Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leslie Claire Licari | -- | 1796 | 2023-06-15 20:07:01 | | | |
| 2 | Fanny Huang | Meta information modification | 1796 | 2023-06-19 08:05:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Licari, L.C.; Bologna, E.; Proietti, F.; Flammia, R.S.; Bove, A.M.; D’annunzio, S.; Tuderti, G.; Leonardo, C. Near-Infrared Fluorescent-Guided Robot-Assisted Renal Surgery. Encyclopedia. Available online: https://encyclopedia.pub/entry/45678 (accessed on 24 January 2026).
Licari LC, Bologna E, Proietti F, Flammia RS, Bove AM, D’annunzio S, et al. Near-Infrared Fluorescent-Guided Robot-Assisted Renal Surgery. Encyclopedia. Available at: https://encyclopedia.pub/entry/45678. Accessed January 24, 2026.
Licari, Leslie Claire, Eugenio Bologna, Flavia Proietti, Rocco Simone Flammia, Alfredo Maria Bove, Simone D’annunzio, Gabriele Tuderti, Costantino Leonardo. "Near-Infrared Fluorescent-Guided Robot-Assisted Renal Surgery" Encyclopedia, https://encyclopedia.pub/entry/45678 (accessed January 24, 2026).
Licari, L.C., Bologna, E., Proietti, F., Flammia, R.S., Bove, A.M., D’annunzio, S., Tuderti, G., & Leonardo, C. (2023, June 15). Near-Infrared Fluorescent-Guided Robot-Assisted Renal Surgery. In Encyclopedia. https://encyclopedia.pub/entry/45678
Licari, Leslie Claire, et al. "Near-Infrared Fluorescent-Guided Robot-Assisted Renal Surgery." Encyclopedia. Web. 15 June, 2023.
Copy Citation
The use of Indocyanine Green in nephron-sparing robotic surgery has gained widespread recognition as a versatile, valuable, and safe technique. With its support in delineating tumor margins and vascular anatomy, in guiding selective or super selective clamping and in assessing kidney perfusion after resection and renorrhaphy, Indocyanine Green (ICG) offers real-time guidance to promote the better preservation of renal function.
indocyanine green
ICG
NIRF
near-infrared fluorescent
robotic surgery
1. Introduction
Over the years, technology has significantly changed surgical procedures.
Fluorescence-guided surgery (FGS) was first developed in 1947 with the introduction of fluorescein, a near-infrared fluorescent (NIRF) dye, for brain tumor resections [1]. Since then, many fluorescence-based techniques and fluorescent probes have been developed and used in different clinical scenarios [2]. Offering real-time feedback and supporting surgeons’ intraoperative decision-making, FGS has consolidated its role in changing surgical procedures.
In the past two decades, robot-assisted surgery (RAS) has revolutionized medicine prospects, allowing greater surgical precision along with a magnified view of tissues and anatomical structures [3][4][5].
Despite different clinical and surgical impacts, RAS and FGS, during their development, shared the same goals: improving surgeons’ accuracy, encouraging new surgical applications, and providing better possibilities in patients’ treatment. Since 2010, fluorescence imaging has been integrated into the Da Vinci® Robotic System. The Firefly® technology allows surgeons to switch vision modality from normal light to near-infrared light at any time during procedures, combining their respective benefits and improving the surgical experience.
Robotic surgery and fluorescence applications perfectly match the extreme heterogeneity offered by urological surgery.
2. Indocyanine Green Overview
Indocyanine Green (ICG) is a water-soluble fluorophore widely used in clinical research since its approval for intravenous (i.v.) administration by the FDA in 1956 [6]. Its green fluorescence, emitted when excited by near-infrared light, can be detected using dedicated optical systems without affecting the surgical field view [7][8][9].
After i.v. administration, ICG rapidly binds to serum proteins, confining it to the vascular compartments [10].
Characterized by a plasma life of 3–5 min and exclusive biliary excretion within 10–15 min, ICG shows a high-safety index and nonradioactive properties [11].
The recommended safe dose for a standard diagnostic procedure is 0.1–0.5 mg/kg [12]. If injected directly into tissues, ICG migrates in lymphatic vessels and in lymph nodes, where it deposits into macrophages [13] and can provide information about organs’ lymphatic drainage.
