1. Ophthalmic Hydrogels
The first choice for ocular treatment is eye drops, which is the most preferred noninvasive way to deliver drugs for treating ophthalmic diseases. Consequently, it is important for drug transport to overcome multiple ocular barriers, such as the corneal epithelium, choroid, retinal pigmented epithelium, choroidal and conjunctival blood flow, lacrimation, and lymphatic drainage, restricting drug transport into the ocular tissues. Less than 5% of drugs have been reported to enter the aqueous humor, which results in sub-therapeutic drug concentrations in these ocular tissues
[1]. To overcome this situation, as a prominent example, polypseudorotaxane hydrogels were developed by mixing polyvinyl caprolactam-polyvinyl acetate-polyethylene (Soluplus
®) micelles of approximately 99.4 nm with a cyclodextrins solution
[2].
In regard to ophthalmic gels, eye drops exist as viscous solutions before application to the eye and are normally used for dry eyes. In situ gels, by comparison, are liquids that are applied as drops to the eye, and it is only after administration that they experience a transition from sol-gel to gel in the conjunctiva through stimuli, such as pH, temperature, or ions, with a significant improvement in the ocular bioavailability
[3]. Alginate-based nanoparticles were prepared using W/O emulsion technology and coated with CS and Gel. The mean diameter of the prepared nanoparticles was in the range of 150–270 nm, which is a suitable size for ocular drug delivery that does not cause irritation
[4].
Dana, Annabi, and co-workers designed a biocompatible adhesive hydrogel for corneal tissue repair, which can be used for the quick and long-term repair of corneal stromal defects. The bioadhesive hydrogel is made of a chemically modified form of gelatin (Gel) and photoinitiators, which can be photocross-linked after short-time exposure to visible light (450 to 550 nm). Gel was chemically functionalized with methacryloyl groups to form a bioadhesive gel for corneal regeneration (GelCORE). Additionally, the authors show that the physical properties and adhesion strength of GelCORE bioadhesives can be tuned by changing the GelCORE concentration and light exposure time
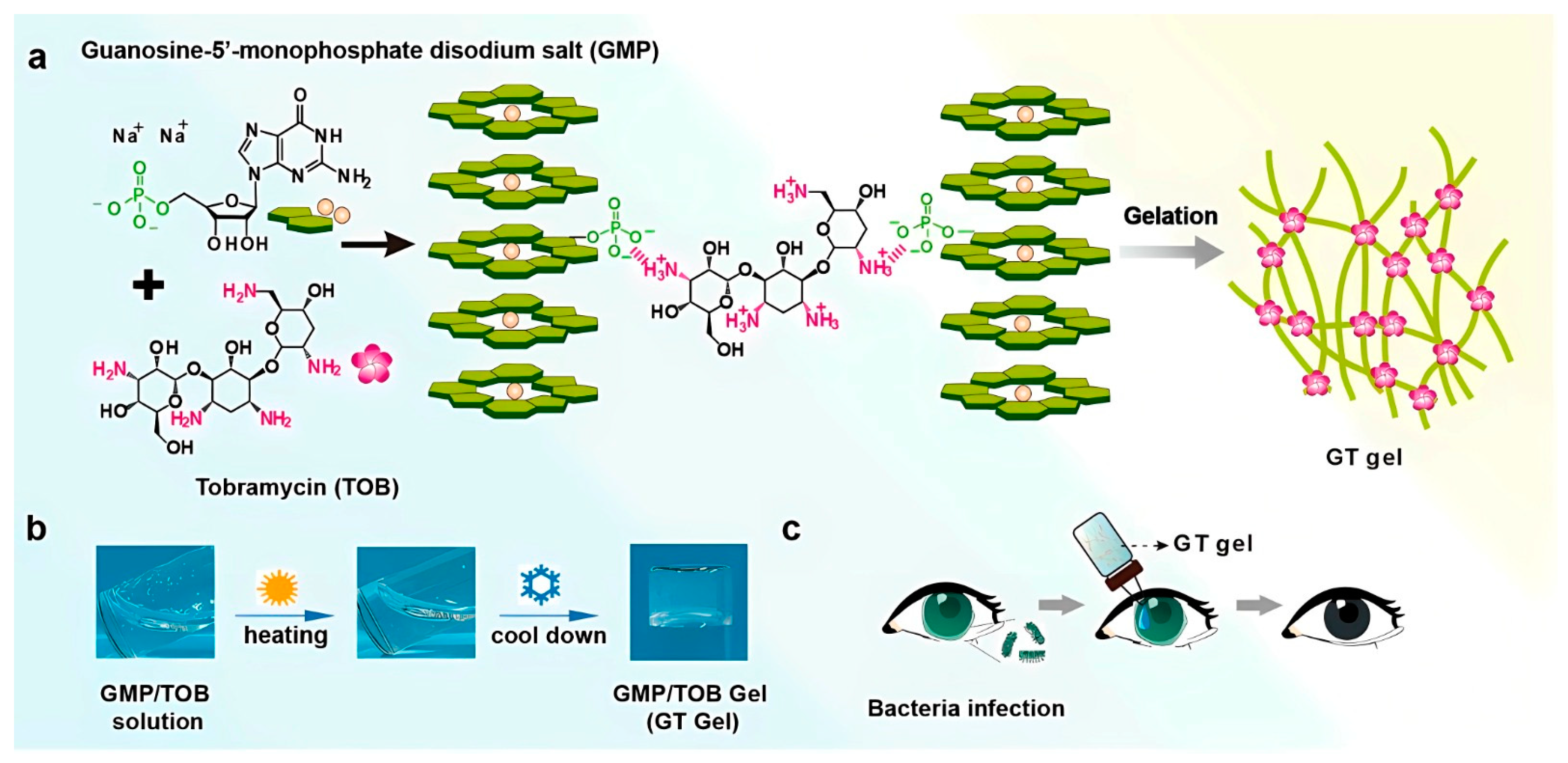
[5]. In this same situation, an efficient antibiotic gel for the clinical treatment of bacterial keratitis was suggested. For such purposes, a supramolecular hydrogel was fabricated via the electrostatic interactions between guanosine 5′-monophosphate disodium salt (GMP) and tobramycin (TOB), which is a kind of aminoglycoside antibiotic bearing multiple primary amine groups in its structure. Obviously, the electrostatic interaction between the primary amine groups of TOB and the negatively charged phosphates on the assembled GMP nanofibers could facilitate the formation of a supramolecular GMP-TOB gel
[6], as is well illustrated in
Figure 1. Cyclodextrin (CD)-based supramolecular hydrogels were studied for their potential to prolong precorneal retention and increase ocular bioavailability
[7]. This effort also opened a beneficial channel for the thermodynamics of an equilibrium system to achieve the gradual and controlled release and capture of cyclodextrin (guest) using a coordination polymer (Mg-CP) as the host and temperature as the stimulus
[8].
