Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luigi Bellocchio | -- | 2221 | 2023-06-16 03:58:18 | | | |
| 2 | Alfred Zheng | Meta information modification | 2221 | 2023-06-16 04:32:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bellocchio, L.; Patano, A.; Inchingolo, A.D.; Inchingolo, F.; Dipalma, G.; Isacco, C.G.; De Ruvo, E.; Rapone, B.; Mancini, A.; Lorusso, F.; et al. Cannabidiol as a Therapeutical Tool in Dentistry. Encyclopedia. Available online: https://encyclopedia.pub/entry/45688 (accessed on 07 February 2026).
Bellocchio L, Patano A, Inchingolo AD, Inchingolo F, Dipalma G, Isacco CG, et al. Cannabidiol as a Therapeutical Tool in Dentistry. Encyclopedia. Available at: https://encyclopedia.pub/entry/45688. Accessed February 07, 2026.
Bellocchio, Luigi, Assunta Patano, Alessio Danilo Inchingolo, Francesco Inchingolo, Gianna Dipalma, Ciro Gargiulo Isacco, Elisabetta De Ruvo, Biagio Rapone, Antonio Mancini, Felice Lorusso, et al. "Cannabidiol as a Therapeutical Tool in Dentistry" Encyclopedia, https://encyclopedia.pub/entry/45688 (accessed February 07, 2026).
Bellocchio, L., Patano, A., Inchingolo, A.D., Inchingolo, F., Dipalma, G., Isacco, C.G., De Ruvo, E., Rapone, B., Mancini, A., Lorusso, F., Scarano, A., Malcangi, G., & Inchingolo, A.M. (2023, June 16). Cannabidiol as a Therapeutical Tool in Dentistry. In Encyclopedia. https://encyclopedia.pub/entry/45688
Bellocchio, Luigi, et al. "Cannabidiol as a Therapeutical Tool in Dentistry." Encyclopedia. Web. 16 June, 2023.
Copy Citation
The medical use of cannabis has a very long history. In the use of cannabinoids, the oral mucosa is the tissue that primarily comes into contact with them and interacts with them. Cannabidiol (CBD) itself is not responsible for the psychotropic effects of cannabis, since it does not produce the typical behavioral effects associated with the consumption of this drug.
cannabinoids
CBD
dental
1. Introduction
The use of Cannabis sativa L. for medical purposes dates back to an Egyptian medical papyrus (circa 1550 BC) [1]. Among the multitude of cannabinoids present in this plant, Δ9tetrahydrocannabinol (Δ9-THC), cannabidiol (CBD) and cannabinol (CBN) are the three main cannabinoids that are the most present and the best described components due also to their significant presence [2][3]. Cannabis is used in three different forms with different THC concentrations: marijuana, hashish, and hash oil [4].
Cannabinoids recognize and bind to specific receptors, the main ones being recognized in the CB1 and CB2 receptors. They are G-protein-coupled. Their polypeptide chain crosses the cell membrane seven times. The amine end remains on the extracellular side, while the carboxyl end remains on the intracellular side. They are characterized by three extracellular loops and three intracellular loops (Figure 1).
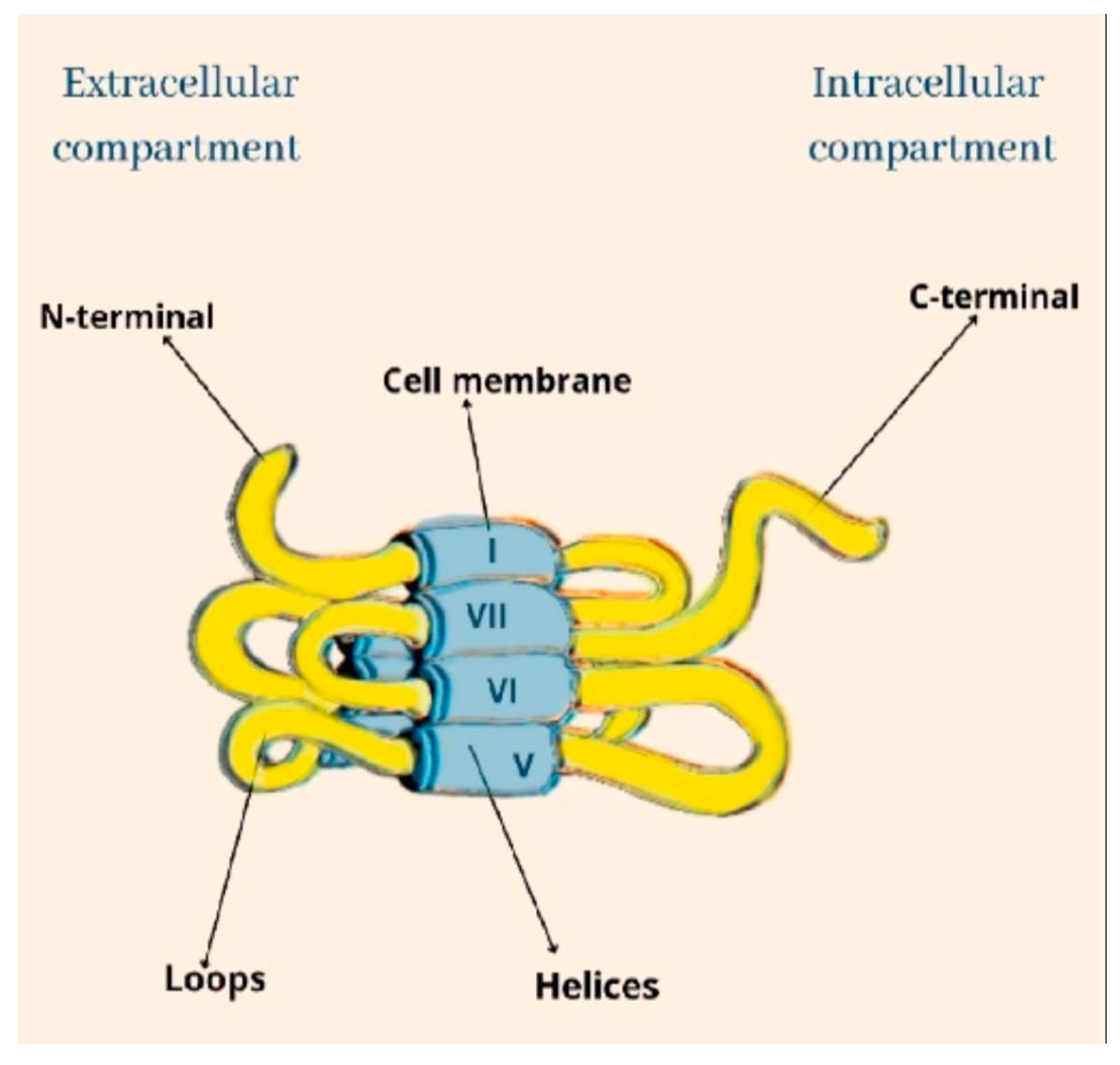
Figure 1. The structural architecture of CB.