Its properties make it useful for the visualization of vascular anatomy, the assessment of tissue perfusion, lesion “tattooing” [14] and lymph node road mapping [15]. Urological surgery is one area where ICG’s versatility and safety profile have made it increasingly popular.
3. NIRF-Guided Robot-Assisted Renal Surgery
The use of Indocyanine Green in nephron-sparing robotic surgery has gained widespread recognition as a versatile, valuable, and safe technique since its introduction about 15 years ago.
With its support in delineating tumor margins and vascular anatomy, in guiding selective or super selective clamping and in assessing kidney perfusion after resection and renorrhaphy, ICG offers real-time guidance to promote the better preservation of renal function (Table 1).
Table 1. Summary of indocyanine green (ICG) applications in robotic renal surgery.
| Robotic Procedure |
Purpose | Potential Pros | Potential Limitations |
ICG Administration |
|---|---|---|---|---|
| Partial Nephrectomy | Differential fluorescence to assess tumor margins [16][17][18][19][20][21] |
Real-time guidance Maximal preservation of renal parenchyma |
Doses of ICG outside an optimal range result in decreased contrast between the lesion and surrounding renal parenchyma Limited tissue penetration |
Intravenous injection prior to arterial clamping |
| Perfusion assessment for selective arterial clamping or test clamping of main artery [22][23][24][25][26][27][28][29][30][31] |
Useful in cases of challenging vascular anatomy or impaired renal function Monitoring segmental perfusion deficits after clamping |
Limited assessment of deep devascularization | Intravenous injection after arterial clamping |
|
| Assess kidney perfusion after resection and renorrhaphy [22][24][29][30][32] |
Checking residual parenchyma blood supply and confirming absence of ischemic injury to healthy parenchyma | Lack of data about specific decision making and clinical impact | Intravenous injection after reperfusion | |
| Intraoperative identification and anatomical dissection of total endophytic renal masses [14][32][33][34] |
Real-time guidance May improve preoperative resection strategy and intraoperative mass identification May promote nephron-sparing surgery |
Needs preoperative renal mass marking No free dye application No benefit in case of avascular renal masses |
Preoperative superselective catheterization of tertiary arterial branches feeding the tumor by interventional uroradiologist +\− embolization | |
| Renal Transplant | Assessment of graft perfusion before and after transplant [35][36] |
Depicting graft microcirculation Evaluating ureteral reperfusion Useful for complex vasculature reconstruction |
Preliminary experience No long-term outcomes evaluation |
Intravenous injection Renal artery injection before the implantation |
3.1. ICG-Guided Renal Mass Differential Fluorescence
The idea behind the use of ICG as a “tumor marker” in kidney cancer surgery is based on experimental evidence. Cortical tumors show a downregulation of bilitranslocase, a carrier protein that allows ICG uptake into the cells [37], resulting in a fluorescence-based visual differentiation from the normal surrounding parenchyma.
Tobis et al. were the first, in 2011 [16], to evaluate the additional value offered by ICG-fluorescence in 11 cases of robot-assisted partial nephrectomy (RAPN). The goal was to utilize the i.v. injection of ICG to differentiate between normal parenchyma and malignant tissue and to highlight the renal vasculature. ICG-guided imaging was deemed potentially useful in eight patients for outlining the resection margin, and in all patients for vessel identification.
The following year, the same group speculated on the potential role of ICG in distinguishing between benign lesions (iso- or hyperfluorescent) and malignant lesions (hypofluorescent) [17], but the results were not supported by subsequent studies [18][19].
What emerged as a possible watershed for the effectiveness of this technique was the ICG dosage. ICG under-dosing “tones down” the normal parenchyma fluorescence, leading to uncertainty in distinguishing between tumor and healthy tissue. On the other hand, ICG overdosing “tones up” all the renal parenchyma, leading to an opposite situation with the same results. Surgeons who have abandoned the use of NIRF or have reported a lack of effectiveness for differential fluorescence and visual margin assessment have probably used excessively high doses of ICG [20].
In 2013, Angell et al. [21] reported a reproducible scheme to achieve a correct ICG dose. They described a first dosage as a test, followed by a second dosage—calibrated on the first one—to achieve the right contrast, reporting a successfully differential fluorescence in 65 out of 79 tumors.