Figure 1. (
a) Illustration of GMP-TOB Gel (GT Gel) formation via electrostatic interactions between GMP-based G-quadruplex and tobramycin. (
b) GT Gel preparation scheme, the process involves heating and cooling. The final objective of the GT gel is the treatment of bacterial keratitis (
c). Reprinted with permission from Ref.
[6].
An excellent supramolecular hydrogel based on β-CD and adamantane for corneal wound healing was well-designed by Fernandes-Cunha et al. In that study, the supramolecular hyaluronic acid hydrogel was formed based on the host–guest interaction. Compared with the free hyaluronic acid solution, the supramolecular hyaluronic acid hydrogel had a prolonged residence time on the cornea without complication
[9]. With similar points of view but with different materials, another design architecture included DNA-based supramolecular hydrogels that could form a gel under physiological conditions and provide a promising candidate for a vitreous body substitute because it is colorless and transparent, with a similar density to that of the natural vitreous body
[10].
2. Adhesive Hydrogels
A cooperative structural effect of hydrogels for adhesive applications falls on creating systems for surgical adhesion, tissue engineering, transdermal drug delivery systems, and soft devices such as sensors and actuators, and in the designing of inks for additive manufacturing
[11][12][13][14][15][16].
With a high potential impact, significant progress has been achieved in the field of adhesive hydrogels, considerably around supramolecular adhesive hydrogels with inherent applications in tissue engineering. In this regard, an exhaustive review of structural strategies for the formation of supramolecular adhesive hydrogels and their application in tissue engineering was reported by Zhao et al.
[17]. Shin et al. demonstrated the feasibility of bioinspired hyaluronic acid modified with a catechol (HA-CA) hydrogel for use in tissue engineering. Furthermore, they showed the adhesiveness of the HA-CA hydrogel
[18]. On the other hand, the outstanding gelation of HA-CA and chitosan modified with catechol (CS-CA) allows highly cross-linked systems and an enhanced adhesive ability to tissues to be obtained
[19].
Due to their dynamism regarding advanced functionality, a key advantage of supplanting stitches with an adhesive is that an adhesive covers an entire wound as a continuum, because conventional suture materials, such as stitches, staples, or wires, are not the ideal choices to achieve minimally invasive surgeries. The other advantages of gluing the tissue are that the method is quick, saves surgery time, is inexpensive, does not require stitch removal, and is waterproof
[20]. In this terrain, the designed adhesive should be biocompatible and nonimmunogenic, should comply with the tissue’s mechanical properties, should develop adhesion over specified time periods, and should adhere strongly, and, in some cases, the adherence must behave reversibly. The designing of hydrogel adhesives is complicated by the inherent nature of these materials, as most of their volume is water and, under physiological conditions, the functional groups needed must have permanent adherence and the covalent junctions be strong and durable; however, they are essentially static and not reversible
[11][21], as shown in
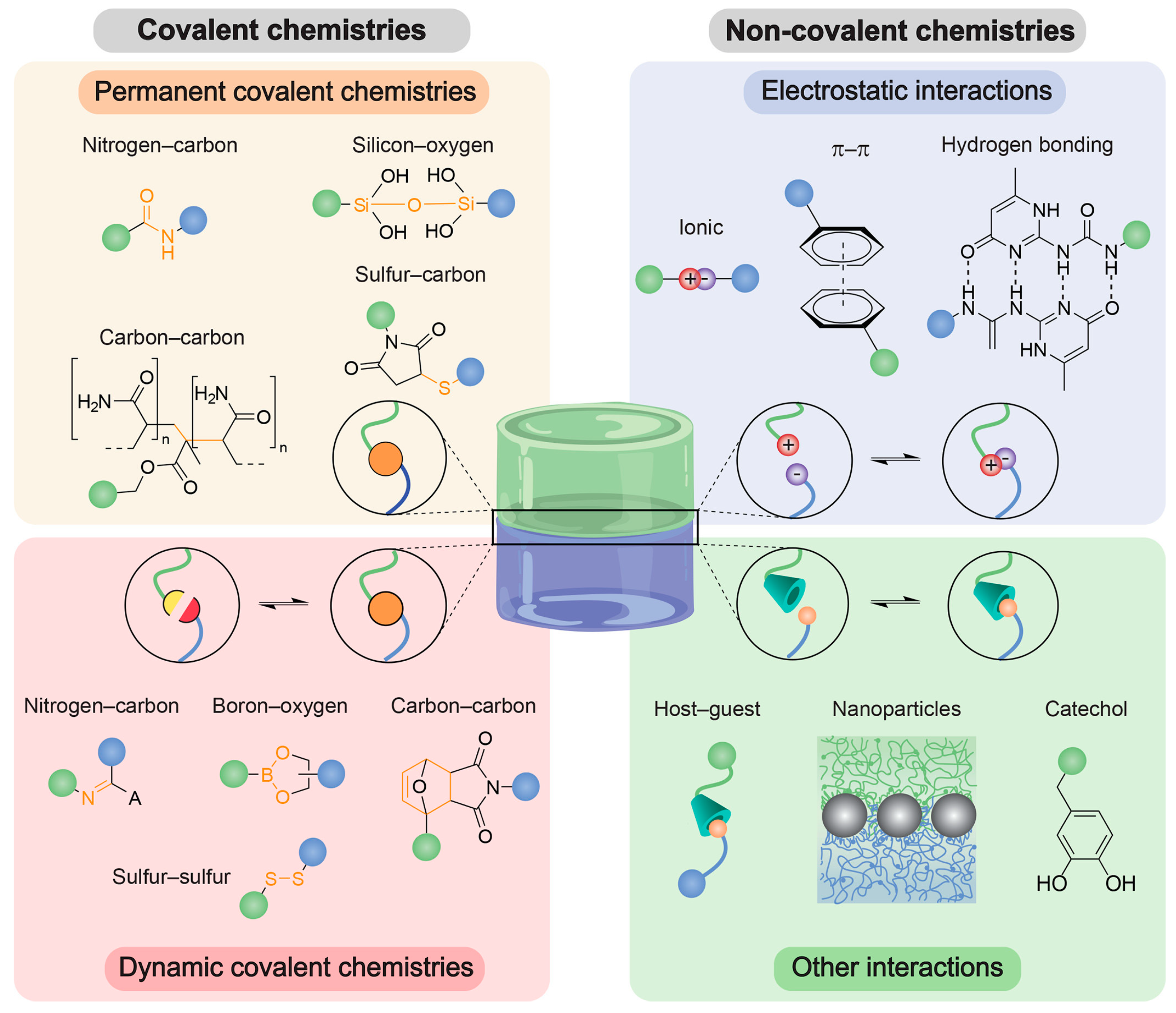
Figure 2.