The CB1 receptor consists of a longer polypeptide chain than CB2 (472 amino acids in CB1, and 360 amino acids in CB2). The amino-terminal (extracellular) domain of CB2 is shorter. The complete amino acid sequence of the two receptors is homologous in 44% of them, while in the transmembrane domains the sequence is equal in 68% of them [5][6] (Figure 2).
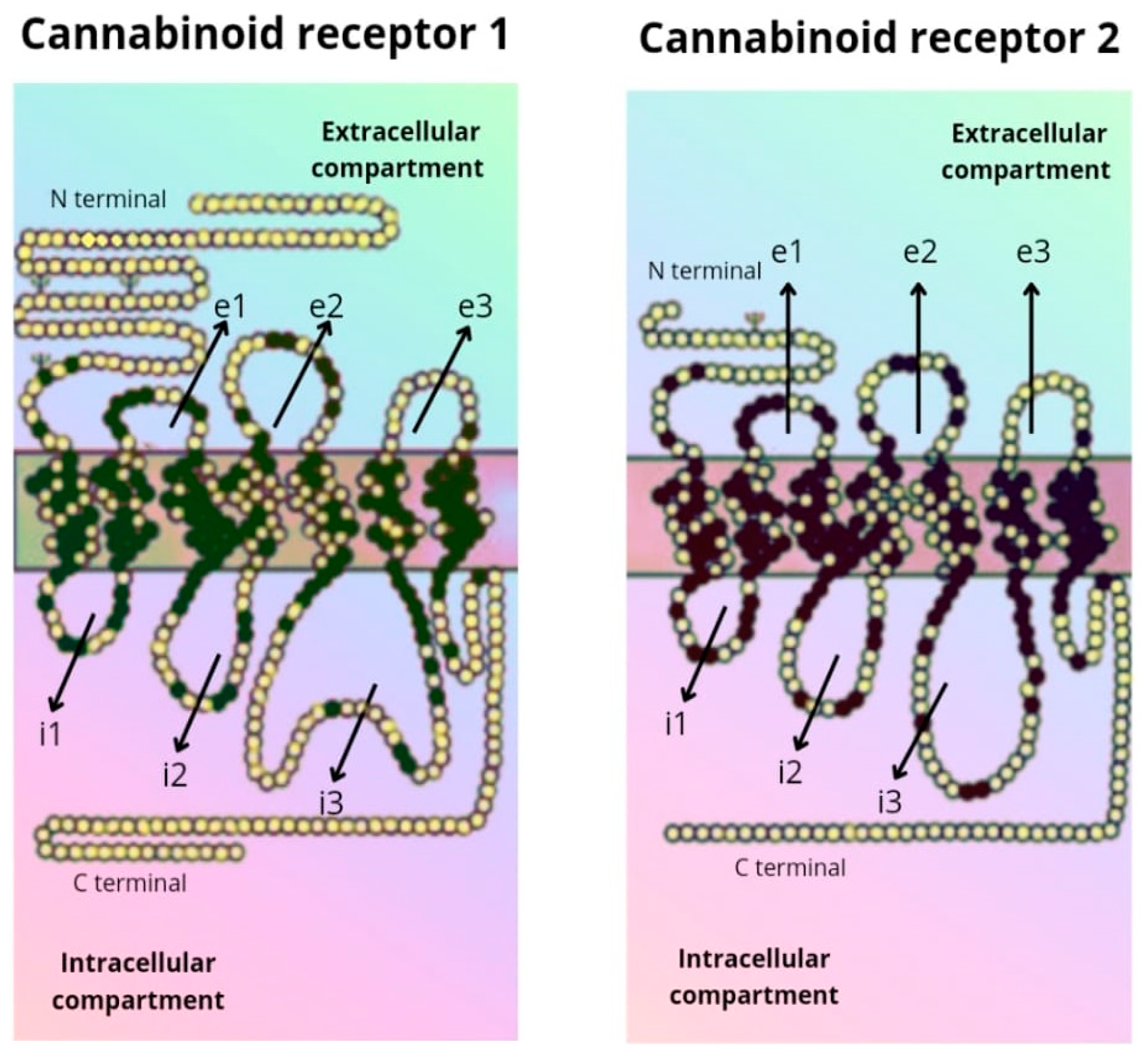
Figure 2. Amino acid sequence of the CB1 and CB2 receptor.
Another receptor belonging to the GPCR family that binds to ECs is the G-protein coupled receptor 55 (GPR55), also known as CB3. It is supposed to modulate memory, motor activity, and cognitive function because of its high expression in the brain, particularly in the cerebellum [7][8]. At the peripheral level, GPR55s, being present in osteoblasts and osteoclasts, would modulate bone metabolism [9].
Widely present in humans is GPR119, which has been shown to represent another cannabinoid receptor that is encoded by the GPR119 gene [8]. It is present predominantly found in pancreatic (beta cells) and gastrointestinal cells. Recent studies attributed to GPR119’s therapeutic effects on diabetes and obesity highlight its direct action on insulin release in the pancreatic cells and indirectly at the level of intestinal enteroendocrine cells on the production of glucagon-like peptide 1 (GLP-1) [10][11].