Similar results came from a more recent prospectively collected database of 325 patients who underwent ICG-RAPN [20]. Using the same concept—an initial low-test dose and an optional second dose immediately prior to vessels clamping—the authors reported an overall success in differentiation rate of 87.3% and an extremely low positive surgical margin rate (0.30%).
3.2. ICG-Guided Renal Selective Perfusion Assessment
To reduce ischemic injury during partial nephrectomy, Gill et al. [38] introduced in 2011 the concept of “zero-ischemia”. An anatomic microdissection of the renal vessels allows a selective or super selective clamping of the tumor-feeding artery, avoiding global ischemia.
The angiographic properties of ICG showed to be very useful in this setting; ICG imaging can confirm tumor devascularization and normal kidney perfusion and can even identify missed arterial branches if the expected regional perfusion deficit is not reached. This allows surgeons to adjust clamping, reduce bleeding and improve tumor excision quality.
Many studies indeed reported that “zero-ischemia” RAPN with NIRF was a safe alternative to conventional on-clamp RAPN, and may improve functional short-term outcomes [22][23][24][25][26]. A statistically significant benefit of a eGFR variation at discharge was reported, supporting the ICG-zero ischemia approach (ΔeGFR ≈ 15%) [22][23][26][27].
A subsequent meta-analysis [28] revealed no significant difference between on-clamp and selective clamping techniques in terms of complications, positive surgical margins (PSM), operative time and estimated blood loss.
In 2020, in a large multi-institutional study of 737 patients, Diana et al. [29] tried to define the role of fluorescence-guided surgery during RAPN. They performed a subgroup analysis comparing ICG-RAPN with standard RAPN. According to the authors’ suggestions, ICG showed a potential advantage for challenging vascular anatomy—such as large tumors with more complex and accessory vascularization and horseshoe, solitary or pelvic kidney—or impaired renal function. However, despite a correlation between ICG and trifecta achievement, the study described no clear functional advantages.
In 2022, Yang et al. [30] described a short-term advantage in preserving eGFR in the ICG-RAPN group, which decreases over time (3 months vs. 6 months). The authors suggested that the standard-RAPN group may be more susceptible to acute tubular necrosis due to increased renal ischemia. This results in an initially poorer eGFR preservation, which gradually rose during the recovery phase. Gradual compensation by the normal contralateral kidney may also minimize the long-term advantage of the ICG-group.
This finding was recently confirmed by the EMERALD randomized single-blind trial [31]. The study was interrupted after 30 cases; at 6 months follow-up, the interim analysis showed no benefit in the ICG super selective RAPN approach evaluated as a combination of eGFR and relative renal function on 99mTc-DMSA scintigraphy (−21.4% vs. −23.4%, p = 0.66). This lack of difference persisted even after adjusting for the percentage of kidney volume preserved—an independent predictor of functional preservation. This trial raises further questions regarding the usefulness of this technique that underlies an intrinsic increased technical risk of vascular damage.
In any case, other studies like the one conducted in 2023 by De Backer [39] highlight how ICG can be used to support the development of new technologies in the field of nephron sparing surgery. Their study utilized ICG administration as a tool to validate their perfusion zone algorithm integrated into a 3D model for planning super selective clamping.
3.3. ICG-Guided Management of Endophytic Renal Tumors
Endophytic renal masses represent a surgical challenge during nephron-sparing surgery due to technical difficulties and a higher risk of complications [40] that potentially narrows the indication for conservative surgery [41]. The standard intravenous ICG use has played a marginal role in this setting because the tumor location reduces the benefits offered by FGS [18].
An innovative application of indocyanine green in this field has been proposed by Simone et al. in a series of 10 totally endophytic renal masses. The patients received a preoperative super selective trans-arterial delivery of an ICG-Lipiodol mixture into tertiary-order arterial branches—feeding the renal mass—prior to transperitoneal “purely” off-clamp RAPN [32]. The ICG-marked area guided not only the tumor localization, but also a safer enucleation. The authors reported several benefits, including preoperative resection strategy improvement, quick intraoperative mass identification, and real-time control of resection margins. The feasibility of this “tattooing” technique, even with different ICG-mixed agents, has been confirmed by subsequent studies [14][33].