Figure 2. Overview of common bonds that are used as adhesion junctions. Adhesion junctions can be formed via permanent or dynamic covalent or noncovalent bond formation. Noncovalent interactions include electrostatic interactions and other interactions such as host–guest chemistries and polymer−nanoparticle (NP) interactions, for instance, catechol-based ones. Reprinted with permission from Ref.
[21].
In biomedicine, there is specific interest in adhering hydrogels to tissues for the closure of wounds, the sealing of damaged sections of organs
[22], and the development of stretchable electronics
[23][24]. In addition, a hydrogels’ adhesion to hard and dry surfaces is useful for engineering adaptive and responsive devices. By virtue of this, tissue adhesives require interdisciplinary efforts that span chemistry, mechanics, and biology, as the performance of an adhesive is primarily determined by the adhesive’s physicochemical properties, chemical and mechanical interactions with the tissue, the host immune response, and local environment characteristics
[25], and, of course, they are often not properly developed for specific tissue applications
[26]. Moreover, it is important to outline that some mechanical relationships do not apply directly to most biological tissues
[27]. In contrast, antiadhesion approaches, including the use of impermeable barriers that block fibroblast penetration from surrounding tissues, for preventing postoperative adhesion are, finally, essential, principally, to establish a permanent membrane that can be governed by expanded polytetrafluoroethylene (ePTFE) providing a barrier beneath the sternum, reducing adhesions between the sternum and the epicardium
[28]. A hydrogel has been newly developed for uterine applications and to prevent adhesion, and the compound was developed using 3D printing technology to encapsulate human amnion mesenchymal stem cells (hAMSCs) from a human amniotic membrane. In this setting, using natural biopolymers such as Gel and collagen (Col) due to the importance of their unique biocompatibility and biodegradability properties, methacrylated gelatin (GelMA) and methacrylated collagen (ColMA) polymers were synthesized
[29]. In addition, Yang et al. studied the effects of imidazolidinyl urea (IU) content and the molecular weight of PEG on mechanical properties. The energy dissipation efficiency and shape memory behavior were also investigated. Moreover, they tested the hemolytic activity, cytocompatibility, in vivo retention time, and tissue compatibility of supramolecular hydrogels
[30].
Several studies, such as those reported by Zhang, X. and Zhang, J. and co-workers, detail the reversible adhesive properties of multistimuli-responsive supramolecular hydrogels based on hydrogen bonding. They reported networks formed by adding poly(ethylene polyamine) (PPA) to an aqueous dispersion of oxidized multiwalled carbon nanotubes (ox-MWCNTs). The resulting materials were cross-linked through a combination of weak (N−H···N) and strong (N−H···O) hydrogen bonding
[31]. Other findings provided by Burattini et al. include the designing of healable supramolecular polymers from chain-folding polyimide and pyrenyl-functionalized polyurethanes. These materials are held together by aromatic π–π stacking between the π–electron-deficient diimide groups and the π–electron-rich pyrenyl units. It is noteworthy that the π–π interactions are complemented by the hydrogen bonding between the urea and diimide residues
[32].
A comprehensive study directed by Liu and his group was focused on using hydroxypropyl-modified α-cyclodextrin (Hy-α-CD) and acrylamide–PEG
20000–acrylamide (ACA-PEG
20000-ACA) to construct a polypseudorotaxane with good water solubility. The attractiveness of this research was that through the photo-initiated polymerization of polypseudorotaxane with acrylamide in situ, a capped polyrotaxane cross-linked with 1,4-butanediol diglycidyl ether in a basic solution was obtained to form a slide-ring supramolecular hydrogel that can be stretched to 25.4 times its original length, which recovers rapidly upon unloading. Furthermore, these Ca
2+-doped hydrogels are used to prepare wearable strain sensors for monitoring human motion
[33]. In another line of research, peptide-DNA-based hydrogels that are organized into interlaced superstructures that deconstruct upon the addition of molecules or changes in charge density have been well reported. In this regard, experimental simulations suggest that chemically reversible structures can only occur within a limited range of supramolecular cohesive energies
[34].
3. Self-Healing Hydrogels Systems
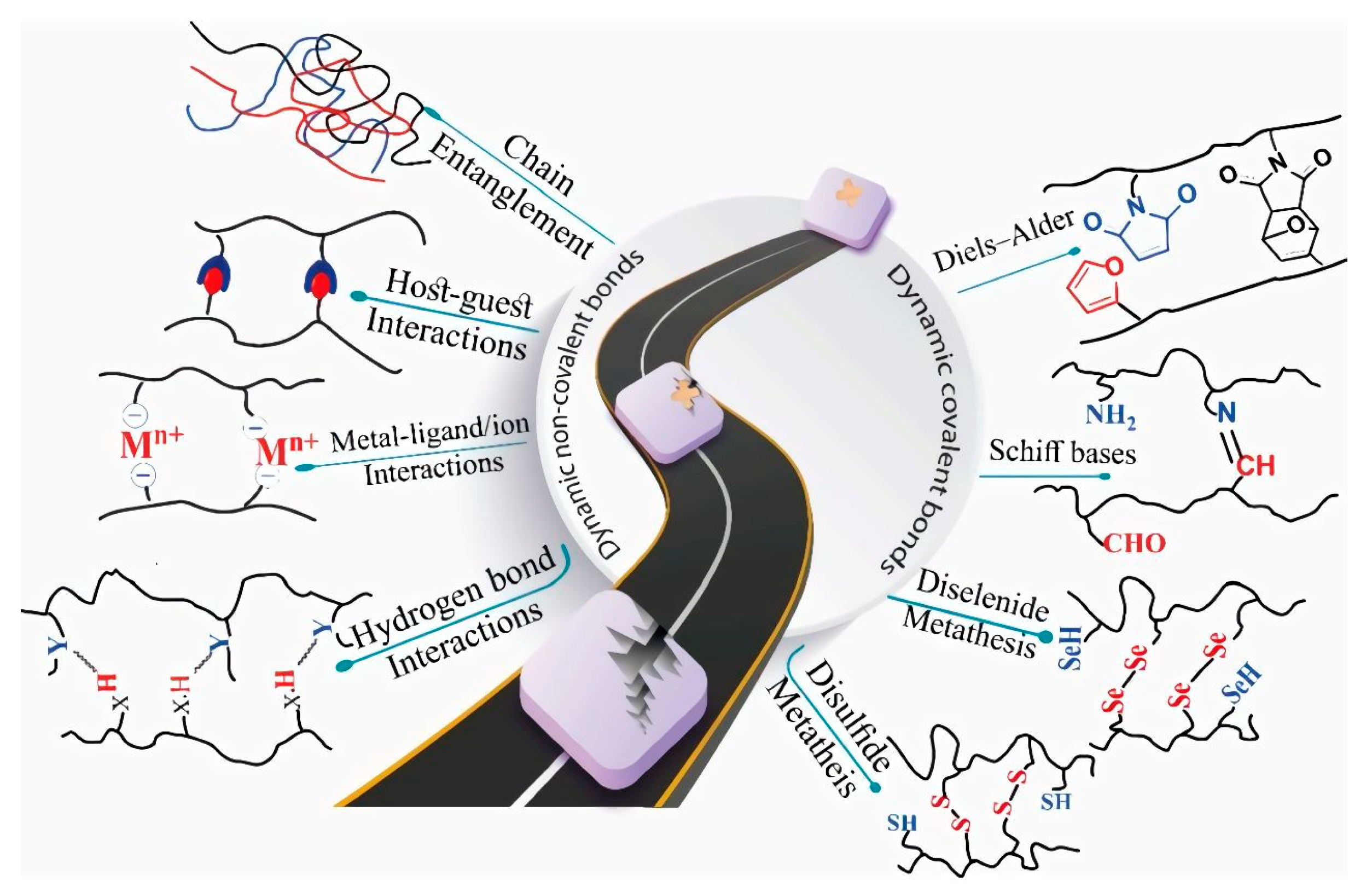
Self-repairing behavior originating from dynamic covalent bonds can include disulfide exchange, diselenide exchange, and Schiff bases (imine bonds). Consequently, breaking and remaking covalent bonds require appropriate conditions of reaction. Conversely, in dynamic non-covalent hydrogels, mostly known as autonomous self-repairing hydrogels, the mechanism is based on hydrogen bond interactions, metal–ligand interactions, host–guest interactions, hydrophobic interactions, supramolecular interactions, and the entanglement of polymeric chains, as illustrated in
Figure 3 [35].