Δ9-THC is the main psychoactive principle of cannabis and is known as the canonical agonist of both cannabinoid receptors, namely the CB1 and CB2 receptors, but with a relatively higher intrinsic affinity for CB1 than for CB2. THC is a hydrophobic and lipophilic compound [12][13]. Thus, many studies have been performed focusing on the pharmacology, therapeutic potential, and toxicity of Δ9-THC as a classical cannabinoid molecule in the last 70 years. These studies promoted the discovery and characterization of the endocannabinoid system (ECS) [14]. The ECS is made up of G-protein coupled (GPCR) cannabinoid receptors (CB1 and CB2) and their endogenous ligands, anandamide (AEA) and 2-arachidonoylglycerol (2AG), which fall into the category of endocannabinoids (ECs) [14]. In addition to the CB1 and CB2 receptors, the ECS also includes the peroxisome proliferator-activated receptor alpha (PPARα), GPR119, GPR55, and the transient receptor potential vanilloid 1 (TRPV1) receptors [15]. ECs are metabolized by multiple specific and non-specific enzymes. Those in the former category include fatty acid amide hydrolase (FAAH, for metabolism of AEA) and monoacylglycerol lipase (MAGL, for metabolism of 2-AG) [16]. Interestingly, since ECs share many structural similarities with prostaglandins, several interactions have between shown between the metabolic pathways for endocannabinoids and inflammatory lipids, including lipoxygenases and cyclooxygenases [16].
The CB1 and CB2 receptors, encoded by the CNR1 and CNR2 genes, respectively, are the main receptors of ECs. They have different functionalities despite sharing more than 44% of amino acid sequences [17]. CB1 receptors are present in the central nervous system (the cerebellum, cerebral cortex, hippocampus, etc.), and act on cognitive functions, including memory, locomotion, and pain. At the peripheral level, CB1 receptors are present in multiple locations, cardiac cells, lung cells, immune cells, reproductive tissues, gastrointestinal system tissues, in the ganglia of the sympathetic nervous system, in the urinary bladder, and in adrenal gland cells, where their functions have been recognized but not well defined [18][19]. Peripherally, CB2 receptors are localized in monocytes/macrophages and poly-morphonuclear neutrophils, lymphocytes and natural killer cells in the testis, skeleton, liver and spleen. In the central nervous system (CNS), CB2 receptors are localized in the hippocampus and substantia nigra and the neuronal, glial, and endothelial cells of the cortex. At the CNS level, the functions of the CB2 receptor are not yet clear, but it is assumed that it may affect the neuro-immunological system [19] (Figure 3).
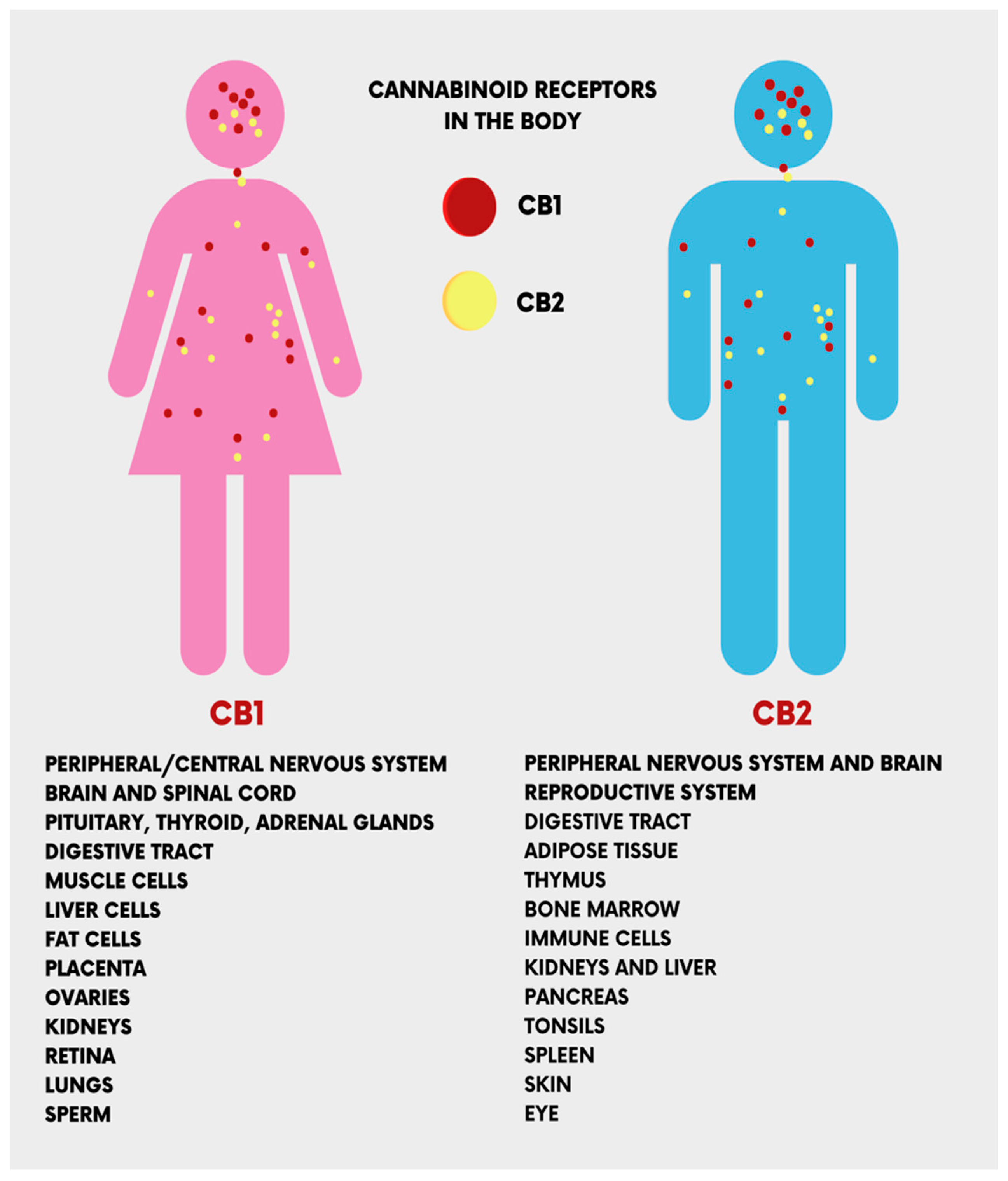
Figure 3. The location of the cannabinoid receptors (CB1 and CB2) in the human body.