In 2022, Nardis and colleagues evaluated the clinical impact of ICG combined with Lipiodol in the context of trans-arterial super selective embolization (Figure 1), in a cohort of 41 patients with totally endophytic masses [34]. The study reported a procedure success rate of 100%, and 63.4% of the tumors were considered “visible with well-defined margins” intra-operatively. Combining ICG with other emerging technological tools for preoperative surgical strategies, such as three-dimensional (3D) reconstructions, may further enhance the potential of RAPN. An example of this application was illustrated in a pilot study by Amparore et al. published in 2023 [42], where ICG was used to overlay a 3D virtual model of the kidney onto the real organ during surgery, with promising preliminary results.
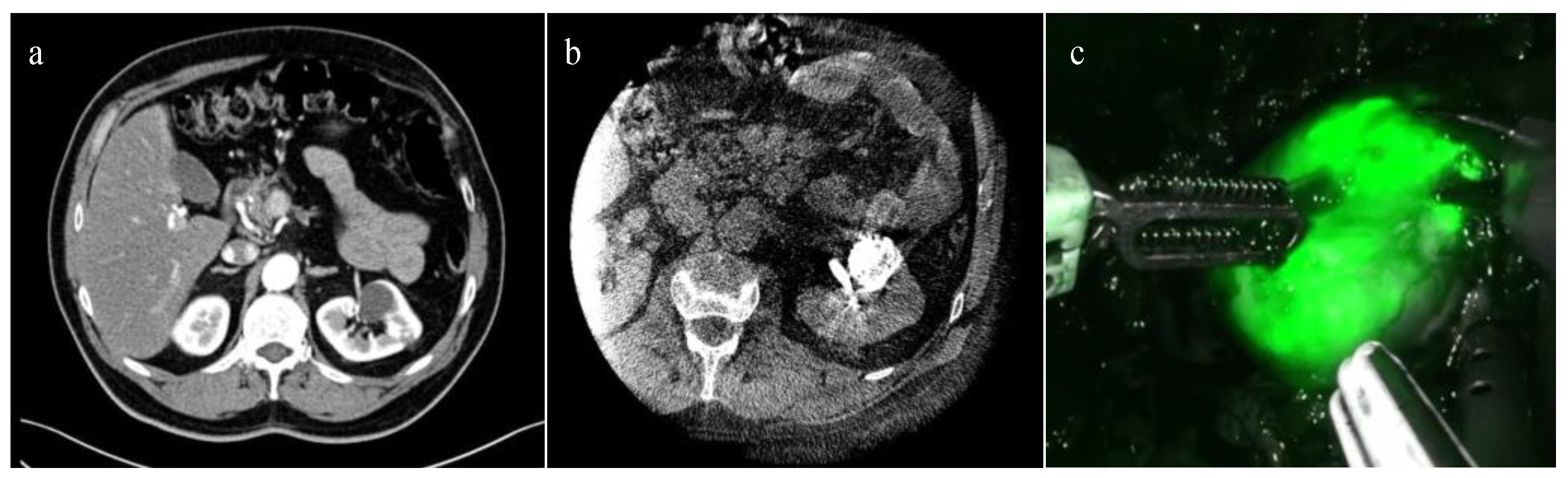
Figure 1. ICG-guided Management of Endophytic Renal Tumors: (a) CT-scan showing a totally endophytic left renal tumor; (b) trans-arterial super selective embolization “tagging” with ICG-Lipiodol; and (c) renal tumor intraoperative ICG near-infrared imaging identification.
References
- Moore, G.E. Fluorescein as an Agent in the Differentiation of Normal and Malignant Tissues. Science 1947, 106, 130–131.
- Stewart, H.L.; Birch, D.J.S. Fluorescence Guided Surgery. Methods Appl. Fluoresc. 2021, 9, 042002.
- Seo, H.-J.; Lee, N.R.; Son, S.K.; Kim, D.K.; Rha, K.H.; Lee, S.H. Comparison of Robot-Assisted Radical Prostatectomy and Open Radical Prostatectomy Outcomes: A Systematic Review and Meta-Analysis. Yonsei Med. J. 2016, 57, 1165–1177.
