Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marwan Habiba | -- | 1773 | 2023-06-15 23:47:57 | | | |
| 2 | Jessie Wu | Meta information modification | 1773 | 2023-06-16 05:24:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Habiba, M.; Benagiano, G.; Guo, S. Pathogenesis of Adenomyosis and Endometriosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/45686 (accessed on 07 February 2026).
Habiba M, Benagiano G, Guo S. Pathogenesis of Adenomyosis and Endometriosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/45686. Accessed February 07, 2026.
Habiba, Marwan, Giuseppe Benagiano, Sun-Wei Guo. "Pathogenesis of Adenomyosis and Endometriosis" Encyclopedia, https://encyclopedia.pub/entry/45686 (accessed February 07, 2026).
Habiba, M., Benagiano, G., & Guo, S. (2023, June 15). Pathogenesis of Adenomyosis and Endometriosis. In Encyclopedia. https://encyclopedia.pub/entry/45686
Habiba, Marwan, et al. "Pathogenesis of Adenomyosis and Endometriosis." Encyclopedia. Web. 15 June, 2023.
Copy Citation
Both endometriosis and adenomyosis are often referred to as “enigmatic diseases” since understanding their pathogenesis remains elusive. Both endometriosis and adenomyosis are relatively common among women of reproductive age and are leading causes of pelvic pain, infertility, and menstrual disorders. They impact negatively on patients’ quality of life, productivity, and well-being. Endometriosis, and perhaps adenomyosis as well, entails a heavy economic burden to society as a whole.
adenomyosis
archimetra
archimetrosis
archimetrium
endometriosis
pathogenesis
tissue injury and repair
1. Structural Aspects
There is evidence that the myometrium is not uniform. The orientation of the muscle fibers in the uterus has been reported to vary from the innermost layers, where the muscle direction is mostly circular, to the interconnected crisscross middle and main layer of the uterus. Leyendecker and co-workers referred to the main component (the middle layer) layer as the stratum vasculare [1]. This separates the subserosa (the outermost layer) or the stratum supravasculare from the innermost layer, which they referred to as the stratum subvasculare. However, the boundaries between these layers are not defined histologically [1]. Structural studies have shown that the transition in the myometrial layers is gradual, with no discernable histological demarcation between the inner and outer zones [2]. As an example, the concept of the junctional zone that designates the endometrium–myometrium interface (EMI) is based on MR imaging [3], not on histologically identifiable features. The classic studies of uterine vasculature, such as those reported by Sampson, show that the rich vascular network of anastomosis that contains the arcuate arteries lies between the outer and middle third of the myometrium [4][5][6]. Blood vessels run medially from that network to supply the myometrium and terminate by supplying the endometrium. Based on its vasculature, Sampson divided the myometrium into three zones: the peripheral or outer third, which is supplied by the peripheral arteries; the arcuate zone, which is the narrow area containing the main vessels; the radial zone, which corresponds to the inner two-thirds of the myometrium, supplied by the radial vessels. This vascular distribution is at variance with the description of the stratum vasculare as forming the main bulk of the myometrium.
Leyendecker et al. [7] proposed to designate as “archimetra” the endometrial–subendometrial region together with the main bulk of the muscle layer (the stratum subvasculare) and the term “neometra” to be applied to the outer layers of the myometrium. They argued that only the archimetra is of paramesonephric origin and that the neometra is of non-Müllerian origin. However, a different embryonic origin is difficult to substantiate. It is commonly accepted that the mesonephric (Wolffian) duct first develops from the intermediate mesoderm and is critical to the development of the paramesonephric (Müllerian) duct [8][9]. Epithelial cells of the Müllerian duct develop adjacent to the rostral mesonephric epithelium as invaginations of the coelomic epithelium [10]. The mesenchyme that surrounds the Müllerian duct epithelium is derived from mesonephric (Wolffian) mesenchyme and coelomic epithelial cells localized along the length of the mesonephros [11][12]. Thus, the myometrial layers in the context of “archimetra” and “neometra”, as depicted by Leyendecker et al. [1], are at direct odds with that depicted by Sampson [5].