CBD has been isolated and described earlier than Δ9-THC [20]. However, it has remained a less studied molecule of cannabis, forgetting its participation in the psychotropic effects of this plant, since CBD use is not associated with the typical behavioral effects of cannabinoids [21]. CBD is a unisomer of THC, and is known to have a better effect on anxiety, cognition, and pain, with little psychoactivity. Compared with THC, CBD has a better affinity for CB1 and CB2 receptors, with the predominance of the latter, and could also interfere with the activity of THC [22][23][24].
Cannabinoids are mainly synthetized as acidic forms (A), thus Δ9-THC(A) and CBD(A) are the end-products of the enzymatic biosynthesis of cannabinoids. When exposed to heat (pyrolysis during smoking or baking), radiation, or spontaneously during storage, the compounds undergo decarboxylation and ‘spontaneous rearrangement’ reactions [25]. C. sativa accumulates THC and CBD in glandular trichomes in the aerial parts of the plant, but not on the root surface. Upon trichome thermal or mechanical ruptures, its contents form a sticky coating on the plant surface due to the viscous, non-crystallizing properties of cannabinoids, which will protect the plant from desiccation and/or potential herbivores [26]. The amount of cannabinoids formed in the trichomes correlates positively with increased temperatures and imposed heat stress, as well as with low soil moisture and poor mineral nutrient content [27]. Cannabinoid production may also provide an evolutionary advantage by functioning as sunscreens that absorb biologically destructive UV-B radiation (280–315 nm), as significantly increased cannabinoid production was measured in cannabis flowers after UV-B-induced stress [28]. Furthermore, cannabinoids in general [29], and CBD in particular [30], have a significant antimicrobial action, which confers high climate resistance and soil adaptability to cannabis. Thus, phytocannabinoids convey various biologically beneficial properties for the plant.
The use of CBD has always represented a complicated legal issue worldwide, and this has often restricted the scientific studies and professional awareness about its therapeutic applications. Apart from this, numerous individual findings suggest some therapeutic effects of CBD, which have been reported to include antipsychotic, anticonvulsant, neuroprotective, anxiolytic, and sleep-promoting effects [31]. Furthermore, pre-clinical and clinical studies attributed a desirable safety profile to CBD [31], associated with its anti-inflammatory effects [32]. However, elucidating the pharmacodynamics of CBD has always proven to be difficult for scientists, beginning with initial reports in which CBD was shown to weakly bind to cannabinoid receptor orthosteric sites when compared to canonical agonists [33], indicating that CBD’s effects might be independent of the cannabinoid receptors. This conclusion was proved to be partially true by other studies, where researchers found a direct interaction of CBD with several receptors, enzymes and ion channels. Recently, however, some reports found both a direct and an indirect modulation of ECS activity from CBD [33]. Taken together, these findings point out CBD as a novel promising phytocannabinoid-based medicine. Indeed, the therapeutic uses of CBD are mostly linked to its anti-inflammatory, antioxidant, and analgesic properties [34]. Thus, CBD is endowed with many potential applications, such as in bone tissue processes [35][36], neuroprotection, epilepsy, anxiety, and cancer [37]. CBD also has some other effects that have not been fully studied, including relaxation, improved sleep, and stress relief, given edible, tincture, and vape formulations of the drug (Figure 4).
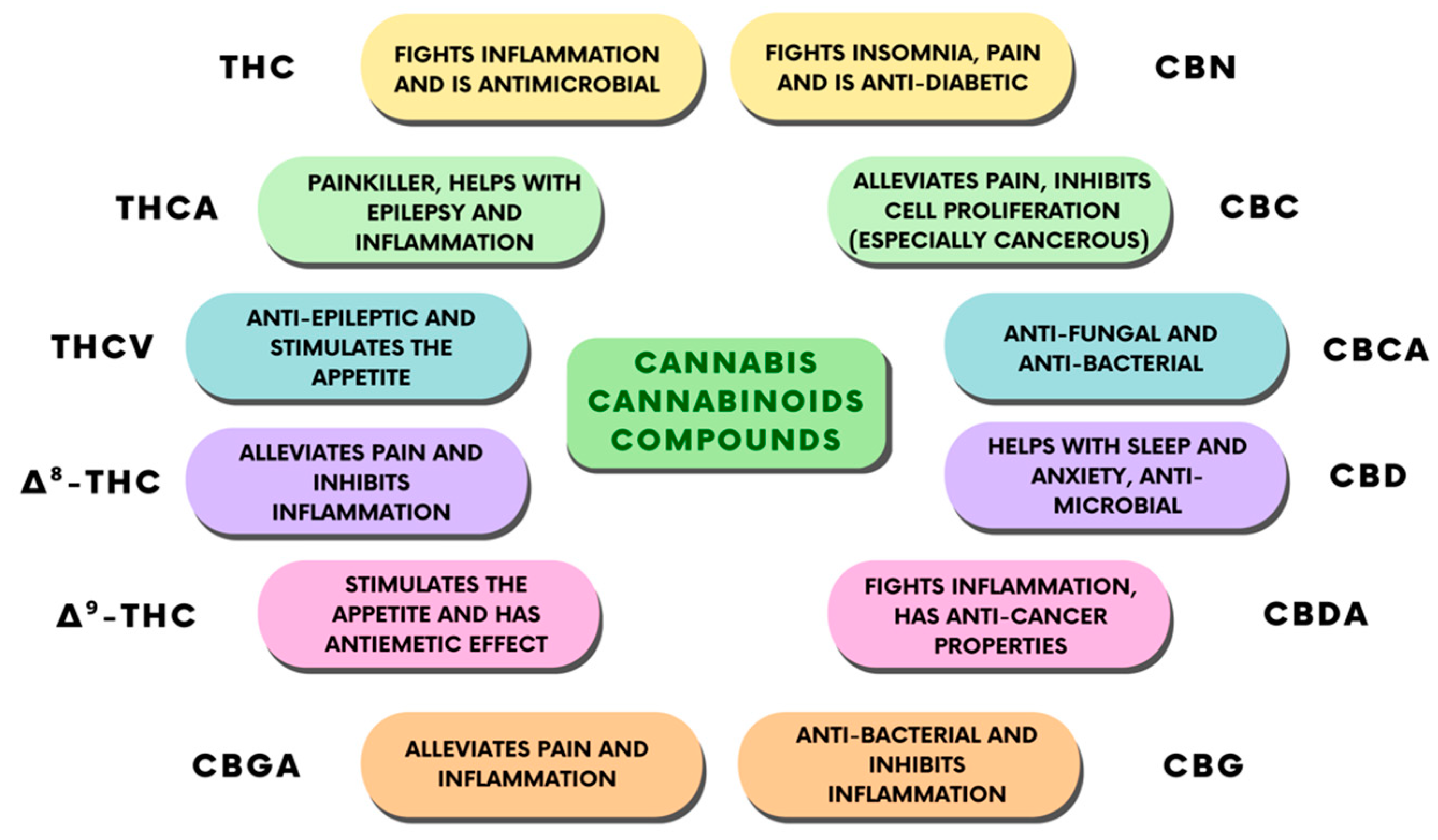
Figure 4. Classification of the different cannabinoid compounds derived from cannabis and their therapeutic properties.