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.-U.; et al. Systematic Review and Meta-analysis of Studies Reporting Urinary Continence Recovery After Robot-assisted Radical Prostatectomy. Eur. Urol. 2012, 62, 405–417.
- Ghezzi, T.L.; Corleta, O.C. 30 Years of Robotic Surgery. World J. Surg. 2016, 40, 2550–2557.
- Bates, A.S.; Patel, V.R. Applications of indocyanine green in robotic urology. J. Robot. Surg. 2016, 10, 357.
- Landsman, M.L.; Kwant, G.; Mook, G.A.; Zijlstra, W.G. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583.
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 7.
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2015, 23, 166–175.
- Kochubey, V.I.; Kulyabina, T.V.; Tuchin, V.V.; Altshuler, G.B. Spectral Characteristics of Indocyanine Green upon Its Interaction with Biological Tissues. Opt. Spectrosc. 2005, 99, 560–566.
- Speich, R.; Saesseli, B.; Hoffmann, U.; Neftel, K.A.; Reichen, J. Anaphylactoid Reactions after Indocyanine-Green Administration. Ann. Intern. Med. 1988, 109, 345–346.
- Wakabayashi, T.; Cacciaguerra, A.B.; Abe, Y.; Bona, E.D.; Nicolini, D.; Mocchegiani, F.; Kabeshima, Y.; Vivarelli, M.; Wakabayashi, G.; Kitagawa, Y. Indocyanine Green Fluorescence Navigation in Liver Surgery: A Systematic Review on Dose and Timing of Administration. Ann. Surg. 2022, 275, 1025–1034.
- van der Pas, M.H.G.M.; van Dongen, G.A.M.S.; Cailler, F.; Pèlegrin, A.; Meijerink, W.J.H.J. Sentinel node procedure of the sigmoid using indocyanine green: Feasibility study in a goat model. Surg. Endosc. 2010, 24, 2182–2187.
- Faiella, E.; Calabrese, A.; Santucci, D.; Corti, R.; Cionfoli, N.; Pusceddu, C.; de Felice, C.; Bozzini, G.; Mazzoleni, F.; Muraca, R.M.; et al. Green Tattoo Pre-Operative Renal Embolization for Robotic-Assisted and Laparoscopic Partial Nephrectomy: A Practical Proof of a New Technique. J. Clin. Med. 2022, 11, 6816.
- Schaafsma, B.E.; Mieog, J.S.D.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.; Löwik, C.W.; Frangioni, J.V.; Van De Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332.
- Tobis, S.; Knopf, J.; Silvers, C.; Yao, J.; Rashid, H.; Wu, G.; Golijanin, D. Near Infrared Fluorescence Imaging with Robotic Assisted Laparoscopic Partial Nephrectomy: Initial Clinical Experience for Renal Cortical Tumors. J. Urol. 2011, 186, 47–52.
- Tobis, S.; Knopf, J.K.; Silvers, C.; Messing, E.; Yao, J.; Rashid, H.; Wu, G.; Golijanin, D. Robot-Assisted and Laparoscopic Partial Nephrectomy with Near Infrared Fluorescence Imaging. J. Endourol. 2012, 26, 797–802.
- Krane, L.S.; Manny, T.B.; Hemal, A.K. Is Near Infrared Fluorescence Imaging Using Indocyanine Green Dye Useful in Robotic Partial Nephrectomy: A Prospective Comparative Study of 94 Patients. Urology 2012, 80, 110–118.
- Manny, T.B.; Krane, L.S.; Hemal, A.K. Indocyanine Green Cannot Predict Malignancy in Partial Nephrectomy: Histopathologic Correlation with Fluorescence Pattern in 100 Patients. J. Endourol. 2013, 27, 918–921.
- Sentell, K.T.; Ferroni, M.C.; Abaza, R. Near-infrared fluorescence imaging for intraoperative margin assessment during robot-assisted partial nephrectomy. BJU Int. 2020, 126, 259–264.
- Angell, J.E.; Khemees, T.A.; Abaza, R. Optimization of Near Infrared Fluorescence Tumor Localization during Robotic Partial Nephrectomy. J. Urol. 2013, 190, 1668–1673.
