oliveira, J.R.; Rocha, Y.M.; de Oliveira Freitas, J.; Rodrigues, J.P.V.; Ferreira, V.P.G.; Nicolete, R. Antitumor and Antiparasitic Activity of Antimicrobial Peptides Derived from Snake Venom: A Systematic Review Approach. Curr. Med. Chem. 2022, 29, 5358–5368.
- Ramazi, S.; Mohammadi, N.; Allahverdi, A.; Khalili, E.; Abdolmaleki, P. A Review on Antimicrobial Peptides Databases and the Computational Tools. Database 2022, 2022, baac011.
- Bell, A. Antimalarial Peptides: The Long and the Short of It. Curr. Pharm. Des. 2011, 17, 2719–2731.
- Lacerda, A.F.; Pelegrini, P.B.; de Oliveira, D.M.; Vasconcelos, É.A.R.; Grossi-de-Sá, M.F. Anti-Parasitic Peptides from Arthropods and Their Application in Drug Therapy. Front. Microbiol. 2016, 7, 91.
- Parapep ParaPep-Database of Anti-Parasitic Peptides. Available online: http://crdd.osdd.net/raghava/parapep/ (accessed on 20 October 2022).
- Jaynes, J.M.; Burton, C.A.; Barr, S.B.; Jeffers, G.W.; Julian, G.R.; White, K.L.; Enright, F.M.; Klei, T.R.; Laine, R.A. In Vitro Cytocidal Effect of Novel Lytic Peptides on Plasmodium Falciparum and Trypanosoma Cruzi1. FASEB J. 1988, 2, 2878–2883.
- Gumila, C.; Ancelin, M.L.; Jeminet, G.; Delort, A.M.; Miquel, G.; Vial, H.J. Differential in Vitro Activities of Ionophore Compounds against Plasmodium Falciparum and Mammalian Cells. Antimicrob. Agents Chemother. 1996, 40, 602–608.
- Ghosh, J.K.; Shaool, D.; Guillaud, P.; Cicéron, L.; Mazier, D.; Kustanovich, I.; Shai, Y.; Mor, A. Selective Cytotoxicity of Dermaseptin S3 toward Intraerythrocytic Plasmodium Falciparum and the Underlying Molecular Basis. J. Biol. Chem. 1997, 272, 31609–31616.
- Krugliak, M.; Feder, R.; Zolotarev, V.Y.; Gaidukov, L.; Dagan, A.; Ginsburg, H.; Mor, A. Antimalarial Activities of Dermaseptin S4 Derivatives. Antimicrob. Agents Chemother. 2000, 44, 2442–2451.
- Moreira, C.K.; Rodrigues, F.G.; Ghosh, A.; Varotti, F.d.P.; Miranda, A.; Daffre, S.; Jacobs-Lorena, M.; Moreira, L.A. Effect of the Antimicrobial Peptide Gomesin against Different Life Stages of Plasmodium spp. Exp. Parasitol. 2007, 116, 346–353.
- Couto, J.; Tonk, M.; Ferrolho, J.; Antunes, S.; Vilcinskas, A.; de la Fuente, J.; Domingos, A.; Cabezas-Cruz, A. Antiplasmodial Activity of Tick Defensins in a Mouse Model of Malaria. Ticks Tick Borne Dis. 2018, 9, 844–849.
- Darkin-Rattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bednarek, M.A.; et al. Apicidin: A Novel Antiprotozoal Agent That Inhibits Parasite Histone Deacetylase. Proc. Natl. Acad. Sci. USA 1996, 93, 13143–13147.
- Kreidenweiss, A.; Kremsner, P.G.; Mordmüller, B. Comprehensive Study of Proteasome Inhibitors against Plasmodium Falciparum Laboratory Strains and Field Isolates from Gabon. Malar. J 2008, 7, 187.
- Schoof, S.; Pradel, G.; Aminake, M.N.; Ellinger, B.; Baumann, S.; Potowski, M.; Najajreh, Y.; Kirschner, M.; Arndt, H.-D. Antiplasmodial Thiostrepton Derivatives: Proteasome Inhibitors with a Dual Mode of Action. Angew. Chem. Int. Ed. Engl. 2010, 49, 3317–3321.
- Rogers, M.J.; Bukhman, Y.V.; McCutchan, T.F.; Draper, D.E. Interaction of Thiostrepton with an RNA Fragment Derived from the Plastid-Encoded Ribosomal RNA of the Malaria Parasite. RNA 1997, 3, 815–820.
- Rosenthal, P.J.; Wollish, W.S.; Palmer, J.T.; Rasnick, D. Antimalarial Effects of Peptide Inhibitors of a Plasmodium Falciparum Cysteine Proteinase. J. Clin. Investig. 1991, 88, 1467–1472.
