
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilaria Desideri | -- | 5365 | 2023-06-15 17:19:39 | | | |
| 2 | Catherine Yang | Meta information modification | 5365 | 2023-06-16 02:51:42 | | |
Video Upload Options
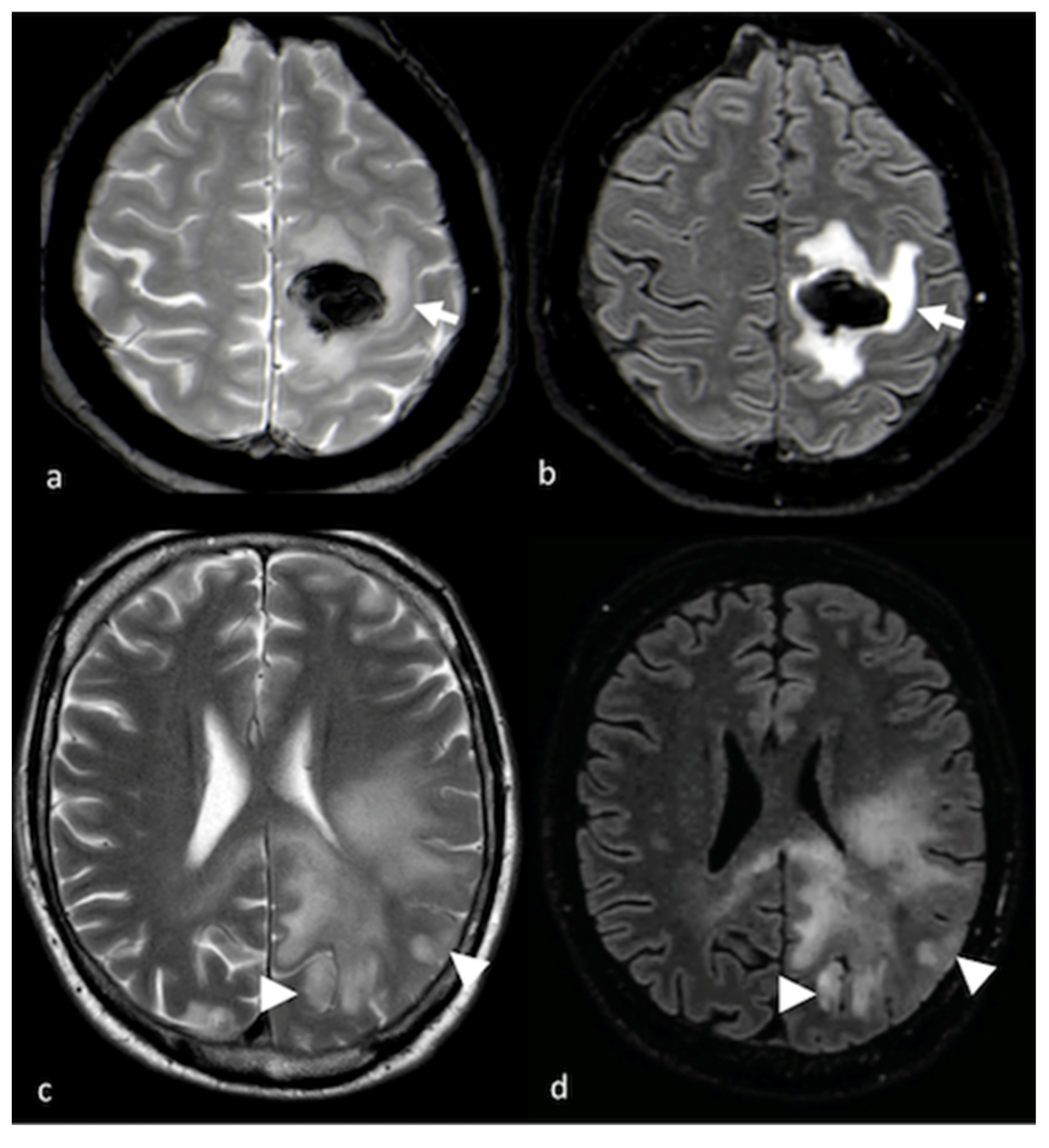
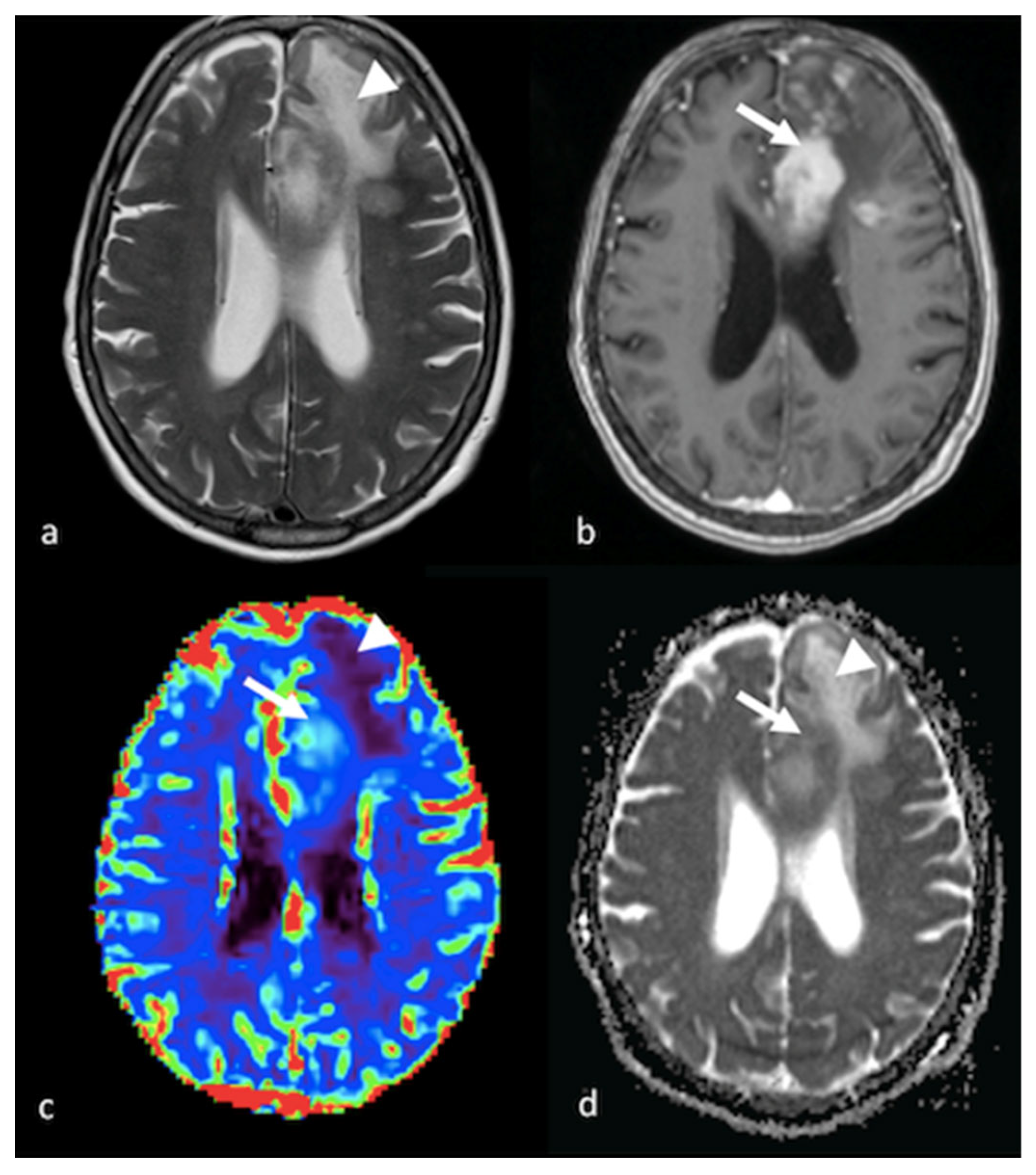
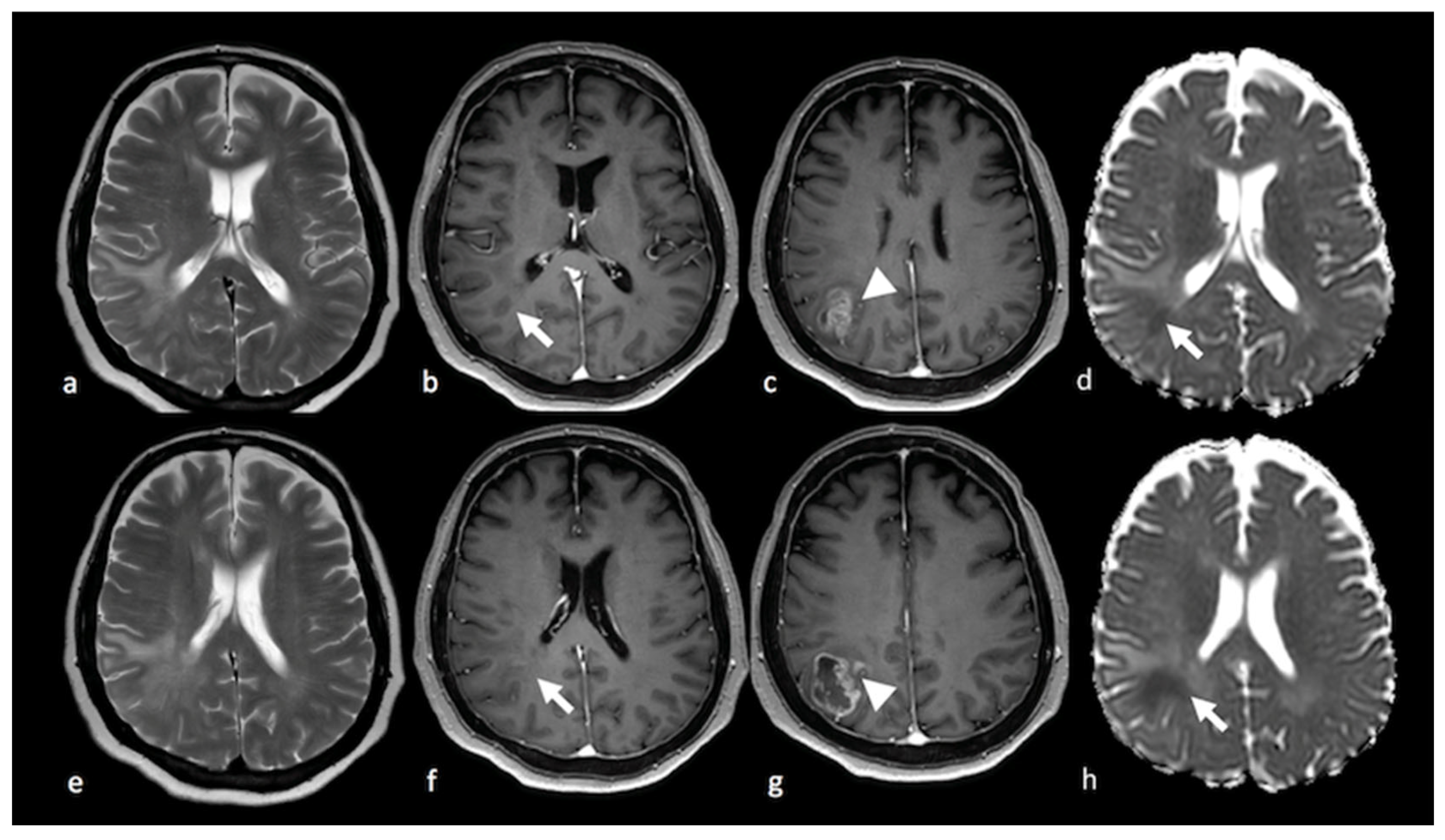
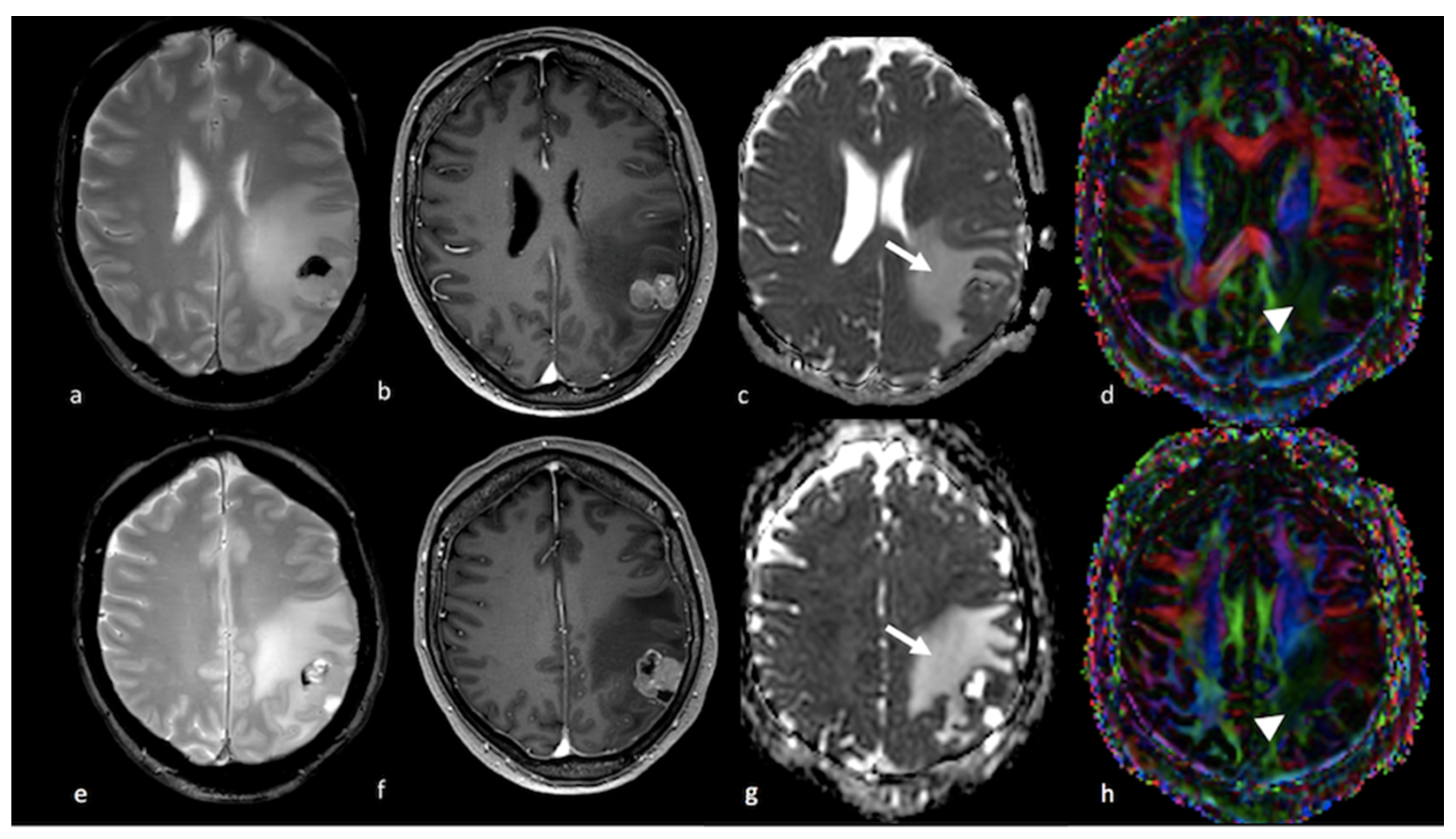
The non-enhancing peritumoral area (NEPA) is defined as the hyperintense region in T2-weighted and fluid-attenuated inversion recovery (FLAIR) images surrounding a brain tumor. The NEPA corresponds to different pathological processes, including vasogenic edema and infiltrative edema. The analysis of the NEPA with conventional and advanced magnetic resonance imaging (MRI) was proposed in the differential diagnosis of solid brain tumors, showing higher accuracy than MRI evaluation of the enhancing part of the tumor. In particular, MRI assessment of the NEPA was demonstrated to be a promising tool for distinguishing high-grade gliomas from primary lymphoma and brain metastases. Additionally, the MRI characteristics of the NEPA were found to correlate with prognosis and treatment response.