Figure 3. Schematic of dynamic covalent and no-covalent bond operation in the self-healing process. Reprinted with permission from Ref.
[35].
The specificity of hydrogels for self-repair is due to their reversible properties after being subjected to stress and, evidently, in the nature of their physical bonds. This is how a hydrogel structured using hyaluronic acid behaves together with gallol when exhibiting the spontaneous loading of approximately 93% of a solution of proteins with a concentration of 270 ug/mL in phosphate-buffered saline (PBS, pH 7.4)
[36]. In this regard, concerns are reported by Hardman and coworkers, particularly about hydrogels for soft-sensing applications. They explain very accurately the electrical and mechanical properties of an ionically sensorized self-healing Gel hydrogel that at room temperature can undergo strains of up to 454% and, furthermore, presents stability over long periods of time and formidable biocompatibility and biodegradability. The hydrogel is Gel- and glycerol-based and can be 3D-printed to create customizable sensor networks
[37].
New research based on supramolecular chemistry establishes that ionic gel self-healing does not require any external stimuli. For instance, polymer design and architecture have a great impact on the creation of ion gels with self-healing abilities as well as other functionalities, such as high stretchability and toughness and compatibility with ionic liquids; herein, the interactions between synthetic polymers such as polyacrylamides and polymethacrylates and an ionic liquid are relevant
[38]. In addition, self-healing sodium–cellulose ionic conducting hydrogels (Na-CICH) with an ionic conductivity of ≈ 10
−4–10
−3 S cm
−1 have been developed using an aqueous NaOH/urea cellulose solution system
[39].
In another sense, which is no less relevant, the formation of Cu(II) metallohydrogel compounds with third-order nonlinear optical activity based on the self-repairing protein bovine serum albumin (BSA) is pursued in order to produce semiconductor devices due to the large contribution of π-electrons to photosensitivity
[40].
Coordination bonds have utility for constructing self-healing polymers and, evidently, because of the presence of metal ligands and metal–ligand dynamic bonds, self-healing polymers can exhibit various functions such as dielectric materials, luminescence, magnetism, catalysis, responsiveness to stimuli, and shape-memory behavior. However, there are coordination compounds that are labile and can undergo a variety of reactions, including electron transfer, ligand exchange, and associated processes. All of these depend on the d-electron configuration of the central metal ion
[41][42]. In the event of mechanical damage, chain deformation, or chemical degradation suffered by self-healing hydrogels made up of reversible dynamic covalent or non-covalent bonds, they can easily recover their original properties
[35]. This process is the product of the division and reformation of dynamic links in response to various external triggers or initiated autonomously.
The characteristics of a self-healing hydrogel based on the specific coordination of Ni
2+ with a polymeric ligand made via the polyaddition of 2,6-diethynylpyridine and diazido-poly(ethylene glycol) have been developed. The choice of Ni
+2 is due to the great selectivity due to the high chain length of the polymeric linker, which can hinder the formation of efficient intermolecular cross-links in Ni
+2 and 2,6-bis(1,2,3-triazol-4-yl)-pyridine (Ni
+2-btp) complexes with high thermodynamic stability. The impact on hydrogelation is selective due to metal–ligand coordination interactions between the Ni
2+ and btp residues, while the additions of other divalent metal ions, such as Zn
2+, Fe
2+, Cu
2+, Mg
2+, Mn
2+, Ca
2+, and Cd
2+, only induce color changes or precipitation
[43].
4. Electrically Conductiveg Hydrogels
Conductive hydrogels are being used in different scientific settings. Indeed, versatile biochemical sensors using conventional hydrogels based on carbohydrates, polymers, DNA, and peptides are being expansively studied. An illustrative example of the use of a 3D hydrogel based on poly(ethylene glycol) diacrylate through the use of the amyloid beta 42 (Aβ42) peptide for prostate-specific antigen (PSA) detection was reported. In this way, an interdigitated microelectrode (IME) with a dynamic range was substantially improved with an enviable sensitivity for biosensing and has been structured for peptides such as Aβ42
[44].
All cellular communication occurs through electrical signals, impacting critical mechanisms and the functionality of biological tissues. This principle was addressed in skeletal muscle tissue engineering (SMTE) to fabricate in vitro bioartificial muscle tissue to assist and accelerate the regeneration process using electrically conductive hydrogels as the established engineered platforms, which can guarantee a tissue-like microenvironment
[45].
Conductive hydrogels can also be obtained by integrating electrical fillers into the hydrogel network. As described by Farr et al., by including graphene oxide (GO) and reduced GO (rGO), metal nanowires, and carbon nanotubes (CNTs) as alternatives, they were able to promote skeletal muscle repair and regeneration
[46].