There is also evidence that the inner myometrium can develop from endometrial stroma and of the potential existence of smooth muscle metaplasia, i.e., endometrial stromal cells can be coaxed to transdifferentiate to smooth muscle cells [13][14][15]. These findings suggest that the whole myometrium shares a common embryonic origin. It is also well-recognized that adenomyosis is not confined to the inner or to the mid-myometrial layers. Both observations challenge the view that endometriosis and adenomyosis are diseases of “archimetra”.
The archimetra theory is partly based on the work of Werth and Grusdew [16], who reported on the features of uterine development from the fetal stage to maturity. This research included five samples from the end of the third month to the fifth month of gestation. This publication, however, does not contain any claims as to the mesonephric/paramesonephric origin of the myometrium. Interestingly, the authors observed that comparative studies with other species (which they referred to as “genetic studies”, employing the terminology used at the time) add little, if any, to our understanding of the mature human uterus [16].
Two additional publications adopted the notion that adenomyosis and endometriosis are “diseases of archimetra” [17][18]. However, neither of these studies added any original information to support the archimetra hypothesis beyond the TIAR theory.
It has been recently suggested that the stratum vasculare in humans constitutes the main musculature of the uterus and that its (hyper)-contraction manifests as primary dysmenorrhea and leads to uterine injury and also the expulsion of the basal endometrium and the development of endometriosis [19]. In support of this hypothesis, it was proposed that the human uterine structure is unique [19]. However, there are considerable similarities between the structure of the myometrium in the mouse and the human [20]. While it is plausible that the mesh-like structure of the middle layer of the human myometrium evolved secondarily to the fusion between the Müllerian ducts, recent research using three-dimensional reconstruction of the mouse uterus identified a middle myometrial layer that connects the inner circular and the outer longitudinal muscle layers in the bicornuate uterus [21]. This finding is critical as it demonstrates that the three-layer myometrium is not unique to Haplorrhines (one of the two suborders of primates, which includes monkeys, apes, and humans) but that it is also shared with some Euarchontoglires (a superorder of mammals, also called “supraprimates”, that includes rodents. See Figure 1). This raises doubt about the proposed notion that the human myometrial structure played a unique role in primate evolution [19].