1. Conventional MRI and Advanced Techniques in the Differential Diagnosis of HGGs, Lymphoma and BMs
1.1. Conventional MRI
1.2. Diffusion Imaging Techniques
1.2.1. Diffusion-Weighted Imaging (DWI)
1.2.2. Diffusion Tensor Imaging (DTI)
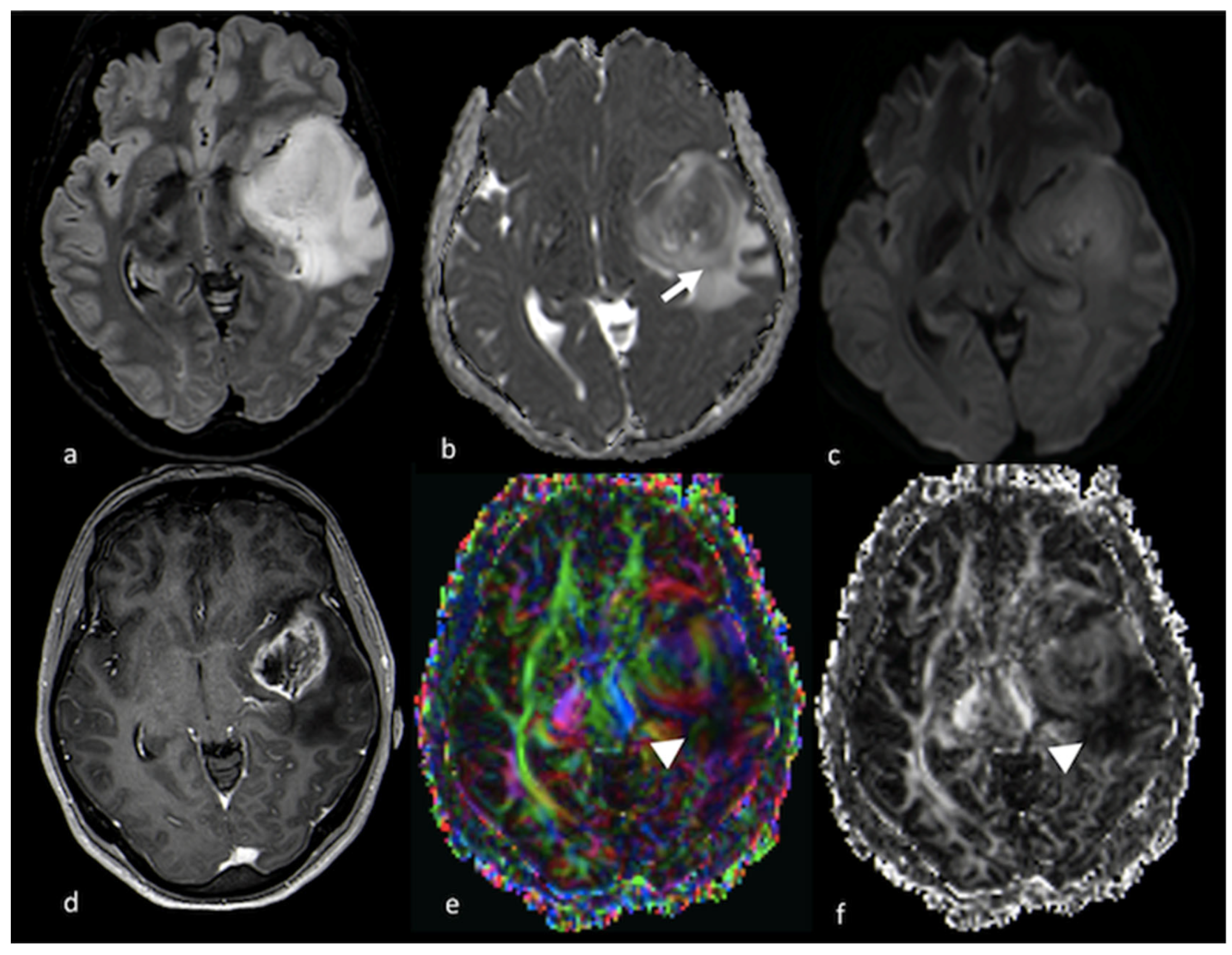
1.2.3. Diffusion Kurtosis Imaging (DKI)
1.3. Perfusion Imaging Techniques
1.4. H-Magnetic Resonance Spectroscopy
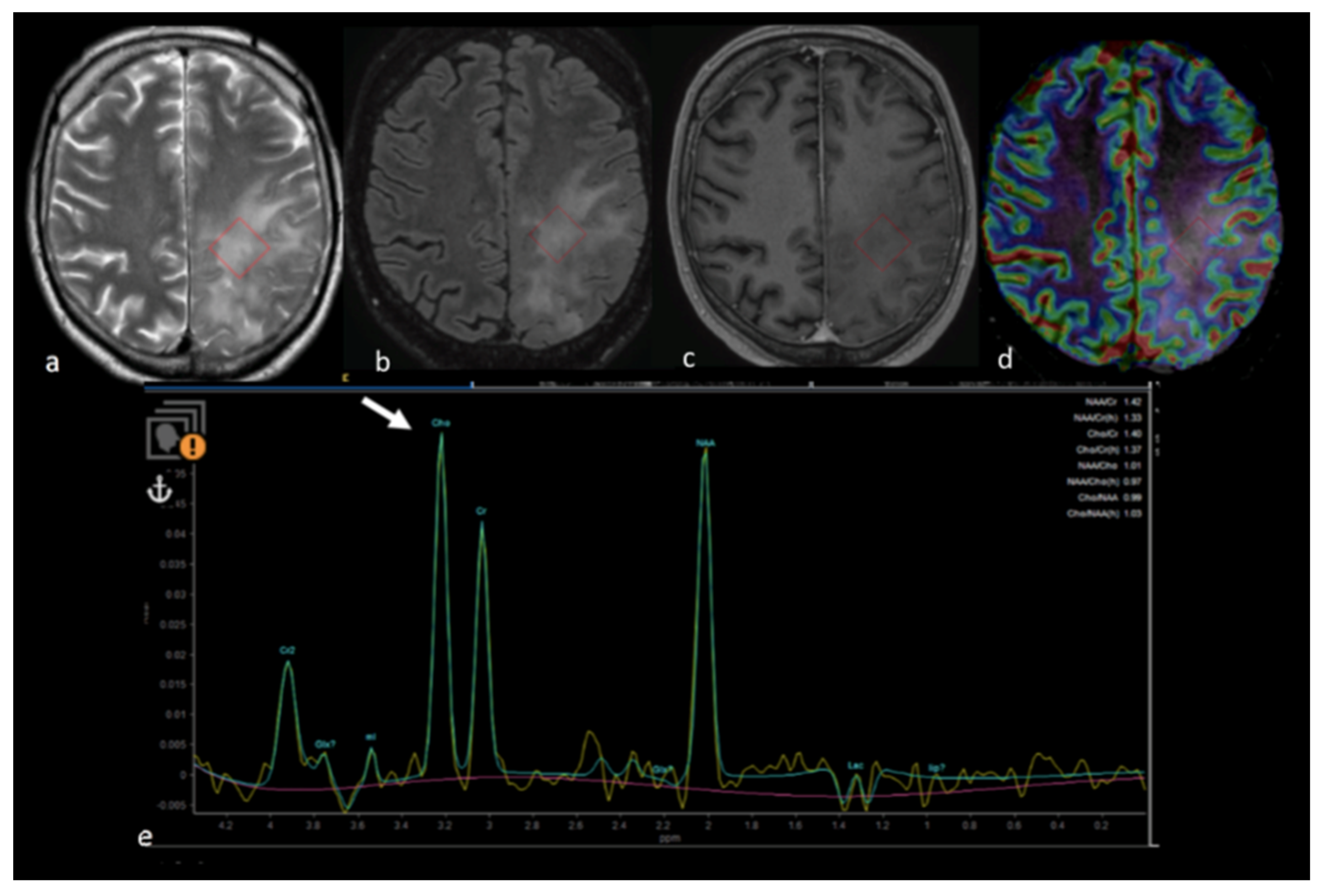
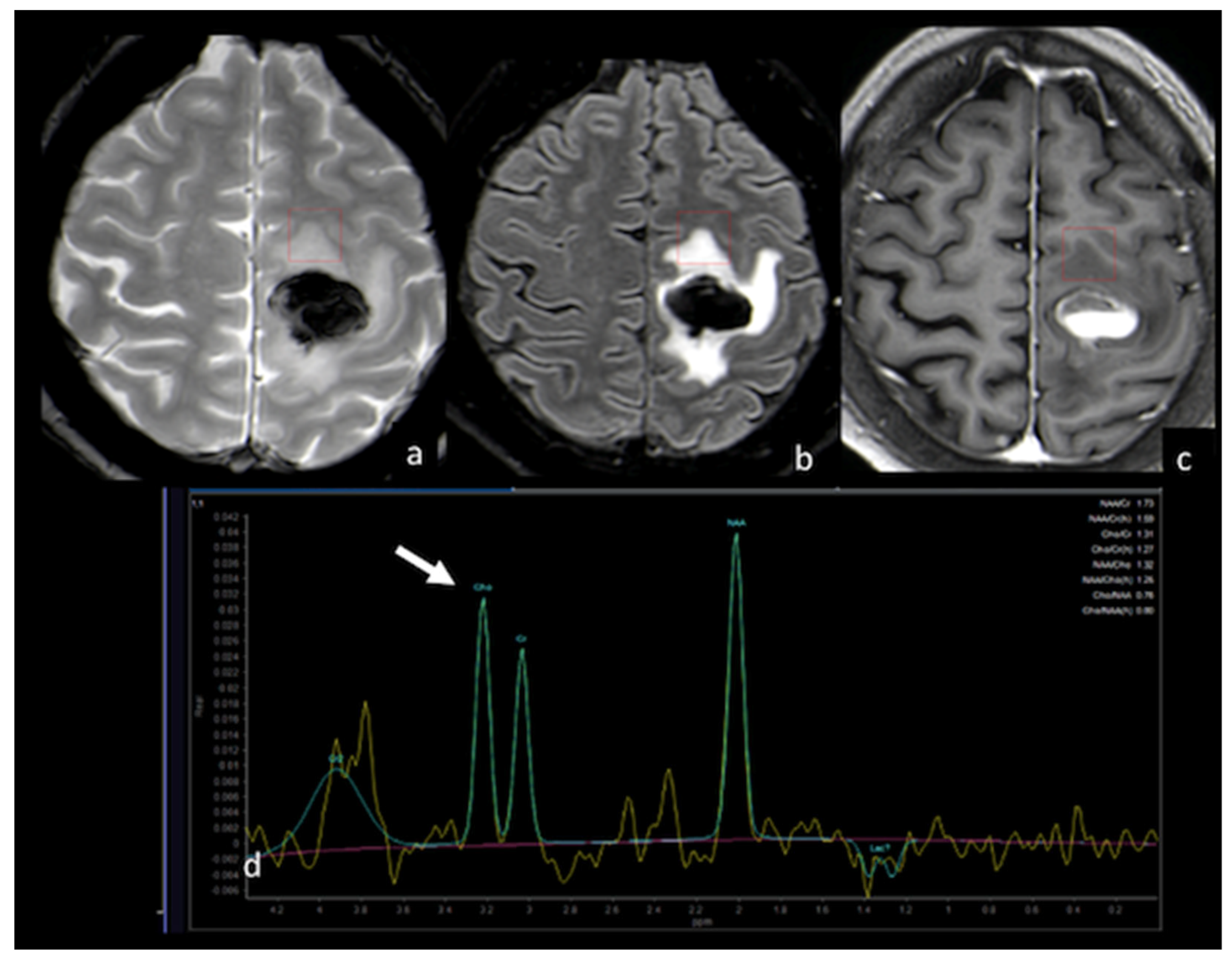
1.5. Amide Proton Transfer
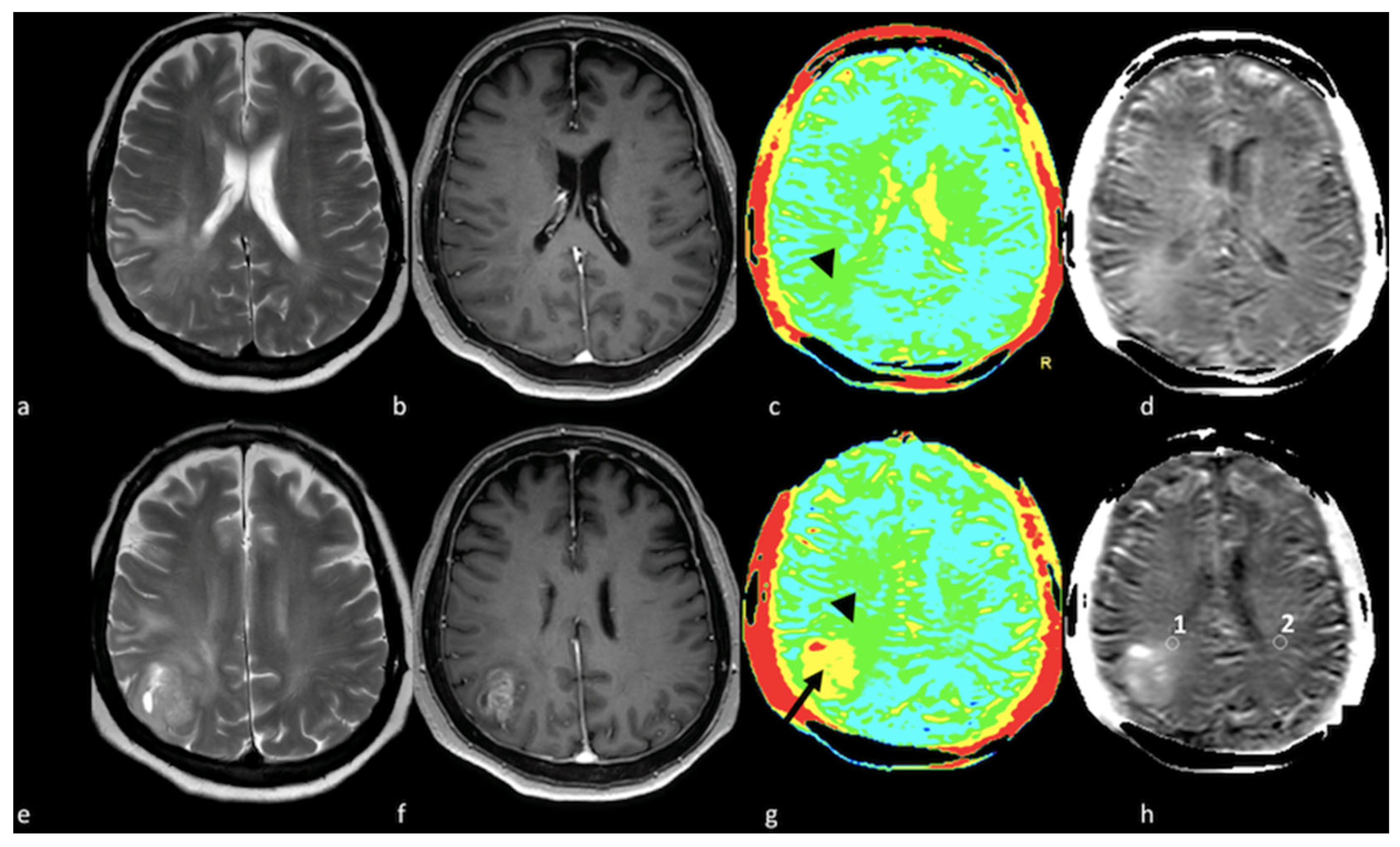
1.6. Multiparametric Approaches, Diagnostic Algorithms and Tumor Segmentation
2. Conventional and Advanced MRI Techniques in the Assessment of NEPAs for Prognosis and Treatment Response
2.1. Correlation between NEPAs and Prognosis
2.2. Correlation between NEPAs and Treatment Response
References
- Baris, M.M.; Celik, A.O.; Gezer, N.S.; Ada, E. Role of mass effect, tumor volume and peritumoral edema volume in the differential diagnosis of primary brain tumor and metastasis. Clin. Neurol. Neurosurg. 2016, 148, 67–71.