There are three reviews with extensive information regarding the performance of electrically conductive hydrogels in technological applications such as biosensing, flexible electronics, and tissue engineering
[47][48][49]. It is noteworthy that the focus of the strategies for producing conductive hydrogels includes the polymerization of a conducting monomer in a prefabricated hydrogel, the mixture of conductive systems via, for instance, the simultaneous or stepwise cross-linking of monomers to synthesize conductive hydrogels, and the self-assembling of modified electrically conductive materials
[48].
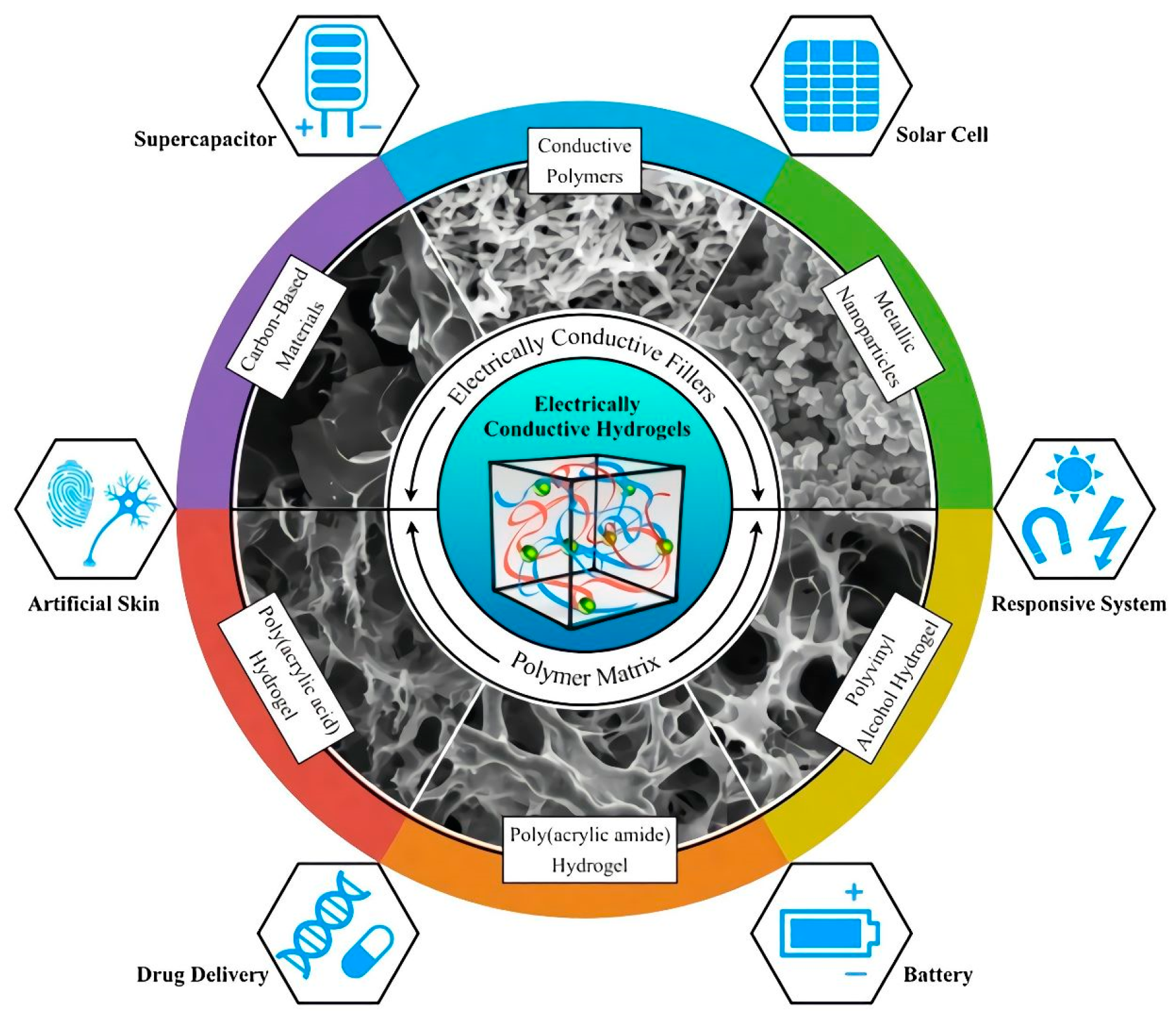
Figure 4 illustrates the main approaches employed, in another instance, to design electrically conductive hydrogels
[49].
Figure 4. Electrically conductive hydrogels are an emerging class of hydrogels combining a hydrophilic matrix with conductive fillers, and they have exceptional promise in a wide range of applications. Reprinted with permission from Ref.
[49].
In the same direction, electrically conductive hydrogels have been designed for active electrode materials for supercapacitors and lithium-ion batteries with specific morphologies and compositions. Some classic examples of electrically conductive hydrogels are encompassed in
Figure 5 [49].