- Pandey, A.V.; Joshi, R.; Tekwani, B.L.; Singh, R.L.; Chauhan, V.S. Synthetic Peptides Corresponding to a Repetitive Sequence of Malarial Histidine Rich Protein Bind Haem and Inhibit Haemozoin Formation in Vitro. Mol. Biochem. Parasitol. 1997, 90, 281–287.
- Semenov, A.; Olson, J.E.; Rosenthal, P.J. Antimalarial Synergy of Cysteine and Aspartic Protease Inhibitors. Antimicrob. Agents Chemother. 1998, 42, 2254–2258.
- Roy, A.; D’Annessa, I.; Nielsen, C.J.F.; Tordrup, D.; Laursen, R.R.; Knudsen, B.R.; Desideri, A.; Andersen, F.F. Peptide Inhibition of Topoisomerase IB from Plasmodium Falciparum. Mol. Biol. Int. 2011, 2011, 854626.
- Arrighi, R.B.G.; Nakamura, C.; Miyake, J.; Hurd, H.; Burgess, J.G. Design and Activity of Antimicrobial Peptides against Sporogonic-Stage Parasites Causing Murine Malarias. Antimicrob. Agents Chemother. 2002, 46, 2104–2110.
- Chalk, R.; Townson, H.; Ham, P.J. Brugia Pahangi: The Effects of Cecropins on Microfilariae in Vitro and in Aedes Aegypti. Exp. Parasitol. 1995, 80, 401–406.
- de Moraes, J.; Nascimento, C.; Miura, L.M.C.V.; Leite, J.R.S.A.; Nakano, E.; Kawano, T. Evaluation of the in Vitro Activity of Dermaseptin 01, a Cationic Antimicrobial Peptide, against Schistosoma Mansoni. Chem. Biodivers 2011, 8, 548–558.
- de Moraes, J.; Keiser, J.; Ingram, K.; Nascimento, C.; Yamaguchi, L.F.; Bittencourt, C.R.; Bemquerer, M.P.; Leite, J.R.; Kato, M.J.; Nakano, E. In Vitro Synergistic Interaction between Amide Piplartine and Antimicrobial Peptide Dermaseptin against Schistosoma Mansoni Schistosomula and Adult Worms. Curr. Med. Chem. 2013, 20, 301–309.
- Aruleba, R.T.; Tincho, M.B.; Pretorius, A.; Kappo, A.P. In Silico Prediction of New Antimicrobial Peptides and Proteins as Druggable Targets towards Alternative Anti-Schistosomal Therapy. Sci. Afr. 2021, 12, e00804.
- Fogarty, C.E.; Suwansa-ard, S.; Phan, P.; McManus, D.P.; Duke, M.G.; Wyeth, R.C.; Cummins, S.F.; Wang, T. Identification of Putative Neuropeptides That Alter the Behaviour of Schistosoma Mansoni Cercariae. Biology 2022, 11, 1344.
- Park, Y.; Jang, S.-H.; Lee, D.G.; Hahm, K.-S. Antinematodal Effect of Antimicrobial Peptide, PMAP-23, Isolated from Porcine Myeloid against Caenorhabditis Elegans. J. Pept. Sci. 2004, 10, 304–311.
- Santos, B.P.O.; Alves, E.S.F.; Ferreira, C.S.; Ferreira-Silva, A.; Góes-Neto, A.; Verly, R.M.; Lião, L.M.; Oliveira, S.C.; de Magalhães, M.T.Q. Schistocins: Novel Antimicrobial Peptides Encrypted in the Schistosoma Mansoni Kunitz Inhibitor SmKI-1. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129989.
- Castro, G.A. Helminths: Structure, Classification, Growth, and Development. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2.
- Smyth, D.J.; Glanfield, A.; McManus, D.P.; Hacker, E.; Blair, D.; Anderson, G.J.; Jones, M.K. Two Isoforms of a Divalent Metal Transporter (DMT1) in Schistosoma Mansoni Suggest a Surface-Associated Pathway for Iron Absorption in Schistosomes. J. Biol. Chem. 2006, 281, 2242–2248.
- Retra, K.; deWalick, S.; Schmitz, M.; Yazdanbakhsh, M.; Tielens, A.G.M.; Brouwers, J.F.H.M.; van Hellemond, J.J. The Tegumental Surface Membranes of Schistosoma Mansoni Are Enriched in Parasite-Specific Phospholipid Species. Int. J. Parasitol. 2015, 45, 629–636.