In recent years, thanks to various public and private institutions, much research and development of CBD have been performed, especially with regard to its therapeutic uses. Indeed, approximately USD 30 bilions is expected to be reached by the CBD market in 2025 [38]. Amongst the various potential uses of cannabinoids [39], and CBD in particular [40], dentistry and oral medicine have recently attracted greater attention [15]. In particular, studies have been exploring the possible medical applications of CBD use in the oral cavity [41], together with the functional and anatomical characterization of the ECS in this part of the body [15][41][42][43], in addition to its modulation by pathological status [15][44].
2. CBD in Dentistry
2.1. Oral Mucosa
In the use of cannabinoids, the oral mucosa is the tissue that primarily comes into contact with them and interacts with them. Studying their physiological, therapeutic and non-therapeutic role in more detail and evaluating their effects is of significant importance.
CB1 and CB2 receptors have been detected on oral mucosal epithelial cells. They modulate their functions: CB2 receptors stimulate the proliferation and differentiation of human epithelial keratinocytes, while CB1 receptors have the opposite effect [18].
The cannabinoid receptors, CB1 and CB2, have also been identified at the level of the connective tissue of the lamina propria. However, the current scientific data regarding cannabinoids on receptors in oral mucosal tissues are still scarce [45]. Interestingly, CB1 and CB2 receptors are also present in the epithelial cells and taste buds of the tongue, where their function would appear to be regulated by the physiological-pathological conditions of the tongue [45]. One example is the presence of burning mouth syndrome, which is associated with a decrease of CB1 receptor expression, while the CB2 receptor expression increases. Furthermore, oncological conditions, such as mobile tongue squamous cell carcinoma, seems to involve the activation of ECS, since the expression of both CB1 and CB2 receptors has been shown to be increased [46][47]. There is little data on the presence of CB1 and CB2 receptors in dental pulp, where CB1 receptors have been detected in the sympathetic nerve fibers and on the surface of the pulp, bordering dentin [48]. This would suggest a possible therapeutic target against dental pain, although this possible role of cannabinoids requires further study. CB1s are also present on human odontoblasts, where they are hypothesized to respond to immune challenges [49][50]. Indeed, their activation and subsequent cyclic adenosine mono-phosphate (cAMP) signaling enables the TRPV1 mediated extracellular Ca(2+)ion passage (TRPV1) via extrusion Na(+)-Ca(2+) exchangers (NCXs), promoting the production of a secondary dentin bridge in response to odontoblast stimuli [51]. In the salivary glands, CB1 and CB2 receptors have specific localizations. In the major salivary glands, CB1 expression is found at the striatal duct cells, while CB2 is found in the acinar cells, especially in the myoepithelial cells, which are responsible for the secretion of saliva [52][53]. Interestingly, the presence and distribution of CB1s in salivary glands would appear to be regulated by the type and amount of food [54] and, furthermore, salivary secretions are modulated by both CB1 and CB2 receptors [55][56][57].
2.2. Periodontal Tissue
The CB1 and CB2 receptors are expressed in the periodontium, and their distribution changes based on periodontal tissue conditions [42]. In a healthy periodontium, CB1s are more highly expressed in the periodontal ligament (PDL), and are more active in the epithelium than in the PDL [58][59]. Interestingly, the presence of bacteria increases the expression of CB2 receptors, whereas in a situation of sterile inflammation, both receptors are more highly expressed in PDL, but not in cementum and alveolar bone [60]. Thus, different expression patterns of the two receptors would appear to be related to different cellular activity (differentiation and proliferation), the control of inflammation, and the healing of the affected site [61]. Several reports seem to suggest a role for CB2 in periodontal tissue healing, especially in terms of modulating the migration and adhesion of periodontal cells upon input from the focal adhesion kinase (FAK) and mitogen-activated protein kinase (MAPK) systems [59][61][62][63].
Consistent beneficial effects of CBD have been described in vitro and in vivo, in addition to conventional periodontal therapy. Using the CBD analog HU-308, the authors found a role for the CB2 receptor in modulating the extent of periodontal damage and its impact on the gingival tissue, alveolar bone, and salivary function [64]. In the same study, CBD demonstrated anti-inflammatory and anti-bone resorption properties by inhibiting the RANK/RANKL system and reducing the levels of pro-inflammatory cytokines [64]. An alternative approach to periodontal therapy might be the CBD-mediated activation of gingival fibroblasts with repairing growth factors and/or the inhibition of metalloproteinases [65][66]. CBD has also been observed to attenuate bacterial inflammatory periodontal diseases thanks to its antimicrobial properties [67][68]. This drug might also be a suitable medicinal alternative in oral mucositis, given its anti-inflammatory properties, which can reduce the severity and extent of lesions [36][69] since CBD also promotes the curative process of common ulcers. Similarly to synthetic oral medicines, CBD is effective in reducing the bacterial charge in dental plaque [68]. Furthermore, CBD is endowed with biocompatibility and osteoinductivity [61][70] as it has been shown to promote fracture healing, possibly activating the p42/44 pathway in mesenchymal cells, which then differentiate into osteoblasts at the lesion site [71]. Thus, analgesic, anti-inflammatory, biological, antimicrobial, and osteoinductive properties of CBD might underlie its positive effects in dentistry, as suggested by most of the recent literature. This has paved the way for the development of patents for the implementing of CBD formulations in dentistry.