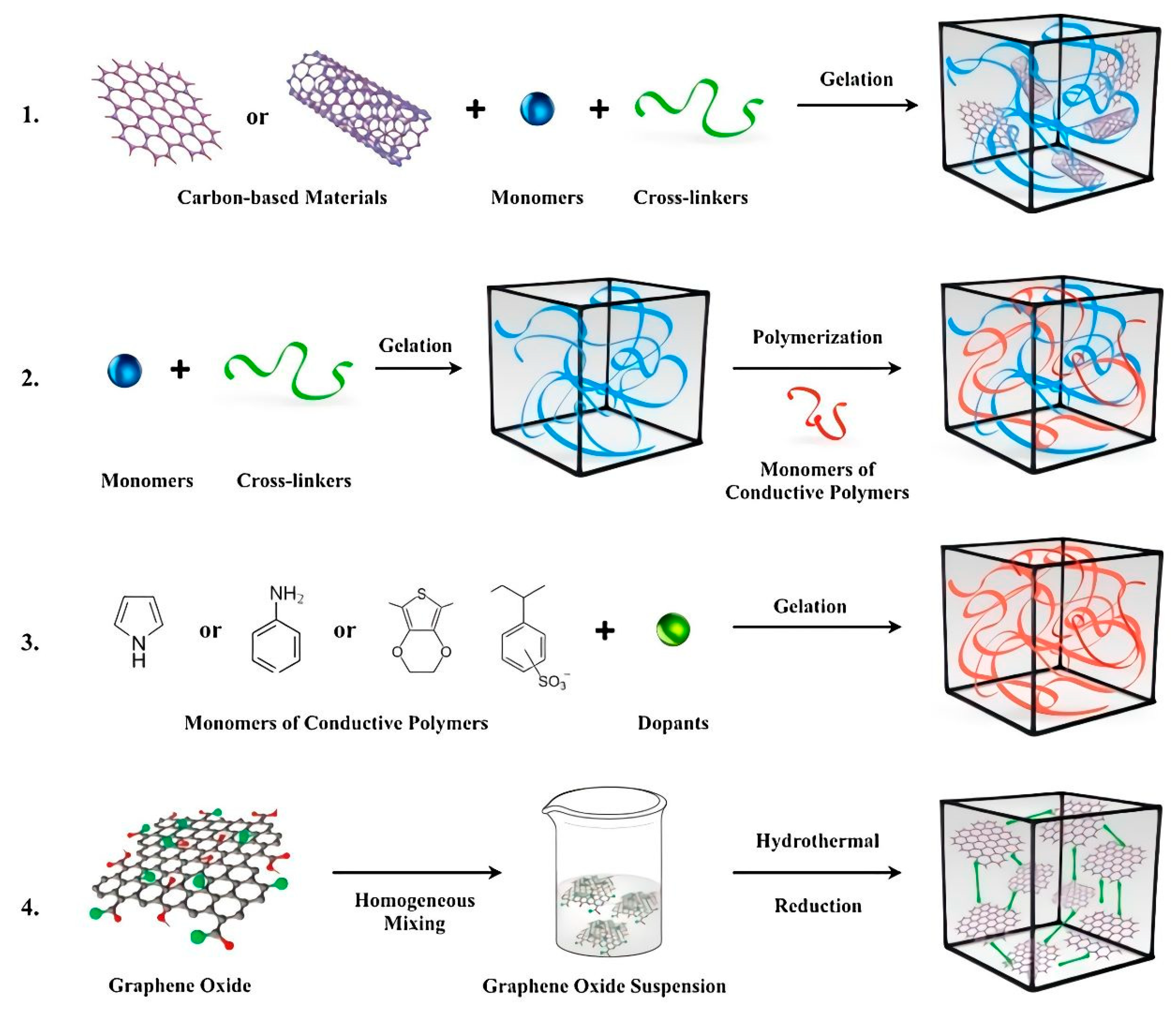
Figure 5. Four main approaches used to obtain ECAs: (
1) hydrogel formation via a conductive filler suspension; (
2) polymerization within a preformed hydrogel matrix; (
3) cross-linking conductive polymers with dopant molecules; and (
4) self-assembly of graphene hydrogel via supramolecular interactions. Reprinted with permission from Ref.
[49].
Great developments in conductive nanocomposites have come true, especially those based on CNTs, graphene, transition metal carbides (MXenes), and metal nanofibers. Of these, carbonitrides, nitrides, and transition metal carbides are the most attractive 2D materials, which, together, are known as MXenes. There are various synthetic routes to MXene derived from its precursor MAX phase: M represents a transition metal; A is the main group element, generally, Si or Al; and X is N or C. Through MXene, some ordered-oriented intelligent tunable hydrogel materials (PMZn) with biocompatible polymers and ZnSO
4 solution as the precursor have been produced. This has led to the manufacture of PMZn-GL conductive hydrogels that can be used as wearable flexible sensors for detecting a series of human activities, such as hand and facial movements
[50]. Similarly, the conductive material Ti
3AlC
2 precursor is processed into MXene nanosheets that are mixed at the same time with a solution of PVA and ZnSO
4. The anisotropic PMZn-GL hydrogel obtained presents good tensile properties and conductive faculties and could well be used as a wearable flexible sensor for comprehensive human motion biomonitoring
[51]. There is no doubt that the transistor technology used in bioelectronic interfaces, as well as its high spatial resolution in bioelectronic signal recording, is expanding its unprecedented coverage degree
[52].
A way to make a patch a biomarker for sweat is to incorporate a transducing layer of nanoporous carbon and MXene (NPC@MXene). The composition of the resulting product is composed of a glucose biosensor along with pH and temperature sensors to precisely quantify glucose that, together with biopotential electrodes, is feasible for recording electrophysiological signals in real-time
[53].
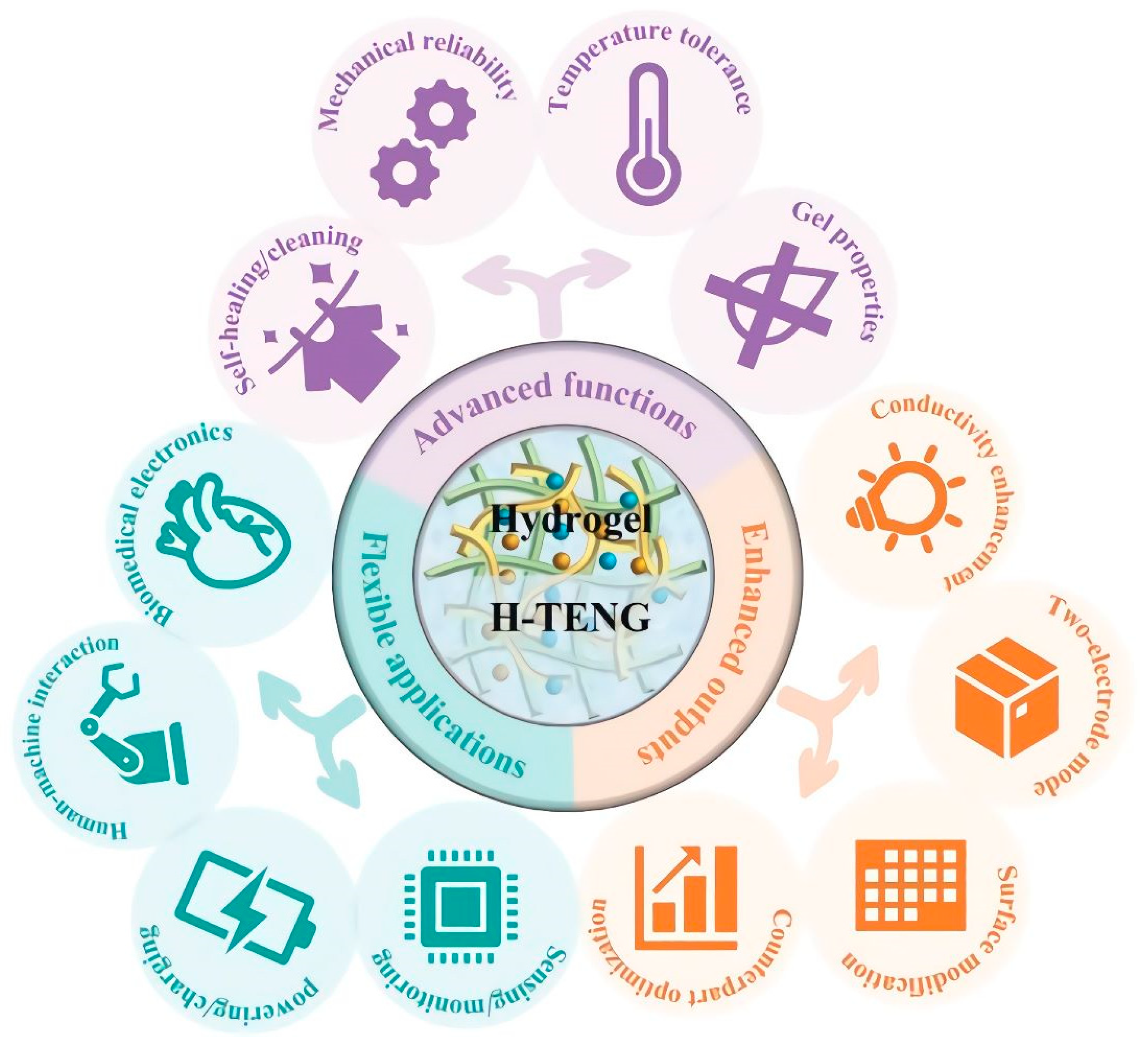
Recently, Wu et al. exposed new developments in hydrogel-based triboelectric nanogenerators (H-TENGs). They explain in a pertinent review the recent progress on the subject of flexible TENGs. The authors emphasize the advanced functions to enhance the outputs and stability of H-TENGs in practical applications, such as biomedical electronics, as represented in
Figure 6 [54].