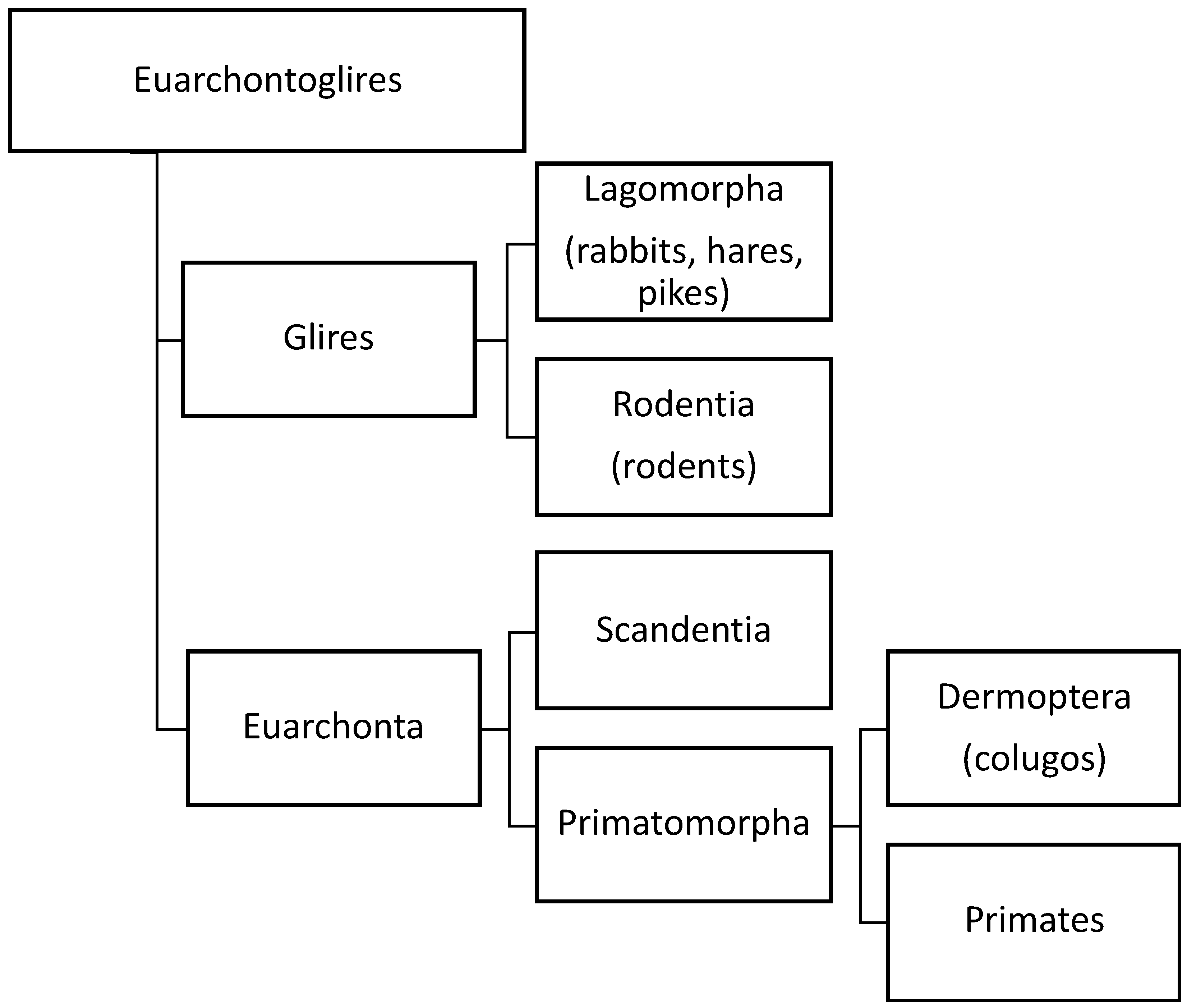
Figure 1. Orders of different species based on our current knowledge of evolution of species. Three-layer myometrium is not unique to Haplorrhines (one of the two suborders of primates, which includes monkeys, apes, and humans), but it is also shared with other Euarchontoglires (a superorder of mammals, also called “supraprimates”, that includes rodents. Adapted from Wikipedia (https://en.wikipedia.org/wiki/Euarchontoglires, accessed on 10 January 2023).
On the other hand, it is not clear whether there is a causal link between myometrial structure and menstruation, and there is no direct information available to compare the myometrium in the majority of mice species, which do not menstruate and the Egyptian spiny mouse (Acomys cahirinus), the only mouse species known to menstruate spontaneously. Thus, the claim that the human uterine structure is unique needs to be substantiated, particularly given that adenomyosis does occur spontaneously in the animal kingdom [22]. Furthermore, endometriosis also occurs spontaneously in primates, such as baboons [23] and cynomolgus monkeys [24], as well as in mice with oncogenic mutations [25]. In other words, the putative uniqueness cannot be used as a justification for the hypothesis.
2. The Archimetra Theory
An important facet of the “archimetra theory” is that adenomyosis and endometriosis are two phenotypes of the same disease. The idea is certainly not new, and, at one time, it was proposed by researcher's group [26][27] based on the observation that the two diseases are characterized by dysfunction in both the eutopic and heterotopic endometrium. A recent review [28] collated the similarities, as well as differences, between the endometrium in adenomyosis and endometriosis. It is also recognized that the two conditions often coexist.
In this regard, it should be borne in mind that, even if proven, a common origin does not equate with endometriosis and adenomyosis, representing the same disease. The commonality of structure also calls into question the value of the term archimetrosis. Adenomyosis and endometriosis differ in their gross, histological, clinical manifestations, and risk factors, including patient age, parity, and the history of iatrogenic uterine procedures in adenomyosis [29][30][31][32][33][34]. This suggests that, at the very least, the triggering events for the two diseases are likely to be different.
3. The Tissue Injury and Repair Theory
An essential component of the unifying theory of adenomyosis and endometriosis [35][36] involves differences in local production of estrogens in the eutopic and ectopic endometrium in affected women.