References
- Russo, E.B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4, 1614–1648.
- Ladha, K.S.; Ajrawat, P.; Yang, Y.; Clarke, H. Understanding the Medical Chemistry of the Cannabis Plant Is Critical to Guiding Real World Clinical Evidence. Molecules 2020, 25, 4042.
- Lowe, H.; Steele, B.; Bryant, J.; Toyang, N.; Ngwa, W. Non-Cannabinoid Metabolites of Cannabis Sativa L. with Therapeutic Potential. Plants 2021, 10, 400.
- Joshi, S.; Ashley, M. Cannabis: A Joint Problem for Patients and the Dental Profession. Br. Dent. J. 2016, 220, 597–601.
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–762.e14.
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.-H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467.e13.
- Moriconi, A.; Cerbara, I.; Maccarrone, M.; Topai, A. GPR55: Current Knowledge and Future Perspectives of a Purported “Type-3” Cannabinoid Receptor. Curr. Med. Chem. 2010, 17, 1411–1429.
- Godlewski, G.; Offertáler, L.; Wagner, J.A.; Kunos, G. Receptors for Acylethanolamides—GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009, 89, 105–111.
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The Putative Cannabinoid Receptor GPR55 Affects Osteoclast Function in Vitro and Bone Mass in Vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516.
- Overton, H.A.; Fyfe, M.C.T.; Reynet, C. GPR119, a Novel G Protein-Coupled Receptor Target for the Treatment of Type 2 Diabetes and Obesity. Br. J. Pharmacol. 2008, 153, S76–S81.
- Chu, Z.-L.; Jones, R.M.; He, H.; Carroll, C.; Gutierrez, V.; Lucman, A.; Moloney, M.; Gao, H.; Mondala, H.; Bagnol, D.; et al. A Role for β-Cell-Expressed G Protein-Coupled Receptor 119 in Glycemic Control by Enhancing Glucose-Dependent Insulin Release. Endocrinology 2007, 148, 2601–2609.
- Howlett, A.C. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev. 2002, 54, 161–202.
- Garrett, E.R.; Hunt, C.A. Physicochemical Properties, Solubility, and Protein Binding of Δ9-Tetrahydrocannabinol. J. Pharm. Sci. 1974, 63, 1056–1064.
- Piomelli, D. The Molecular Logic of Endocannabinoid Signalling. Nat. Rev. Neurosci. 2003, 4, 873–884.
- Bellocchio, L.; Inchingolo, A.D.; Inchingolo, A.M.; Lorusso, F.; Malcangi, G.; Santacroce, L.; Scarano, A.; Bordea, I.R.; Hazballa, D.; D’Oria, M.T.; et al. Cannabinoids Drugs and Oral Health—From Recreational Side-Effects to Medicinal Purposes: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8329.
- Toczek, M.; Malinowska, B. Enhanced Endocannabinoid Tone as a Potential Target of Pharmacotherapy. Life Sci. 2018, 204, 20–45.
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the Pharmacology and Signal Transduction of the Human Cannabinoid CB1 and CB2 Receptors. Mol. Pharmacol. 1995, 48, 443–450.
- Maccarrone, M.; Di Rienzo, M.; Battista, N.; Gasperi, V.; Guerrieri, P.; Rossi, A.; Finazzi-Agrò, A. The Endocannabinoid System in Human Keratinocytes. J. Biol. Chem. 2003, 278, 33896–33903.
- Howlett, A.C.; Abood, M.E. CB1 and CB2 Receptor Pharmacology. Adv. Pharmacol. 2017, 80, 169–206.
- Adams, R.; Hunt, M.; Clark, J.H. Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200.
- Mechoulam, R.; Shani, A.; Edery, H.; Grunfeld, Y. Chemical Basis of Hashish Activity. Science 1970, 169, 611–612.
- Hall, W.; Degenhardt, L. Adverse Health Effects of Non-Medical Cannabis Use. Lancet 2009, 374, 1383–1391.
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early Phytocannabinoid Chemistry to Endocannabinoids and Beyond. Nat. Rev. Neurosci. 2014, 15, 757–764.
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the Art and New Challenges for Therapeutic Applications. Pharmacol. Ther. 2017, 175, 133–150.
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The Biosynthesis of the Cannabinoids. J. Cannabis Res. 2021, 3, 7.
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004.
- Bazzaz, F.A.; Dusek, D.; Seigler, D.S.; Haney, A.W. Photosynthesis and Cannabinoid Content of Temperate and Tropical Populations of Cannabis Sativa. Biochem. Syst. Ecol. 1975, 3, 15–18.
- Eichhorn Bilodeau, S.; Wu, B.-S.; Rufyikiri, A.-S.; MacPherson, S.; Lefsrud, M. An Update on Plant Photobiology and Implications for Cannabis Production. Front. Plant Sci. 2019, 10, 296.
- van Klingeren, B.; ten Ham, M. Antibacterial Activity of Δ9-Tetrahydrocannabinol and Cannabidiol. Antonie Van Leeuwenhoek 1976, 42, 9–12.
- Schofs, L.; Sparo, M.D.; Sánchez Bruni, S.F. The Antimicrobial Effect behind Cannabis Sativa. Pharmacol. Res. Perspect. 2021, 9, e00761.
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154.
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21.