Figure 6. Development of H-TENGs: advanced functions, enhanced outputs, and flexible and wearable applications. Reprinted from Ref.
[54] with permission.
In a very eloquent work, 1D-fiber-shaped supercapacitor (FSC) structures were designed with cellulose, composed to produce recyclable ionic hydrogels to establish the foundations of a system of a fully sustainable energy storage fibers made from reused/recycled materials for a wearable Internet of Things (IoT). Such hydrogels were produced with aqueous system cellulose and lithium hydroxide (LiOH) in the presence of urea, and, later, a regeneration process was carried out directly in carbon fiber wire electrodes. In this way, a pair of stretch-broken carbon fiber yarns (SBCFYs) were obtained as current collectors to fabricate 1D FSCs. Additionally, a specific capacitance of up to 433.02 μF·cm
−2 at 5 μA·cm
−2 was reported, and, simultaneously, the specific energy density reached a value of 1.73 × 10
−2 μWh·cm
−2. Moreover, the maximum achieved specific power density was 5.33 × 10
−1 mW·cm
−2 at 1 mA·cm
−2 [55].
Recently, great advances in the impact of complex and intelligent machines are more noticeable, especially when it comes to the biomedical engineering sciences. Such is the case for computers, mobile devices, sensors, actuators, and robots. Because of this, unconventional materials have been designed for bridging between humans and machines through hydrogel interfaces in a broad range of applications
[56].
5. Metallo-Supramolecular Hydrogels
One paper demonstrating ion size variation is that presented by Dubey and co-workers, who reported on a series of fluorescent metallohydrogels with attached alkali metal ions that show large conductance and rheological properties consistent with alkali metal ion size variation
[57].
Following this order of ideas, the use of 3,6-bis(2-pyridyl)-1,2,4,5-Tetrazines (bptz) ligands for the synthesis of supramolecular gels based on metal–ligand coordination has been described, showing notable dynamic mechanical properties. These materials have the advantage of being easily functionalized via Diels–Alder reactions; therefore, metallohydrogel scaffolds have been prepared for the release of small molecules activated via photoactivation and enzymatically. The Diels–Alder functionalization of the bptz ligands attached to the ends of the PEG chains is ensured via the gelation induced by metal coordination in the presence of Ni
2+ and Fe
2+ cations
[58].
One aspect of complex peptides is obtained via the coordination of the metal ions that impact their functionality; however, they are also participants in the self-assembly and, at the same time, in the structuring of supramolecular gels. In this context, His–copper (His-Cu) coordination in the supramolecular assembly of gelators is used to improve the understanding and development of these materials. Metal–gel coordination mimics biologically relevant metal–peptide coordination, influencing hydrogel self-assembly and their mechanical properties, biodegradability, biocompatibility, adjustability, and recycling, while metal coordination allows their widespread applications in the biomedical, waste management, and catalysis industries
[59]. This has led to studies reporting a peptide-based simple drug delivery system (DDS) for doxorubicin (DOX) delivery as an anticancer system. This DDS is structured with tripeptides that form a metal–peptide coordination center with Cu(II) to self-assemble and thus generate structures whose morphology allows the controlled and sustained release of DOX from the His residue at the site
[60]. The choice of His to displace drug molecules from the metallopeptide network is because, among the essential amino acids, His can easily bind strongly to the Cu
2+ center. Regarding the above, for example, it is noteworthy that one of the four peripheral ZnII ions is anchored by the Glu and His side chains from one monomer and from another His
[61]. Thus, the Lys amine group completes the tetrahedral coordination geometry. In another study, the kinetic and thermodynamic characteristics of pyridinedicarboxamide (PDCA) complexes with a series of transition metal ions were established, which could provide the basis for the development of new metallo-supramolecular polymeric networks and hydrogels. By means of the condensation of linear PEG segments with the PDCA ligand via urethane bonds and the complexation of PDCA with various metal ions under basic conditions, the rheological peculiarities were investigated
[62].
The strength, stability, and connectivity of coordination bonds can be tuned largely without significant synthetic effort, solely through the simple choice of metal ion. Thus, it has been shown that energy dissipation modes are relevant in metallo-supramolecular systems, even more so by introducing a mixture of metal ions with different complex stabilities
[63]. To a large extent, different binding constants for bis(terpyridine) and Mn(II) and Zn(II) complexes have been demonstrated. Multivalent effects are influenced by increasing the binding affinity
[42]. Through connectivity mismatches, supramolecular hydrogels have been developed by systematically introducing different percentages of connectivity defects into a model supramolecular network, and the resulting macroscopic elastic response via oscillatory shear rheology, microscopic autodiffusivity via fluorescence recovery after photobleaching (FRAP), and characterizing and testing the specific type of defects via double-quantum NMR (DQ-NMR). In the work developed by Nicolella et al., it is highlighted that with connectivity defects, a hydrogel becomes softer and autodiffusivity increases inside
[64].
In order to visualize the modes of energy dissipation, hydrogel models with metal–ligand coordination have been developed with divalent metal ions in the formation of a stable biscomplex, evidencing significant linearity in the energy dissipation. Deviation from linear behavior is to be expected in cases where metal ions and ligands show weak coordination affinity and even more so if metal ions compete to form mono, bis, or tris complexes
[65].