The theory seems to be based largely on the premise that endometriosis and adenomyosis are caused by trauma due to chronic uterine peristaltic activity (or to phases of hyperperistalsis) and that this induces micro-traumatization at the EMI activating TIAR, resulting in an increased local production of estrogen, which propagates the cycle causing disease. The theory also attempts to account for the absence of endometriosis against the universal phenomenon of retrograde menstruation. One proposition is that endometriosis occurs in women with hyperperistalsis, which leads to the dislocation of basal endometrium and propagation of a cycle of TIAR [37].
The ability to heal after the injury is fundamental to the survival of all organisms and involves an evolutionarily conserved mechanism of tissue regeneration and repair. Regeneration entails the replacement of damaged tissue through the proliferation of surrounding undamaged tissue. Repair entails the formation of granulation tissue and its maturation in the form of scarring. The endometrium has a unique ability to regenerate after menstrual desquamation without progressive scarring, but this does not seem to be shared by the myometrium, which heals by fibrosis [38][39].
Over the years, the mechanism of TIAR has been gradually advanced (see [19][35][36]) to account for both endometriosis and adenomyosis. The theory is built on the occurrence of “auto traumatization” secondary to uterine hyperperistalsis and that this is self-perpetuating secondary to the release of local estrogen and relevant cytokines. The trigger of hyperperistalsis is said to reside in an increased or prolonged estradiol stimulation leading to prolonged supraphysiological mechanical strain on the cells near the fundo-cornual raphe. The theory postulates that such increased estrogen production is caused by prolonged follicular phase, anovulatory cycles, follicular persistency, or the presence of large antral follicles. While there is a plausible relation between endometriosis and adenomyosis and systemic or local estrogen production, surprisingly, there is no documented evidence for the occurrence of uterine (endometrial or myometrial) trauma or for the complete detachment of the endometrium. An important missing link is the demonstration of the time sequence of TIAR; in other words, it has not been shown that the presence of different stages of TIAR, including hemostasis, inflammation, proliferation, and remodeling, can precede the implantation of ectopic endometrium, or exist in its absence. The extent of endometrial shedding at menstruation may vary, and there is no convincing evidence that this varies in women with or without endometriosis [40][41][42].
At the core of the TIAR theory is a local, injury-induced overproduction of estrogen [19][35][36]. Unfortunately, most of the arguments presented to support the theory [19][35][36] are based on the feed-forward loop model that is true for ectopic endometrium [43], not myometrium or normal endometrium, as elaborated previously [44]. Of course, injury involving the vasculature may lead to estrogen production due to platelet activation [45][46], but whether hyperperistalsis can lead to such injury is unclear. Overall, there is no supportive experimental evidence.
References
- Noe, M.; Kunz, G.; Herbertz, M.; Mall, G.; Leyendecker, G. The cyclic pattern of the immunocytochemical expression of oestrogen and progesterone receptors in human myometrial and endometrial layers: Characterization of the endometrial-subendometrial unit. Hum. Reprod. 1999, 14, 190–197.
- Mehasseb, M.K.; Bell, S.C.; Brown, L.; Pringle, J.H.; Habiba, M. Phenotypic characterisation of the inner and outer myometrium in normal and adenomyotic uteri. Gynecol. Obstet. Investig. 2011, 71, 217–224.
- Hricak, H.; Alpers, C.; Crooks, L.E.; Sheldon, P.E. Magnetic resonance imaging of the female pelvis: Initial experience. AJR Am. J. Roentgenol. 1983, 141, 1119–1128.
- Sampson, J.A. The blood supply of uterine myomata. Surg. Gynecol. Obstet. 1912, 14, 215–234.
- Sampson, J.A. The influence of myomata on the blood supply of the uterus, with special reference to abnormal uterine bleeding. Surg. Gynecol. Obstet. 1913, 16, 144–180.
- Yovich, J.L.; Rowlands, P.K.; Lingham, S.; Sillender, M.; Srinivasan, S. Pathogenesis of endometriosis: Look no further than John Sampson. Reprod. Biomed. Online 2020, 40, 7–11.
- Leyendecker, G.; Kunz, G.; Noe, M.; Herbertz, M.; Mall, G. Endometriosis: A dysfunction and disease of the archimetra. Hum. Reprod. Update 1998, 4, 752–762.