- Maurer, M.H.; Synowitz, M.; Badakshi, H.; Lohkamp, L.N.; Wustefeld, J.; Schafer, M.L.; Wiener, E. Glioblastoma multiforme versus solitary supratentorial brain metastasis: Differentiation based on morphology and magnetic resonance signal characteristics. Rofo 2013, 185, 235–240.
- Tang, Y.M.; Ngai, S.; Stuckey, S. The solitary enhancing cerebral lesion: Can FLAIR aid the differentiation between glioma and metastasis? AJNR Am. J. Neuroradiol. 2006, 27, 609–611.
- Wang, P.; Shi, Y.H.; Li, J.Y.; Zhang, C.Z. Differentiating Glioblastoma from Primary Central Nervous System Lymphoma: The Value of Shaping and Nonenhancing Peritumoral Hyperintense Gyral Lesion on FLAIR Imaging. World Neurosurg. 2021, 149, e696–e704.
- Stejskal, E.O.; Tanner, J.E. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J. Chem. Phys. 2004, 42, 288–292.
- Latour, L.L.; Svoboda, K.; Mitra, P.P.; Sotak, C.H. Time-dependent diffusion of water in a biological model system. Proc. Natl. Acad. Sci. USA 1994, 91, 1229–1233.
- Bauer, A.H.; Erly, W.; Moser, F.G.; Maya, M.; Nael, K. Differentiation of solitary brain metastasis from glioblastoma multiforme: A predictive multiparametric approach using combined MR diffusion and perfusion. Neuroradiology 2015, 57, 697–703.
- Pang, H.; Ren, Y.; Dang, X.; Feng, X.; Yao, Z.; Wu, J.; Yao, C.; Di, N.; Ghinda, D.C.; Zhang, Y. Diffusional kurtosis imaging for differentiating between high-grade glioma and primary central nervous system lymphoma. J. Magn. Reson. Imaging 2016, 44, 30–40.
- Lee, E.J.; Ahn, K.J.; Lee, E.K.; Lee, Y.S.; Kim, D.B. Potential role of advanced MRI techniques for the peritumoural region in differentiating glioblastoma multiforme and solitary metastatic lesions. Clin. Radiol. 2013, 68, e689–e697.
- Byrnes, T.J.; Barrick, T.R.; Bell, B.A.; Clark, C.A. Diffusion tensor imaging discriminates between glioblastoma and cerebral metastases in vivo. NMR Biomed. 2011, 24, 54–60.
- Ko, C.C.; Tai, M.H.; Li, C.F.; Chen, T.Y.; Chen, J.H.; Shu, G.; Kuo, Y.T.; Lee, Y.C. Differentiation between Glioblastoma Multiforme and Primary Cerebral Lymphoma: Additional Benefits of Quantitative Diffusion-Weighted MR Imaging. PLoS ONE 2016, 11, e0162565.
- Caravan, I.; Ciortea, C.A.; Contis, A.; Lebovici, A. Diagnostic value of apparent diffusion coefficient in differentiating between high-grade gliomas and brain metastases. Acta Radiol. 2018, 59, 599–605.
- Han, C.; Huang, S.; Guo, J.; Zhuang, X.; Han, H. Use of a high b-value for diffusion weighted imaging of peritumoral regions to differentiate high-grade gliomas and solitary metastases. J. Magn. Reson. Imaging 2015, 42, 80–86.
- Lee, E.J.; terBrugge, K.; Mikulis, D.; Choi, D.S.; Bae, J.M.; Lee, S.K.; Moon, S.Y. Diagnostic value of peritumoral minimum apparent diffusion coefficient for differentiation of glioblastoma multiforme from solitary metastatic lesions. AJR Am. J. Roentgenol. 2011, 196, 71–76.
- Basser, P.J.; Mattiello, J.; LeBihan, D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B. 1994, 103, 247–254.
- Sternberg, E.J.; Lipton, M.L.; Burns, J. Utility of diffusion tensor imaging in evaluation of the peritumoral region in patients with primary and metastatic brain tumors. AJNR Am. J. Neuroradiol. 2014, 35, 439–444.
- Van Cauter, S.; Veraart, J.; Sijbers, J.; Peeters, R.R.; Himmelreich, U.; De Keyzer, F.; Van Gool, S.W.; Van Calenbergh, F.; De Vleeschouwer, S.; Van Hecke, W.; et al. Gliomas: Diffusion kurtosis MR imaging in grading. Radiology 2012, 263, 492–501.
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440.
- Hempel, J.M.; Bisdas, S.; Schittenhelm, J.; Brendle, C.; Bender, B.; Wassmann, H.; Skardelly, M.; Tabatabai, G.; Vega, S.C.; Ernemann, U.; et al. In vivo molecular profiling of human glioma using diffusion kurtosis imaging. J. Neurooncol. 2017, 131, 93–101.
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109.
- Haopeng, P.; Xuefei, D.; Yan, R.; Zhenwei, Y.; Wei, H.; Ziyin, W.; Qungang, S.; Chaojie, L.; Linyan, Y.; Zhongmin, W.; et al. Diffusion kurtosis imaging differs between primary central nervous system lymphoma and high-grade glioma and is correlated with the diverse nuclear-to-cytoplasmic ratio: A histopathologic, biopsy-based study. Eur. Radiol. 2020, 30, 2125–2137.
- Sha, Z.; Song, Y.; Wu, Y.; Sha, P.; Ye, C.; Fan, G.; Gao, S.; Yu, R. The value of texture analysis in peritumoral edema of differentiating diagnosis between glioblastoma and primary brain lymphoma. Br. J. Neurosurg. 2020, 1–4.
- Tan, Y.; Wang, X.C.; Zhang, H.; Wang, J.; Qin, J.B.; Wu, X.F.; Zhang, L.; Wang, L. Differentiation of high-grade-astrocytomas from solitary-brain-metastases: Comparing diffusion kurtosis imaging and diffusion tensor imaging. Eur. J. Radiol. 2015, 84, 2618–2624.
- Rosen, B.R.; Belliveau, J.W.; Vevea, J.M.; Brady, T.J. Perfusion imaging with NMR contrast agents. Magn. Reson. Med. 1990, 14, 249–265.
- Jain, R.K.; di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 2007, 8, 610–622.
- Aronen, H.J.; Gazit, I.E.; Louis, D.N.; Buchbinder, B.R.; Pardo, F.S.; Weisskoff, R.M.; Harsh, G.R.; Cosgrove, G.R.; Halpern, E.F.; Hochberg, F.H.; et al. Cerebral blood volume maps of gliomas: Comparison with tumor grade and histologic findings. Radiology 1994, 191, 41–51.
- Cho, S.K.; Na, D.G.; Ryoo, J.W.; Roh, H.G.; Moon, C.H.; Byun, H.S.; Kim, J.H. Perfusion MR imaging: Clinical utility for the differential diagnosis of various brain tumors. Korean J. Radiol. 2002, 3, 171–179.
- Lupo, J.M.; Cha, S.; Chang, S.M.; Nelson, S.J. Dynamic susceptibility-weighted perfusion imaging of high-grade gliomas: Characterization of spatial heterogeneity. AJNR Am. J. Neuroradiol. 2005, 26, 1446–1454.