- Tham, M.; Yilmaz, O.; Alaverdashvili, M.; Kelly, M.E.M.; Denovan-Wright, E.M.; Laprairie, R.B. Allosteric and Orthosteric Pharmacology of Cannabidiol and Cannabidiol-Dimethylheptyl at the Type 1 and Type 2 Cannabinoid Receptors. Br. J. Pharmacol. 2019, 176, 1455–1469.
- Burstein, S. Cannabidiol (CBD) and Its Analogs: A Review of Their Effects on Inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385.
- Apostu, D.; Lucaciu, O.; Mester, A.; Benea, H.; Oltean-Dan, D.; Onisor, F.; Baciut, M.; Bran, S. Cannabinoids and Bone Regeneration. Drug Metab. Rev. 2019, 51, 65–75.
- Whyte, L.; Ford, L.; Ridge, S.; Cameron, G.; Rogers, M.; Ross, R. Cannabinoids and Bone: Endocannabinoids Modulate Human Osteoclast Function in Vitro. Br. J. Pharmacol. 2012, 165, 2584–2597.
- Sholler, D.J.; Schoene, L.; Spindle, T.R. Therapeutic Efficacy of Cannabidiol (CBD): A Review of the Evidence From Clinical Trials and Human Laboratory Studies. Curr. Addict. Rep. 2020, 7, 405–412.
- Technavio CBD Oil Market by Product and Geography—Forecast and Analysis 2022–2026. Available online: https://www.technavio.com/report/cbd-oil-market-industry-analysis (accessed on 1 May 2023).
- Abidi, A.H.; Alghamdi, S.S.; Derefinko, K. A Critical Review of Cannabis in Medicine and Dentistry: A Look Back and the Path Forward. Clin. Exp. Dent. Res. 2022, 8, 613–631.
- David, C.; Elizalde-Hernández, A.; Barboza, A.; Cardoso, G.; Santos, M.; Moraes, R. Cannabidiol in Dentistry: A Scoping Review. Dent. J. 2022, 10, 193.
- Cuba, L.d.F.; Salum, F.G.; Guimarães, F.S.; Cherubini, K.; Borghetti, R.L.; Figueiredo, M.A.Z. Cannabidiol on 5-FU-induced Oral Mucositis in Mice. Oral Dis. 2020, 26, 1483–1493.
- Konermann, A.; Jäger, A.; Held, S.A.E.; Brossart, P.; Schmöle, A. In Vivo and In Vitro Identification of Endocannabinoid Signaling in Periodontal Tissues and Their Potential Role in Local Pathophysiology. Cell. Mol. Neurobiol. 2017, 37, 1511–1520.
- Miyashita, K.; Oyama, T.; Sakuta, T.; Tokuda, M.; Torii, M. Anandamide Induces Matrix Metalloproteinase-2 Production through Cannabinoid-1 Receptor and Transient Receptor Potential Vanilloid-1 in Human Dental Pulp Cells in Culture. J. Endod. 2012, 38, 786–790.
- Ataei, A.; Rahim Rezaee, S.A.; Moeintaghavi, A.; Ghanbari, H.; Azizi, M. Evaluation of Cannabinoid Receptors Type 1–2 in Periodontitis Patients. Clin. Exp. Dent. Res. 2022, 8, 1040–1044.
- Borsani, E.; Majorana, A.; Cocchi, M.A.; Conti, G.; Bonadeo, S.; Padovani, A.; Lauria, G.; Bardellini, E.; Rezzani, R.; Rodella, L.F. Epithelial Expression of Vanilloid and Cannabinoid Receptors: A Potential Role in Burning Mouth Syndrome Pathogenesis. Histol. Histopathol. 2014, 29, 523–533.
- Theocharis, S.; Giaginis, C.; Alexandrou, P.; Rodriguez, J.; Tasoulas, J.; Danas, E.; Patsouris, E.; Klijanienko, J. Evaluation of Cannabinoid CB1 and CB2 Receptors Expression in Mobile Tongue Squamous Cell Carcinoma: Associations with Clinicopathological Parameters and Patients’ Survival. Tumor Biol. 2016, 37, 3647–3656.
- Rosenblatt, K.A.; Daling, J.R.; Chen, C.; Sherman, K.J.; Schwartz, S.M. Marijuana Use and Risk of Oral Squamous Cell Carcinoma. Cancer Res. 2004, 64, 4049–4054.
- Beneng, K.; Renton, T.; Yilmaz, Z.; Yiangou, Y.; Anand, P. Cannabinoid Receptor CB1-Immunoreactive Nerve Fibres in Painful and Non-Painful Human Tooth Pulp. J. Clin. Neurosci. 2010, 17, 1476–1479.
- Que, K.; He, D.; Jin, Y.; Wu, L.; Wang, F.; Zhao, Z.; Yang, J.; Deng, J. Expression of Cannabinoid Type 1 Receptors in Human Odontoblast Cells. J. Endod. 2017, 43, 283–288.
- Navarro-Saiz, L.; Bernal-Cepeda, L.; Castellanos, J. Immune Challenges Upregulate the Expression of Cannabinoid Receptors in Cultured Human Odontoblasts and Gingival Fibroblasts. Acta Odontológica Latinoam. 2022, 35, 80–89.
- Tsumura, M.; Sobhan, U.; Muramatsu, T.; Sato, M.; Ichikawa, H.; Sahara, Y.; Tazaki, M.; Shibukawa, Y. TRPV1-Mediated Calcium Signal Couples with Cannabinoid Receptors and Sodium–Calcium Exchangers in Rat Odontoblasts. Cell Calcium 2012, 52, 124–136.
- Thoungseabyoun, W.; Tachow, A.; Pakkarato, S.; Rawangwong, A.; Krongyut, S.; Sakaew, W.; Kondo, H.; Hipkaeo, W. Immunohistochemical Localization of Cannabinoid Receptor 1 (CB1) in the Submandibular Gland of Mice under Normal Conditions and When Stimulated by Isoproterenol or Carbachol. Arch. Oral Biol. 2017, 81, 160–166.
- Pirino, C.; Cappai, M.G.; Maranesi, M.; Tomassoni, D.; Giontella, A.; Pinna, W.; Boiti, C.; Kamphues, J.; Dall’Aglio, C. The Presence and Distribution of Cannabinoid Type 1 and 2 Receptors in the Mandibular Gland: The Influence of Different Physical Forms of Diets on Their Expression in Piglets. J. Anim. Physiol. Anim. Nutr. 2018, 102, e870–e876.