- Müller, J. Bildungsgeschichte der Genitalien aus Anatomischen Untersuchungen an Embryonen des Menschen und der Thiere ; Bei Arnz: Düsseldorf, Germany, 1830.
- Gruenwald, P. Zur Entwicklungsmechanik des Urogenital Systems beim Huhn . Arch. Entw-Mech. 1937, 136, 786–813.
- Gruenwald, P. The relation of the growing Müllerian duct to the Wolffian duct and its importance for the genesis of malformations. Anat. Rec. 1941, 81, 1–19.
- Guioli, S.; Sekido, R.; Lovell-Badge, R. The origin of the Mullerian duct in chick and mouse. Dev. Biol. 2007, 302, 389–398.
- Zhan, Y.; Fujino, A.; MacLaughlin, D.T.; Manganaro, T.F.; Szotek, P.P.; Arango, N.A.; Teixeira, J.; Donahoe, P.K. Mullerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Mullerian duct regression. Development 2006, 133, 2359–2369.
- Zhang, Q.; Duan, J.; Liu, X.; Guo, S.W. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol. Cell. Endocrinol. 2016, 428, 1–16.
- Fujii, S.; Konishi, I.; Mori, T. Smooth muscle differentiation at endometrio-myometrial junction. An ultrastructural study. Virchows Arch. A Pathol. Anat. Histopathol. 1989, 414, 105–112.
- Konishi, I.; Fujii, S.; Okamura, H.; Mori, T. Development of smooth muscle in the human fetal uterus: An ultrastructural study. J. Anat. 1984, 139 Pt 2, 239–252.
- Werth, R.; Grusdew, W. Untersuchungen über die Entwicklung und Morphologie der menschlichen Uterusmuskulatur . Arch. Gynakol. 1898, 55, 325–413.
- Nalbanski, A.B. Endometriosis—A disease of the archimetra? Akush Ginekol. 2004, 43, 44–49.
- Maruyama, S.; Imanaka, S.; Nagayasu, M.; Kimura, M.; Kobayashi, H. Relationship between adenomyosis and endometriosis; Different phenotypes of a single disease? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 191–197.
- Leyendecker, G.; Wildt, L.; Laschke, M.W.; Mall, G. Archimetrosis: The evolution of a disease and its extant presentation: Pathogenesis and pathophysiology of archimetrosis (uterine adenomyosis and endometriosis). Arch. Gynecol. Obstet. 2023, 307, 93–112.
- Brody, J.R.; Cunha, G.R. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development. Am. J. Anat. 1989, 186, 1–20.
- Kagami, K.; Ono, M.; Iizuka, T.; Matsumoto, T.; Hosono, T.; Sekizuka-Kagami, N.; Shinmyo, Y.; Kawasaki, H.; Fujiwara, H. A novel third mesh-like myometrial layer connects the longitudinal and circular muscle fibers -A potential stratum to coordinate uterine contractions. Sci. Rep. 2020, 10, 8274.
- Wang, X.; Benagiano, G.; Liu, X.; Guo, S.-W. Unveiling the pathogenesis of adenomyosis through animal models. J. Clin. Med. 2022, 11, 1744.
- Merrill, J.A. Spontaneous endometriosis in the Kenya baboon (Papio doguera). Am. J. Obstet. Gynecol. 1968, 101, 569–570.
- Nishimoto-Kakiuchi, A.; Netsu, S.; Okabayashi, S.; Taniguchi, K.; Tanimura, H.; Kato, A.; Suzuki, M.; Sankai, T.; Konno, R. Spontaneous endometriosis in cynomolgus monkeys as a clinically relevant experimental model. Hum. Reprod. 2018, 33, 1228–1236.
- Dinulescu, D.M.; Ince, T.A.; Quade, B.J.; Shafer, S.A.; Crowley, D.; Jacks, T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat. Med. 2005, 11, 63–70.
- Benagiano, G.; Brosens, I. Adenomyosis and Endometriosis have a common origin. J. Gynaecol. Obstet. India 2011, 61, 146–153.
- Brosens, I.; Kunz, G.; Benagiano, G. Is adenomyosis the neglected phenotype of an endomyometrial dysfunction syndrome? Gynecol. Surg. 2012, 9, 131–137.