- Calli, C.; Kitis, O.; Yunten, N.; Yurtseven, T.; Islekel, S.; Akalin, T. Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur. J. Radiol. 2006, 58, 394–403.
- Kremer, S.; Grand, S.; Remy, C.; Esteve, F.; Lefournier, V.; Pasquier, B.; Hoffmann, D.; Benabid, A.L.; Le Bas, J.F. Cerebral blood volume mapping by MR imaging in the initial evaluation of brain tumors. J. Neuroradiol. 2002, 29, 105–113.
- Cha, S. Update on brain tumor imaging: From anatomy to physiology. AJNR Am. J. Neuroradiol. 2006, 27, 475–487.
- Warmuth, C.; Gunther, M.; Zimmer, C. Quantification of blood flow in brain tumors: Comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology 2003, 228, 523–532.
- Ferre, J.C.; Bannier, E.; Raoult, H.; Mineur, G.; Carsin-Nicol, B.; Gauvrit, J.Y. Arterial spin labeling (ASL) perfusion: Techniques and clinical use. Diagn. Interv. Imaging 2013, 94, 1211–1223.
- Haller, S.; Zaharchuk, G.; Thomas, D.L.; Lovblad, K.O.; Barkhof, F.; Golay, X. Arterial Spin Labeling Perfusion of the Brain: Emerging Clinical Applications. Radiology 2016, 281, 337–356.
- Halshtok Neiman, O.; Sadetzki, S.; Chetrit, A.; Raskin, S.; Yaniv, G.; Hoffmann, C. Perfusion-weighted imaging of peritumoral edema can aid in the differential diagnosis of glioblastoma mulltiforme versus brain metastasis. Isr. Med. Assoc. J. 2013, 15, 103–105.
- Neska-Matuszewska, M.; Bladowska, J.; Sasiadek, M.; Zimny, A. Differentiation of glioblastoma multiforme, metastases and primary central nervous system lymphomas using multiparametric perfusion and diffusion MR imaging of a tumor core and a peritumoral zone-Searching for a practical approach. PLoS ONE 2018, 13, e0191341.
- Askaner, K.; Rydelius, A.; Engelholm, S.; Knutsson, L.; Latt, J.; Abul-Kasim, K.; Sundgren, P.C. Differentiation between glioblastomas and brain metastases and regarding their primary site of malignancy using dynamic susceptibility contrast MRI at 3T. J. Neuroradiol. 2019, 46, 367–372.
- Hakyemez, B.; Erdogan, C.; Gokalp, G.; Dusak, A.; Parlak, M. Solitary metastases and high-grade gliomas: Radiological differentiation by morphometric analysis and perfusion-weighted MRI. Clin. Radiol. 2010, 65, 15–20.
- Toh, C.H.; Wei, K.C.; Chang, C.N.; Ng, S.H.; Wong, H.F.; Lin, C.P. Differentiation of brain abscesses from glioblastomas and metastatic brain tumors: Comparisons of diagnostic performance of dynamic susceptibility contrast-enhanced perfusion MR imaging before and after mathematic contrast leakage correction. PLoS ONE 2014, 9, e109172.
- Wilson, M.; Andronesi, O.; Barker, P.B.; Bartha, R.; Bizzi, A.; Bolan, P.J.; Brindle, K.M.; Choi, I.Y.; Cudalbu, C.; Dydak, U.; et al. Methodological consensus on clinical proton MRS of the brain: Review and recommendations. Magn. Reson. Med. 2019, 82, 527–550.
- Luyten, P.R.; Marien, A.J.; Heindel, W.; van Gerwen, P.H.; Herholz, K.; den Hollander, J.A.; Friedmann, G.; Heiss, W.D. Metabolic imaging of patients with intracranial tumors: H-1 MR spectroscopic imaging and PET. Radiology 1990, 176, 791–799.
- Posse, S.; DeCarli, C.; Le Bihan, D. Three-dimensional echo-planar MR spectroscopic imaging at short echo times in the human brain. Radiology 1994, 192, 733–738.
- Tsougos, I.; Svolos, P.; Kousi, E.; Fountas, K.; Theodorou, K.; Fezoulidis, I.; Kapsalaki, E. Differentiation of glioblastoma multiforme from metastatic brain tumor using proton magnetic resonance spectroscopy, diffusion and perfusion metrics at 3 T. Cancer Imaging 2012, 12, 423–436.
- Chiang, I.C.; Kuo, Y.T.; Lu, C.Y.; Yeung, K.W.; Lin, W.C.; Sheu, F.O.; Liu, G.C. Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 2004, 46, 619–627.
- Bendini, M.; Marton, E.; Feletti, A.; Rossi, S.; Curtolo, S.; Inches, I.; Ronzon, M.; Longatti, P.; Di Paola, F. Primary and metastatic intraaxial brain tumors: Prospective comparison of multivoxel 2D chemical-shift imaging (CSI) proton MR spectroscopy, perfusion MRI, and histopathological findings in a group of 159 patients. Acta Neurochir. 2011, 153, 403–412.
- Tsolaki, E.; Svolos, P.; Kousi, E.; Kapsalaki, E.; Fountas, K.; Theodorou, K.; Tsougos, I. Automated differentiation of glioblastomas from intracranial metastases using 3T MR spectroscopic and perfusion data. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 751–761.
- Wijnen, J.P.; Idema, A.J.; Stawicki, M.; Lagemaat, M.W.; Wesseling, P.; Wright, A.J.; Scheenen, T.W.; Heerschap, A. Quantitative short echo time 1H MRSI of the peripheral edematous region of human brain tumors in the differentiation between glioblastoma, metastasis, and meningioma. J. Magn. Reson. Imaging 2012, 36, 1072–1082.
- Hattingen, E.; Raab, P.; Franz, K.; Zanella, F.E.; Lanfermann, H.; Pilatus, U. Myo-inositol: A marker of reactive astrogliosis in glial tumors? NMR Biomed. 2008, 21, 233–241.
- Kallenberg, K.; Bock, H.C.; Helms, G.; Jung, K.; Wrede, A.; Buhk, J.H.; Giese, A.; Frahm, J.; Strik, H.; Dechent, P.; et al. Untreated glioblastoma multiforme: Increased myo-inositol and glutamine levels in the contralateral cerebral hemisphere at proton MR spectroscopy. Radiology 2009, 253, 805–812.
- Chawla, S.; Zhang, Y.; Wang, S.; Chaudhary, S.; Chou, C.; O’Rourke, D.M.; Vossough, A.; Melhem, E.R.; Poptani, H. Proton magnetic resonance spectroscopy in differentiating glioblastomas from primary cerebral lymphomas and brain metastases. J. Comput. Assist. Tomogr. 2010, 34, 836–841.
- Ricci, R.; Bacci, A.; Tugnoli, V.; Battaglia, S.; Maffei, M.; Agati, R.; Leonardi, M. Metabolic findings on 3T 1H-MR spectroscopy in peritumoral brain edema. AJNR Am. J. Neuroradiol. 2007, 28, 1287–1291.
- Di Costanzo, A.; Trojsi, F.; Tosetti, M.; Giannatempo, G.M.; Nemore, F.; Piccirillo, M.; Bonavita, S.; Tedeschi, G.; Scarabino, T. High-field proton MRS of human brain. Eur. J. Radiol. 2003, 48, 146–153.
- Schurr, A. Lactate: The ultimate cerebral oxidative energy substrate? J. Cereb. Blood Flow Metab. 2006, 26, 142–152.
- Danielsen, E.R.; Ross, B.D. Magnetic Resonance Spectroscopy Diagnosis of Neurological Diseases; CRC Press: Boca Raton, FL, USA, 1999.
- Kural, C.; Atac, G.K.; Tehli, O.; Solmaz, I.; Temiz, C.; Hodaj, I.; Izci, Y. The evaluation of the effects of steroid treatment on the tumor and peritumoral edema by DWI and MR spectroscopy in brain tumors. Neurol. Neurochir. Pol. 2018, 52, 495–504.