- Yoshida, R.; Ohkuri, T.; Jyotaki, M.; Yasuo, T.; Horio, N.; Yasumatsu, K.; Sanematsu, K.; Shigemura, N.; Yamamoto, T.; Margolskee, R.F.; et al. Endocannabinoids Selectively Enhance Sweet Taste. Proc. Natl. Acad. Sci. USA 2010, 107, 935–939.
- Prestifilippo, J.P.; Fernandez-Solari, J.; Medina, V.; Rettori, V.; Elverdin, J.C. Role of the Endocannabinoid System in Ethanol-Induced Inhibition of Salivary Secretion. Alcohol Alcohol. 2009, 44, 443–448.
- Prestifilippo, J.P.; Fernández-Solari, J.; de la Cal, C.; Iribarne, M.; Suburo, A.M.; Rettori, V.; McCann, S.M.; Elverdin, J.C. Inhibition of Salivary Secretion by Activation of Cannabinoid Receptors. Exp. Biol. Med. 2006, 231, 1421–1429.
- Andreis, K.; Billingsley, J.; Naimi Shirazi, K.; Wager-Miller, J.; Johnson, C.; Bradshaw, H.; Straiker, A. Cannabinoid CB1 Receptors Regulate Salivation. Sci. Rep. 2022, 12, 14182.
- Jirasek, P.; Jusku, A.; Simanek, V.; Frankova, J.; Storch, J.; Vacek, J. Cannabidiol and Periodontal Inflammatory Disease: A Critical Assessment. Biomed. Pap. 2022, 166, 155–160.
- Liu, C.; Qi, X.; Alhabeil, J.; Lu, H.; Zhou, Z. Activation of Cannabinoid Receptors Promote Periodontal Cell Adhesion and Migration. J. Clin. Periodontol. 2019, 46, 1264–1272.
- Raphael-Mizrahi, B.; Gabet, Y. The Cannabinoids Effect on Bone Formation and Bone Healing. Curr. Osteoporos. Rep. 2020, 18, 433–438.
- Kozono, S.; Matsuyama, T.; Biwasa, K.K.; Kawahara, K.; Nakajima, Y.; Yoshimoto, T.; Yonamine, Y.; Kadomatsu, H.; Tancharoen, S.; Hashiguchi, T.; et al. Involvement of the Endocannabinoid System in Periodontal Healing. Biochem. Biophys. Res. Commun. 2010, 394, 928–933.
- Wang, J.G.; Miyazu, M.; Xiang, P.; Li, S.N.; Sokabe, M.; Naruse, K. Stretch-Induced Cell Proliferation Is Mediated by FAK-MAPK Pathway. Life Sci. 2005, 76, 2817–2825.
- Lanza Cariccio, V.; Scionti, D.; Raffa, A.; Iori, R.; Pollastro, F.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Treatment of Periodontal Ligament Stem Cells with MOR and CBD Promotes Cell Survival and Neuronal Differentiation via the PI3K/Akt/MTOR Pathway. Int. J. Mol. Sci. 2018, 19, 2341.
- Ossola, C.A.; Surkin, P.N.; Mohn, C.E.; Elverdin, J.C.; Fernández-Solari, J. Anti-Inflammatory and Osteoprotective Effects of Cannabinoid-2 Receptor Agonist HU-308 in a Rat Model of Lipopolysaccharide-Induced Periodontitis. J. Periodontol. 2016, 87, 725–734.
- Rawal, S.Y.; Dabbous, M.K.; Tipton, D.A. Effect of Cannabidiol on Human Gingival Fibroblast Extracellular Matrix Metabolism: MMP Production and Activity, and Production of Fibronectin and Transforming Growth Factor β. J. Periodontal Res. 2012, 47, 320–329.
- Napimoga, M.H.; Benatti, B.B.; Lima, F.O.; Alves, P.M.; Campos, A.C.; Pena-dos-Santos, D.R.; Severino, F.P.; Cunha, F.Q.; Guimarães, F.S. Cannabidiol Decreases Bone Resorption by Inhibiting RANK/RANKL Expression and pro-Inflammatory Cytokines during Experimental Periodontitis in Rats. Int. Immunopharmacol. 2009, 9, 216–222.
- Gu, Z.; Singh, S.; Niyogi, R.G.; Lamont, G.J.; Wang, H.; Lamont, R.J.; Scott, D.A. Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Front. Immunol. 2019, 10, 2288.
- Stahl, V.; Vasudevan, K. Comparison of Efficacy of Cannabinoids versus Commercial Oral Care Products in Reducing Bacterial Content from Dental Plaque: A Preliminary Observation. Cureus 2020, 12, e6809.
- Klein, M.; de Quadros De Bortolli, J.; Guimarães, F.S.; Salum, F.G.; Cherubini, K.; de Figueiredo, M.A.Z. Effects of Cannabidiol, a Cannabis Sativa Constituent, on Oral Wound Healing Process in Rats: Clinical and Histological Evaluation. Phyther. Res. 2018, 32, 2275–2281.
- Nakajima, Y.; Furuichi, Y.; Biswas, K.K.; Hashiguchi, T.; Kawahara, K.; Yamaji, K.; Uchimura, T.; Izumi, Y.; Maruyama, I. Endocannabinoid, Anandamide in Gingival Tissue Regulates the Periodontal Inflammation through NF-ΚB Pathway Inhibition. FEBS Lett. 2006, 580, 613–619.
- Petrescu, N.B.; Jurj, A.; Sorițău, O.; Lucaciu, O.P.; Dirzu, N.; Raduly, L.; Berindan-Neagoe, I.; Cenariu, M.; Boșca, B.A.; Campian, R.S.; et al. Cannabidiol and Vitamin D3 Impact on Osteogenic Differentiation of Human Dental Mesenchymal Stem Cells. Medicina 2020, 56, 607.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
781
Revisions:
2 times
(View History)
Update Date:
16 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