- Borofsky, M.S.; Gill, I.S.; Hemal, A.K.; Marien, T.P.; Jayaratna, I.; Krane, L.S.; Stifelman, M.D. Near-infrared fluorescence imaging to facilitate super-selective arterial clamping during zero-ischaemia robotic partial nephrectomy. BJU Int. 2013, 111, 604–610.
- McClintock, T.R.; Bjurlin, M.A.; Wysock, J.S.; Borofsky, M.S.; Marien, T.P.; Okoro, C.; Stifelman, M.D. Can Selective Arterial Clamping with Fluorescence Imaging Preserve Kidney Function During Robotic Partial Nephrectomy? Urology 2014, 84, 327–334.
- Mattevi, D.; Luciani, L.G.; Mantovani, W.; Cai, T.; Chiodini, S.; Vattovani, V.; Puglisi, M.; Malossini, G. Fluorescence-guided selective arterial clamping during RAPN provides better early functional outcomes based on renal scan compared to standard clamping. J. Robot. Surg. 2019, 13, 391–396.
- Gadus, L.; Kocarek, J.; Chmelik, F.; Matejkova, M.; Heracek, J. Robotic Partial Nephrectomy with Indocyanine Green Fluorescence Navigation. Contrast Media Mol. Imaging 2020, 2020, 1287530.
- Lanchon, C.; Arnoux, V.; Fiard, G.; Descotes, J.-L.; Rambeaud, J.-J.; Lefrancq, J.-B.; Poncet, D.; Terrier, N.; Overs, C.; Franquet, Q.; et al. Super-selective robot-assisted partial nephrectomy using near-infrared flurorescence versus early-unclamping of the renal artery: Results of a prospective matched-pair analysis. Int. Braz. J. Urol. 2018, 44, 53–62.
- Harke, N.; Schoen, G.; Schiefelbein, F.; Heinrich, E. Selective clamping under the usage of near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: A single-surgeon matched-pair study. World J. Urol. 2014, 32, 1259–1265.
- Veccia, A.; Antonelli, A.; Hampton, L.J.; Greco, F.; Perdonà, S.; Lima, E.; Hemal, A.K.; Derweesh, I.; Porpiglia, F.; Autorino, R. Near-infrared Fluorescence Imaging with Indocyanine Green in Robot-assisted Partial Nephrectomy: Pooled Analysis of Comparative Studies. Eur. Urol. Focus 2020, 6, 505–512.
- Diana, P.; Buffi, N.M.; Lughezzani, G.; Dell’oglio, P.; Mazzone, E.; Porter, J.; Mottrie, A. The Role of Intraoperative Indocyanine Green in Robot-assisted Partial Nephrectomy: Results from a Large, Multi-institutional Series. Eur. Urol. 2020, 78, 743–749.
- Yang, Y.-K.; Hsieh, M.-L.; Chen, S.-Y.; Liu, C.-Y.; Lin, P.-H.; Kan, H.-C.; Pang, S.-T.; Yu, K.-J. Clinical Benefits of Indocyanine Green Fluorescence in Robot-Assisted Partial Nephrectomy. Cancers 2022, 14, 3032.
- Long, J.-A.; Fiard, G.; Giai, J.; Teyssier, Y.; Fontanell, A.; Overs, C.; Poncet, D.; Descotes, J.-L.; Rambeaud, J.-J.; Moreau-Gaudry, A.; et al. Superselective Ischemia in Robotic Partial Nephrectomy Does Not Provide Better Long-term Renal Function than Renal Artery Clamping in a Randomized Controlled Trial (EMERALD): Should We Take the Risk? Eur. Urol. Focus 2022, 8, 769–776.
- Simone, G.; Tuderti, G.; Anceschi, U.; Ferriero, M.; Costantini, M.; Minisola, F.; Vallati, G.; Pizzi, G.; Guaglianone, S.; Misuraca, L.; et al. “Ride the Green Light”: Indocyanine Green–marked Off-clamp Robotic Partial Nephrectomy for Totally Endophytic Renal Masses. Eur. Urol. 2019, 75, 1008–1014.