- Ward, K.M.; Aletras, A.H.; Balaban, R.S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 2000, 143, 79–87.
- Yan, K.; Fu, Z.; Yang, C.; Zhang, K.; Jiang, S.; Lee, D.H.; Heo, H.Y.; Zhang, Y.; Cole, R.N.; Van Eyk, J.E.; et al. Assessing Amide Proton Transfer (APT) MRI Contrast Origins in 9 L Gliosarcoma in the Rat Brain Using Proteomic Analysis. Mol. Imaging Biol. 2015, 17, 479–487.
- Li, J.; Zhuang, Z.; Okamoto, H.; Vortmeyer, A.O.; Park, D.M.; Furuta, M.; Lee, Y.S.; Oldfield, E.H.; Zeng, W.; Weil, R.J. Proteomic profiling distinguishes astrocytomas and identifies differential tumor markers. Neurology 2006, 66, 733–736.
- Jones, C.K.; Polders, D.; Hua, J.; Zhu, H.; Hoogduin, H.J.; Zhou, J.; Luijten, P.; van Zijl, P.C. In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magn. Reson. Med. 2012, 67, 1579–1589.
- Wen, Z.; Hu, S.; Huang, F.; Wang, X.; Guo, L.; Quan, X.; Wang, S.; Zhou, J. MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 2010, 51, 616–622.
- Zheng, S.; van der Bom, I.M.; Zu, Z.; Lin, G.; Zhao, Y.; Gounis, M.J. Chemical exchange saturation transfer effect in blood. Magn. Reson. Med. 2014, 71, 1082–1092.
- Wang, M.; Hong, X.; Chang, C.F.; Li, Q.; Ma, B.; Zhang, H.; Xiang, S.; Heo, H.Y.; Zhang, Y.; Lee, D.H.; et al. Simultaneous detection and separation of hyperacute intracerebral hemorrhage and cerebral ischemia using amide proton transfer MRI. Magn. Reson. Med. 2015, 74, 42–50.
- Yu, H.; Lou, H.; Zou, T.; Wang, X.; Jiang, S.; Huang, Z.; Du, Y.; Jiang, C.; Ma, L.; Zhu, J.; et al. Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur. Radiol. 2017, 27, 4516–4524.
- Wang, S.; Kim, S.; Chawla, S.; Wolf, R.L.; Knipp, D.E.; Vossough, A.; O’Rourke, D.M.; Judy, K.D.; Poptani, H.; Melhem, E.R. Differentiation between Glioblastomas, Solitary Brain Metastases, and Primary Cerebral Lymphomas Using Diffusion Tensor and Dynamic Susceptibility Contrast-Enhanced MR Imaging. Am. J. Neuroradiol. 2011, 32, 507–514.
- Weber, M.A.; Zoubaa, S.; Schlieter, M.; Juttler, E.; Huttner, H.B.; Geletneky, K.; Ittrich, C.; Lichy, M.P.; Kroll, A.; Debus, J.; et al. Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology 2006, 66, 1899–1906.
- Caulo, M.; Panara, V.; Tortora, D.; Mattei, P.A.; Briganti, C.; Pravata, E.; Salice, S.; Cotroneo, A.R.; Tartaro, A. Data-driven grading of brain gliomas: A multiparametric MR imaging study. Radiology 2014, 272, 494–503.
- Lehmann, P.; Saliou, G.; de Marco, G.; Monet, P.; Souraya, S.E.; Bruniau, A.; Vallee, J.N.; Ducreux, D. Cerebral peritumoral oedema study: Does a single dynamic MR sequence assessing perfusion and permeability can help to differentiate glioblastoma from metastasis? Eur. J. Radiol. 2012, 81, 522–527.
- Mouthuy, N.; Cosnard, G.; Abarca-Quinones, J.; Michoux, N. Multiparametric magnetic resonance imaging to differentiate high-grade gliomas and brain metastases. J. Neuroradiol. 2012, 39, 301–307.
- Lemercier, P.; Paz Maya, S.; Patrie, J.T.; Flors, L.; Leiva-Salinas, C. Gradient of apparent diffusion coefficient values in peritumoral edema helps in differentiation of glioblastoma from solitary metastatic lesions. AJR Am. J. Roentgenol. 2014, 203, 163–169.
- Voicu, I.P.; Pravata, E.; Panara, V.; Navarra, R.; Mattei, P.A.; Caulo, M. Differentiating solitary brain metastases from high-grade gliomas with MR: Comparing qualitative versus quantitative diagnostic strategies. Radiol. Med. 2022, 127, 891–898.
- Artzi, M.; Liberman, G.; Blumenthal, D.T.; Aizenstein, O.; Bokstein, F.; Ben Bashat, D. Differentiation between vasogenic edema and infiltrative tumor in patients with high-grade gliomas using texture patch-based analysis. J. Magn. Reson. Imaging 2018, 48, 729–736.
- Iqbal, S.; Ghani Khan, M.U.; Saba, T.; Mehmood, Z.; Javaid, N.; Rehman, A.; Abbasi, R. Deep learning model integrating features and novel classifiers fusion for brain tumor segmentation. Microsc. Res. Tech. 2019, 82, 1302–1315.
- Zhao, X.; Wu, Y.; Song, G.; Li, Z.; Zhang, Y.; Fan, Y. A deep learning model integrating FCNNs and CRFs for brain tumor segmentation. Med. Image Anal. 2018, 43, 98–111.
- Ranjbarzadeh, R.; Bagherian Kasgari, A.; Jafarzadeh Ghoushchi, S.; Anari, S.; Naseri, M.; Bendechache, M. Brain tumor segmentation based on deep learning and an attention mechanism using MRI multi-modalities brain images. Sci. Rep. 2021, 11, 10930.
- Schoenegger, K.; Oberndorfer, S.; Wuschitz, B.; Struhal, W.; Hainfellner, J.; Prayer, D.; Heinzl, H.; Lahrmann, H.; Marosi, C.; Grisold, W. Peritumoral edema on MRI at initial diagnosis: An independent prognostic factor for glioblastoma? Eur. J. Neurol. 2009, 16, 874–878.
- Wu, C.X.; Lin, G.S.; Lin, Z.X.; Zhang, J.D.; Liu, S.Y.; Zhou, C.F. Peritumoral edema shown by MRI predicts poor clinical outcome in glioblastoma. World J. Surg. Oncol. 2015, 13, 97.
- Wang, K.; Wang, Y.Y.; Wang, J.F.; Ma, J.; Jiang, T.; Dai, J.P. Radiologic Features and Expression of Vascular Endothelial Growth Factor Stratify Survival Outcomes in Patients with Glioblastoma. AJNR Am. J. Neuroradiol. 2016, 37, 629–635.
- Carrillo, J.A.; Lai, A.; Nghiemphu, P.L.; Kim, H.J.; Phillips, H.S.; Kharbanda, S.; Moftakhar, P.; Lalaezari, S.; Yong, W.; Ellingson, B.M.; et al. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am. J. Neuroradiol. 2012, 33, 1349–1355.
- Henker, C.; Kriesen, T.; Glass, A.; Schneider, B.; Piek, J. Volumetric quantification of glioblastoma: Experiences with different measurement techniques and impact on survival. J. Neurooncol. 2017, 135, 391–402.
- Ramnarayan, R.; Dodd, S.; Das, K.; Heidecke, V.; Rainov, N.G. Overall survival in patients with malignant glioma may be significantly longer with tumors located in deep grey matter. J. Neurol. Sci. 2007, 260, 49–56.
- Pope, W.B.; Sayre, J.; Perlina, A.; Villablanca, J.P.; Mischel, P.S.; Cloughesy, T.F. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am. J. Neuroradiol. 2005, 26, 2466–2474.
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198.
- Mummareddy, N.; Salwi, S.R.; Ganesh Kumar, N.; Zhao, Z.; Ye, F.; Le, C.H.; Mobley, B.C.; Thompson, R.C.; Chambless, L.B.; Mistry, A.M. Prognostic relevance of CSF and peri-tumoral edema volumes in glioblastoma. J. Clin. Neurosci. 2021, 84, 1–7.