- Ballesteros, C.; Geary, J.F.; Mackenzie, C.D.; Geary, T.G. Characterization of Divalent Metal Transporter 1 (DMT1) in Brugia Malayi Suggests an Intestinal-Associated Pathway for Iron Absorption. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 341–349.
- Glanfield, A.; McManus, D.P.; Anderson, G.J.; Jones, M.K. Pumping Iron: A Potential Target for Novel Therapeutics against Schistosomes. Trends Parasitol. 2007, 23, 583–588.
- Hoeckendorf, A.; Leippe, M. SPP-3, a Saposin-like Protein of Caenorhabditis Elegans, Displays Antimicrobial and Pore-Forming Activity and Is Located in the Intestine and in One Head Neuron. Dev. Comp. Immunol. 2012, 38, 181–186.
- Bruno, R.; Maresca, M.; Canaan, S.; Cavalier, J.-F.; Mabrouk, K.; Boidin-Wichlacz, C.; Olleik, H.; Zeppilli, D.; Brodin, P.; Massol, F.; et al. Worms’ Antimicrobial Peptides. Mar. Drugs 2019, 17, 512.
- Deshwal, S.; Mallon, E.B. Antimicrobial Peptides Play a Functional Role in Bumblebee Anti-Trypanosome Defense. Dev. Comp. Immunol. 2014, 42, 240–243.
- Cauchard, S.; Van Reet, N.; Büscher, P.; Goux, D.; Grötzinger, J.; Leippe, M.; Cattoir, V.; Laugier, C.; Cauchard, J. Killing of Trypanozoon Parasites by the Equine Cathelicidin ECATH1. Antimicrob. Agents Chemother. 2016, 60, 2610–2619.
- Suárez-Quevedo, Y.; Barbosa-Vinasco, H.J.; Gutiérrez-Garnizo, S.A.; Olaya-Morales, J.L.; Zabala-González, D.; Carranza-Martínez, J.C.; Guhl-Nannetti, F.; Cantillo-Barraza, O.; Vallejo, G.A. Innate Trypanolytic Factors in Triatomine Hemolymph against Trypanosoma Rangeli and T. Cruzi: A Comparative Study in Eight Chagas Disease Vectors. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2020, 44, 88–104.
- Harrington, J.M. Antimicrobial Peptide Killing of African Trypanosomes. Parasite Immunol. 2011, 33, 461–469.
- Hu, Y.; Aksoy, S. An Antimicrobial Peptide with Trypanocidal Activity Characterized from Glossina Morsitans Morsitans. Insect Biochem. Mol. Biol. 2005, 35, 105–115.
- Delgado, M.; Anderson, P.; Garcia-Salcedo, J.A.; Caro, M.; Gonzalez-Rey, E. Neuropeptides Kill African Trypanosomes by Targeting Intracellular Compartments and Inducing Autophagic-like Cell Death. Cell Death Differ. 2009, 16, 406–416.
- Souza, A.L.A.; Faria, R.X.; Calabrese, K.S.; Hardoim, D.J.; Taniwaki, N.; Alves, L.A.; De Simone, S.G. Temporizin and Temporizin-1 Peptides as Novel Candidates for Eliminating Trypanosoma Cruzi. PLoS ONE 2016, 11, e0157673.
- Monteiro, M.L.; Lima, D.B.; de Menezes, R.R.P.P.B.; Sampaio, T.L.; Silva, B.P.; Serra Nunes, J.V.; Cavalcanti, M.M.; Morlighem, J.-E.; Martins, A.M.C. Antichagasic Effect of Hemocyanin Derived from Antimicrobial Peptides of Penaeus Monodon Shrimp. Exp. Parasitol. 2020, 215, 107930.
- Pinto, E.G.; Pimenta, D.C.; Antoniazzi, M.M.; Jared, C.; Tempone, A.G. Antimicrobial Peptides Isolated from Phyllomedusa Nordestina (Amphibia) Alter the Permeability of Plasma Membrane of Leishmania and Trypanosoma Cruzi. Exp. Parasitol. 2013, 135, 655–660.
- Memariani, H.; Memariani, M. Melittin as a Promising Anti-Protozoan Peptide: Current Knowledge and Future Prospects. AMB Express 2021, 11, 69.
- Barr, S.C.; Rose, D.; Jaynes, J.M. Activity of Lytic Peptides against Intracellular Trypanosoma Cruzi Amastigotes in Vitro and Parasitemias in Mice. J. Parasitol. 1995, 81, 974–978.
- Löfgren, S.E.; Miletti, L.C.; Steindel, M.; Bachère, E.; Barracco, M.A. Trypanocidal and Leishmanicidal Activities of Different Antimicrobial Peptides (AMPs) Isolated from Aquatic Animals. Exp. Parasitol. 2008, 118, 197–202.