- Benagiano, G.; Brosens, I.; Habiba, M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum. Reprod. Update 2014, 20, 386–402.
- Curtis, K.M.; Hillis, S.D.; Marchbanks, P.A.; Peterson, H.B. Disruption of the endometrial-myometrial border during pregnancy as a risk factor for adenomyosis. Am. J. Obstet. Gynecol. 2002, 187, 543–544.
- Levgur, M.; Abadi, M.A.; Tucker, A. Adenomyosis: Symptoms, histology, and pregnancy terminations. Obstet. Gynecol. 2000, 95, 688–691.
- Panganamamula, U.R.; Harmanli, O.H.; Isik-Akbay, E.F.; Grotegut, C.A.; Dandolu, V.; Gaughan, J.P. Is prior uterine surgery a risk factor for adenomyosis? Obstet. Gynecol. 2004, 104, 1034–1038.
- Parazzini, F.; Vercellini, P.; Panazza, S.; Chatenoud, L.; Oldani, S.; Crosignani, P.G. Risk factors for adenomyosis. Hum. Reprod. 1997, 12, 1275–1279.
- Parazzini, F.; Mais, V.; Cipriani, S.; Busacca, M.; Venturini, P.; GISE. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: Results from a prospective multicentric study in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 143, 103–106.
- Taran, F.A.; Weaver, A.L.; Coddington, C.C.; Stewart, E.A. Understanding adenomyosis: A case control study. Fertil. Steril. 2010, 94, 1223–1228.
- Leyendecker, G.; Wildt, L. A new concept of endometriosis and adenomyosis: Tissue injury and repair (TIAR). Horm. Mol. Biol. Clin. Investig. 2011, 5, 125–142.
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obstet. 2009, 280, 529–538.
- Leyendecker, G.; Herbertz, M.; Kunz, G.; Mall, G. Endometriosis results from the dislocation of basal endometrium. Hum. Reprod. 2002, 17, 2725–2736.
- Salamonsen, L.A. Tissue injury and repair in the female human reproductive tract. Reproduction 2003, 125, 301–311.
- Eremichev, R.; Kulebyakina, M.; Alexandrushkina, N.; Nimiritsky, P.; Basalova, N.; Grigorieva, O.; Egiazaryan, M.; Dyikanov, D.; Tkachuk, V.; Makarevich, P. Scar-Free Healing of Endometrium: Tissue-Specific Program of Stromal Cells and Its Induction by Soluble Factors Produced After Damage. Front. Cell. Dev. Biol. 2021, 9, 616893.
- Maybin, J.A.; Critchley, H.O. Menstrual physiology: Implications for endometrial pathology and beyond. Hum. Reprod. Update 2015, 21, 748–761.
- Salamonsen, L.A.; Hutchison, J.C.; Gargett, C.E. Cyclical endometrial repair and regeneration. Development 2021, 148, dev199577.
- Tempest, N.; Hill, C.J.; Maclean, A.; Marston, K.; Powell, S.G.; Al-Lamee, H.; Hapangama, D.K. Novel microarchitecture of human endometrial glands: Implications in endometrial regeneration and pathologies. Hum. Reprod. Update 2022, 28, 153–171.
- Bulun, S.E.; Lin, Z.; Imir, G.; Amin, S.; Demura, M.; Yilmaz, B.; Martin, R.; Utsunomiya, H.; Thung, S.; Gurates, B.; et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: From bench to treatment. Pharmacol. Rev. 2005, 57, 359–383.
- Guo, S.W. The Pathogenesis of Adenomyosis vis-a-vis Endometriosis. J. Clin. Med. 2020, 9, 485.
- Qi, Q.; Guo, S.-W.; Liu, X. Activated Platelets Induce Hypoxia-Inducible Factor-1α Expression Likely through Transforming Growth Factor-β1 in Human Endometrial Stromal Cells. Reprod. Dev. Med. 2019, 3, 69.
- Qi, Q.; Liu, X.; Zhang, Q.; Guo, S.-W. Platelets induce increased estrogen production through NF-κB and TGF-β1 signaling pathways in endometriotic stromal cells. Sci. Rep. 2019, 10, 1281.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
16 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