- Strugar, J.G.; Criscuolo, G.R.; Rothbart, D.; Harrington, W.N. Vascular endothelial growth/permeability factor expression in human glioma specimens: Correlation with vasogenic brain edema and tumor-associated cysts. J. Neurosurg. 1995, 83, 682–689.
- Seidel, C.; Dorner, N.; Osswald, M.; Wick, A.; Platten, M.; Bendszus, M.; Wick, W. Does age matter?—A MRI study on peritumoral edema in newly diagnosed primary glioblastoma. BMC Cancer 2011, 11, 127.
- Zhou, Q.; Ke, X.; Xue, C.; Li, S.; Huang, X.; Zhang, B.; Zhou, J. A Nomogram for Predicting Early Recurrence in Patients with High-Grade Gliomas. World Neurosurg. 2022, 164, e619–e628.
- Liang, H.T.; Chen, W.Y.; Lai, S.F.; Su, M.Y.; You, S.L.; Chen, L.H.; Tseng, H.M.; Chen, C.M.; Kuo, S.H.; Tseng, W.I. The extent of edema and tumor synchronous invasion into the subventricular zone and corpus callosum classify outcomes and radiotherapy strategies of glioblastomas. Radiother. Oncol. 2017, 125, 248–257.
- Liang, H.T.; Mizumoto, M.; Ishikawa, E.; Matsuda, M.; Tanaka, K.; Kohzuki, H.; Numajiri, H.; Oshiro, Y.; Okumura, T.; Matsumura, A.; et al. Peritumoral edema status of glioblastoma identifies patients reaching long-term disease control with specific progression patterns after tumor resection and high-dose proton boost. J. Cancer Res. Clin. Oncol. 2021, 147, 3503–3516.
- Cui, Y.; Zeng, W.; Jiang, H.; Ren, X.; Lin, S.; Fan, Y.; Liu, Y.; Zhao, J. Higher Cho/NAA Ratio in Postoperative Peritumoral Edema Zone Is Associated With Earlier Recurrence of Glioblastoma. Front. Neurol. 2020, 11, 592155.
- Pirzkall, A.; McKnight, T.R.; Graves, E.E.; Carol, M.P.; Sneed, P.K.; Wara, W.W.; Nelson, S.J.; Verhey, L.J.; Larson, D.A. MR-spectroscopy guided target delineation for high-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 915–928.
- Zhang, M.; Ye, F.; Su, M.; Cui, M.; Chen, H.; Ma, X. The Prognostic Role of Peritumoral Edema in Patients with Newly Diagnosed Glioblastoma: A Retrospective Analysis. J. Clin. Neurosci. 2021, 89, 249–257.
- Chepuri, N.B.; Yen, Y.F.; Burdette, J.H.; Li, H.; Moody, D.M.; Maldjian, J.A. Diffusion anisotropy in the corpus callosum. AJNR Am. J. Neuroradiol. 2002, 23, 803–808.
- Calluaud, G.; Terrier, L.M.; Mathon, B.; Destrieux, C.; Velut, S.; Francois, P.; Zemmoura, I.; Amelot, A. Peritumoral Edema/Tumor Volume Ratio: A Strong Survival Predictor for Posterior Fossa Metastases. Neurosurgery 2019, 85, 117–125.
- Rathore, S.; Akbari, H.; Doshi, J.; Shukla, G.; Rozycki, M.; Bilello, M.; Lustig, R.; Davatzikos, C. Radiomic signature of infiltration in peritumoral edema predicts subsequent recurrence in glioblastoma: Implications for personalized radiotherapy planning. J. Med. Imaging 2018, 5, 021219.
- Akbari, H.; Macyszyn, L.; Da, X.; Bilello, M.; Wolf, R.L.; Martinez-Lage, M.; Biros, G.; Alonso-Basanta, M.; O’Rourke, D.M.; Davatzikos, C. Imaging Surrogates of Infiltration Obtained Via Multiparametric Imaging Pattern Analysis Predict Subsequent Location of Recurrence of Glioblastoma. Neurosurgery 2016, 78, 572–580.
- Cepeda, S.; Luppino, L.T.; Perez-Nunez, A.; Solheim, O.; Garcia-Garcia, S.; Velasco-Casares, M.; Karlberg, A.; Eikenes, L.; Sarabia, R.; Arrese, I.; et al. Predicting Regions of Local Recurrence in Glioblastomas Using Voxel-Based Radiomic Features of Multiparametric Postoperative MRI. Cancers 2023, 15, 1894.
- Chougule, T.; Gupta, R.K.; Saini, J.; Agrawal, S.; Gupta, M.; Vakharia, N.; Singh, A.; Patir, R.; Vaishya, S.; Ingalhalikar, M. Radiomics signature for temporal evolution and recurrence patterns of glioblastoma using multimodal magnetic resonance imaging. NMR Biomed. 2022, 35, e4647.
- Dasgupta, A.; Geraghty, B.; Maralani, P.J.; Malik, N.; Sandhu, M.; Detsky, J.; Tseng, C.L.; Soliman, H.; Myrehaug, S.; Husain, Z.; et al. Quantitative mapping of individual voxels in the peritumoral region of IDH-wildtype glioblastoma to distinguish between tumor infiltration and edema. J. Neurooncol. 2021, 153, 251–261.
- Juan-Albarracin, J.; Fuster-Garcia, E.; Perez-Girbes, A.; Aparici-Robles, F.; Alberich-Bayarri, A.; Revert-Ventura, A.; Marti-Bonmati, L.; Garcia-Gomez, J.M. Glioblastoma: Vascular Habitats Detected at Preoperative Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging Predict Survival. Radiology 2018, 287, 944–954.
- Riahi Samani, Z.; Parker, D.; Akbari, H.; Wolf, R.L.; Brem, S.; Bakas, S.; Verma, R. Artificial intelligence-based locoregional markers of brain peritumoral microenvironment. Sci. Rep. 2023, 13, 963.
- Artzi, M.; Bokstein, F.; Blumenthal, D.T.; Aizenstein, O.; Liberman, G.; Corn, B.W.; Ben Bashat, D. Differentiation between vasogenic-edema versus tumor-infiltrative area in patients with glioblastoma during bevacizumab therapy: A longitudinal MRI study. Eur. J. Radiol. 2014, 83, 1250–1256.
- De Groot, J.F.; Fuller, G.; Kumar, A.J.; Piao, Y.; Eterovic, K.; Ji, Y.; Conrad, C.A. Tumor invasion after treatment of glioblastoma with bevacizumab: Radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010, 12, 233–242.
- Abramovitch, R.; Dafni, H.; Smouha, E.; Benjamin, L.E.; Neeman, M. In vivo prediction of vascular susceptibility to vascular susceptibility endothelial growth factor withdrawal: Magnetic resonance imaging of C6 rat glioma in nude mice. Cancer Res. 1999, 59, 5012–5016.
- Fan, Y.; He, L.; Yang, H.; Wang, Y.; Su, J.; Hou, S.; Luo, Y.; Jiang, X. Preoperative MRI-Based Radiomics of Brain Metastasis to Assess T790M Resistance Mutation After EGFR-TKI Treatment in NSCLC. J. Magn. Reson. Imaging 2022, 57, 1778–1787.
- Leeman, J.E.; Clump, D.A.; Flickinger, J.C.; Mintz, A.H.; Burton, S.A.; Heron, D.E. Extent of perilesional edema differentiates radionecrosis from tumor recurrence following stereotactic radiosurgery for brain metastases. Neuro Oncol. 2013, 15, 1732–1738.
- Dohm, A.E.; Nickles, T.M.; Miller, C.E.; Bowers, H.J.; Miga, M.I.; Attia, A.; Chan, M.D.; Weis, J.A. Clinical assessment of a biophysical model for distinguishing tumor progression from radiation necrosis. Med. Phys. 2021, 48, 3852–3859.