Molecular interactions were observed when producing a metallogel with vanadium pentoxide. The metallogel was obtained by reacting (E)-N’-((2-hydroxynaphthalen-1-yl)methylene)benzohydrazide with vanadium pentoxide in a methanol solution. Surprisingly, a ligand gelation test showed that the ligand could not gel in both polar and nonpolar solvents. However, when the warm DMF solution of the ligand was reacted with the warm vanadium pentoxide solution in DMF/H
2O (2:1
v/
v), spontaneous gelation occurred when cooled to room temperature
[66]. Metallogels show weak C–H---O and N–H---O bonding interactions. In a molecular packing diagram, it is possible to observe that the dioxovanadium (V) molecules are linked by N–H---O intermolecular hydrogen bonds to form a one-dimensional zig-zag linear arrangement.
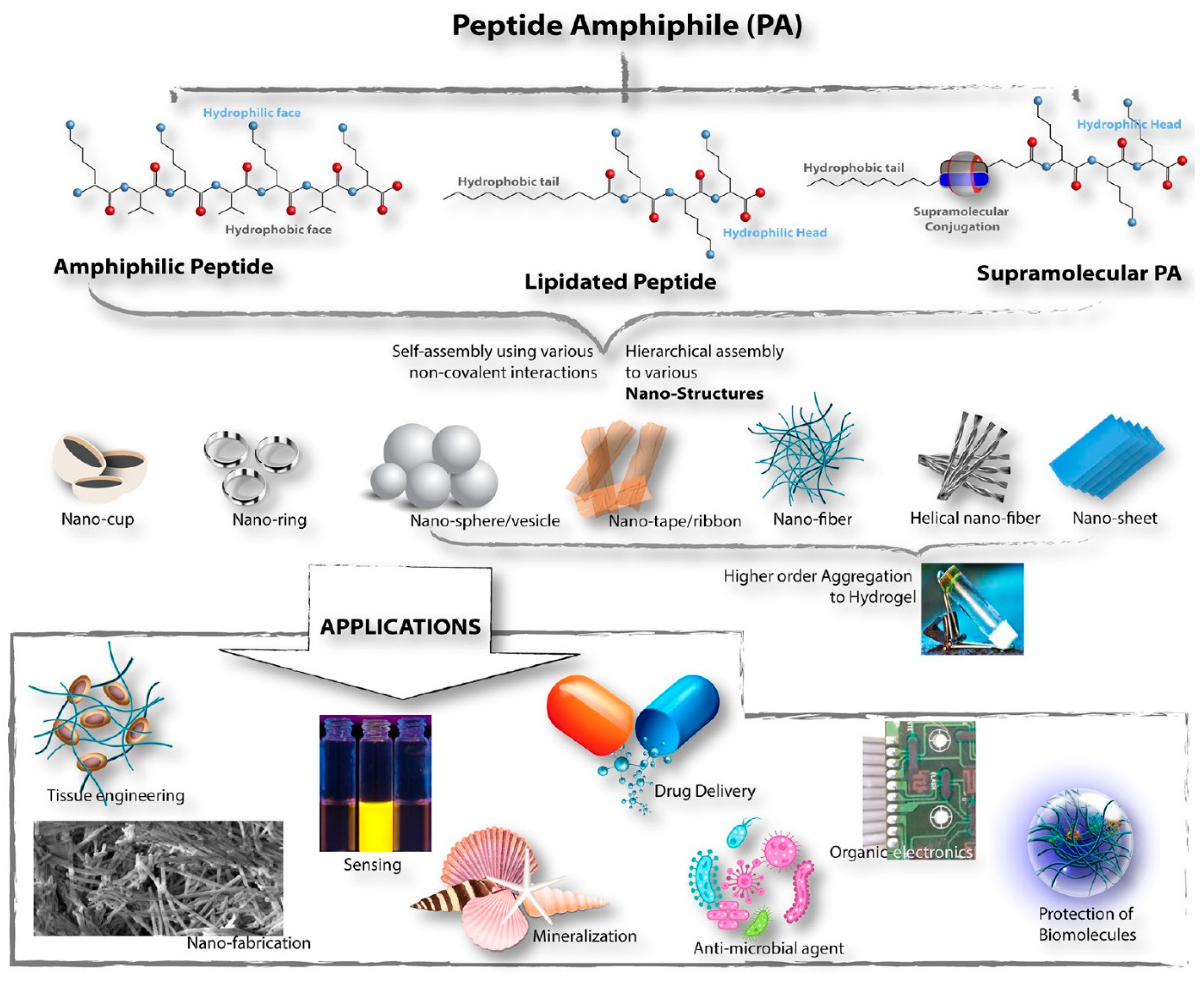
In another case, amphiphilic peptides (APs) were studied for the construction of molecular blocks, especially for the manufacture of supramolecular soft materials with potential for various applications in the fields of science and technology. Especially in recent years, various amphiphilic peptides have been designed and synthesized
[67], which is schematized in
Figure 7. Amphiphilic peptides are divided into two different subclasses, namely, peptides containing alternating hydrophilic/hydrophobic amino acid residues and those containing a long hydrophobic stretch of amino acids attached to a hydrophilic sequence. Obviously, the impacts on the aggregation properties of these designer amphiphiles vary markedly.
Figure 7. Schematic overview of the peptide amphiphile (PA) classification, different self-assembled nanostructures formed, and PA applications
[67].
Metallo-supramolecular polymer networks (MSPNs) are the subject of study in order to detail certain biomimetic functions such as self-healing and stimuli-responsiveness. A good example is the use of His, which is an α-amino acid of great importance in enzyme function. It is found abundantly in the soft, collagen-like cores of the byssus threads of marine mussels. It contains an a-amino group capable of being protonated under biological conditions and a carboxylic acid, in addition to containing an imidazole group. The nitrogen atom of the imidazole group is protonated at a pH of <6 (pKa 6.5), while under neutral and basic conditions, it falls apart, which is a magnificent property. Thus, depending on the pH conditions, His may have the feasibility of forming compounds with various transition metal ions and shaping various coordination geometries.
Metal ions are essential in controlling the structural properties and catalytic functions of many proteins required for proper physiological function. Thus, artificial metalloproteins are built by introducing metallocofactors into a natural protein. Detailed examples of the bioinorganic active sites of cupredoxins, commonly known as blue copper proteins, are presented by Borovik and his team
[68]. The transfer of electrons between the Cu
2+ and Cu
+ redox states causes the active sites of cupredoxins to present a distorted trigonal monopyramidal coordination geometry in the Cu(I) and Cu(II) states. The highly covalent nature of the Cu−S
cys bond in Cys (cysteine) results in charge transfer from the Sπ−Cu ligand to the metal, which is responsible for the characteristic blue color (λ
max ≈ 600 nm) and the small hyperfine coupling constant observed using EPR spectroscopy (A ≈ 180 MHz).