- Tuderti, G.; Brassetti, A.; Mastroianni, R.; Misuraca, L.; Bove, A.; Anceschi, U.; Ferriero, M.; Guaglianone, S.; Gallucci, M.; Simone, G. Expanding the limits of nephron-sparing surgery: Surgical technique and mid-term outcomes of purely off-clamp robotic partial nephrectomy for totally endophytic renal tumors. Int. J. Urol. 2022, 29, 282–288.
- Nardis, P.G.; Cipollari, S.; Lucatelli, P.; Basilico, F.; Rocco, B.; Corona, M.; Cannavale, A.; Leonardo, C.; Flammia, R.S.; Proietti, F.; et al. Cone-Beam CT-Guided Transarterial Tagging of Endophytic Renal Tumors with Indocyanine Green for Robot-Assisted Partial Nephrectomy. J. Vasc. Interv. Radiol. 2022, 33, 934–941.
- Vignolini, G.; Sessa, F.; Greco, I.; Cito, G.; Vanacore, D.; Cocci, A.; Sessa, M.; Grandi, V.; Pili, A.; Giancane, S.; et al. Intraoperative assessment of ureteral and graft reperfusion during robotic kidney transplantation with indocyanine green fluorescence videography. Minerva Urol. Nefrol. 2019, 71, 79–84.
- Ietto, G.; Iori, V.; Inversini, D.; Parise, C.; Zani, E.; Iovino, D.; Tozzi, M.; Gasperina, D.D.; Carcano, G.; Workgroup, I. Indocyanine Green Angiography for Quality Assessment of Renal Graft Before Transplantation: A Pilot Study. Exp. Clin. Transplant. 2023, 21, 110–115.
- Golijanin, D.J.; Marshall, J.; Cardin, A.; A Singer, E.; Wood, R.W.; E Reeder, J.; Wu, G.; Yao, J.L.; Passamonti, S.; Messing, E.M. Bilitranslocase (BTL) is immunolocalised in proximal and distal renal tubules and absent in renal cortical tumors accurately corresponding to intraoperative near infrared fluorescence (NIRF) expression of renal cortical tumors using intravenous indocyanine green (ICG). J. Urol. 2008, 179, 137.
- Gill, I.S.; Eisenberg, M.S.; Aron, M.; Berger, A.; Ukimura, O.; Patil, M.B.; Campese, V.; Thangathurai, D.; Desai, M.M. “Zero Ischemia” Partial Nephrectomy: Novel Laparoscopic and Robotic Technique. Eur. Urol. 2011, 59, 128–134.
- De Backer, P.; Vermijs, S.; Van Praet, C.; De Visschere, P.; Vandenbulcke, S.; Mottaran, A.; Bravi, C.A.; Berquin, C.; Lambert, E.; Dautricourt, S.; et al. A Novel Three-dimensional Planning Tool for Selective Clamping During Partial Nephrectomy: Validation of a Perfusion Zone Algorithm. Eur. Urol. 2023, 83, 413–421.
- Schiavina, R.; Novara, G.; Borghesi, M.; Ficarra, V.; Ahlawat, R.; Moon, D.A.; Porpiglia, F.; Challacombe, B.J.; Dasgupta, P.; Brunocilla, E.; et al. PADUA and R.E.N.A.L. nephrometry scores correlate with perioperative outcomes of robot-assisted partial nephrectomy: Analysis of the Vattikuti Global Quality Initiative in Robotic Urologic Surgery (GQI-RUS) database. BJU Int. 2017, 119, 456–463.
- Khene, Z.-E.; Peyronnet, B.; Gasmi, A.; Verhoest, G.; Mathieu, R.; Bensalah, K. Endophytic Renal Cell Carcinoma Treated with Robot-Assisted Surgery: Functional Outcomes—A Comprehensive Review of the Current Literature. Urol. Int. 2020, 104, 343–350.
- Amparore, D.; Checcucci, E.; Piazzolla, P.; Piramide, F.; De Cillis, S.; Piana, A.; Verri, P.; Manfredi, M.; Fiori, C.; Vezzetti, E.; et al. Indocyanine Green Drives Computer Vision Based 3D Augmented Reality Robot Assisted Partial Nephrectomy: The Beginning of “Automatic” Overlapping Era. Urology 2022, 164, e312–e316.
More
Information
Subjects:
Urology & Nephrology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
934
Revisions:
2 times
(View History)
Update Date:
19 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




