Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Afzal Shah | -- | 2191 | 2023-06-15 16:49:12 | | | |
| 2 | Camila Xu | Meta information modification | 2191 | 2023-06-16 05:43:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khan, K.A.; Shah, A.; Nisar, J.; Haleem, A.; Shah, I. Methods Used for the Degradation of Food Dyes. Encyclopedia. Available online: https://encyclopedia.pub/entry/45672 (accessed on 07 February 2026).
Khan KA, Shah A, Nisar J, Haleem A, Shah I. Methods Used for the Degradation of Food Dyes. Encyclopedia. Available at: https://encyclopedia.pub/entry/45672. Accessed February 07, 2026.
Khan, Kashif Ali, Afzal Shah, Jan Nisar, Abdul Haleem, Iltaf Shah. "Methods Used for the Degradation of Food Dyes" Encyclopedia, https://encyclopedia.pub/entry/45672 (accessed February 07, 2026).
Khan, K.A., Shah, A., Nisar, J., Haleem, A., & Shah, I. (2023, June 15). Methods Used for the Degradation of Food Dyes. In Encyclopedia. https://encyclopedia.pub/entry/45672
Khan, Kashif Ali, et al. "Methods Used for the Degradation of Food Dyes." Encyclopedia. Web. 15 June, 2023.
Copy Citation
Dyes are widely used in various industries, including food, textile, pharmaceutics and cosmetic industries for the addition of color to products to make them more attractive for customers.
green synthesis
nanoparticles
dyes
photodegradation
1. Introduction
Dyes are widely used in various industries, including food, textile, pharmaceutics and cosmetic industries for the addition of color to products to make them more attractive for customers [1]. However, depending on their chemical composition and usage, some dyes may pose toxicity risks for aquatic organisms [2]. Water has been greatly polluted with dyes from industrial wastewater, mainly from the cosmetics, textile, paper and food industries, which companies allow to fall untreated into the natural water resources. These pollutants ultimately pose life-threatening risks to water biota, plants, and human beings, even in low concentrations, due to products produced during their degradation, particularly photodegradation [3]. In the textile industry, some azo dyes have been found to break down into toxic aromatic amines, which may cause cancer and other health problems. As a result, some countries have banned or restricted the use of certain azo dyes in textiles [4]. In the cosmetic industry, some dyes may cause skin irritation, allergic reactions, and other adverse effects, especially when used in high concentrations or on sensitive skin [5].
Dyes are mainly divided into three different categories, namely, anionic, cationic and nonionic dyes. Anionic dyes mainly possess negatively charged groups in their primary structure, such as the SO3− group, while cationic dyes have positively charged groups, such as the protonated amine group. The third type of dyes are called nonionic due to their dissociative behavior in aqueous solutions [6]. Azo-based dyes have more stability than other dyes due to presence of the azo group, which gives them much higher stability when affected by heat, aerobic digestion, and light. However, they may also cause severe problems in human health, such as genetic mutation, allergic problems, vomiting, and cyanosis. Dyes from different industries are discharged into bodies of water where they cause damage to aquatic life. These dyes can cause suffocation in aquatic animals, such as fish, by affecting their gills. Aquatic animals’ breeding and shelter locations may also be affected. Dyes can also affect photosynthesis by blocking the passage of light through the water, causing problems for aquatic animals [7]. Because of these toxic effects, it is very necessary to remove these dyes from water bodies by some technique to save aquatic life [8].
Other types of dyes include: (a) Basic dyes such as thiazine, oxazine, azine, triaryl methyl, and cyanin derivatives. Basic dyes, known for their brilliant intense color, play important role in the textile sector [9]. The positively charged dyes attract the negatively charged anions of nylon, polyester, and acrylic fibers through electrostatic forces. (b) Acidic dyes, including methyl orange, methyl red, and erichrome black T [10]. These dyes are widely used as coloring agents for synthetic (polyamides, acrylic) fibers in acidic conditions (low pH) [11]. These dyes are also employed as food colorants [12]. (c) Reactive dyes, such as remazol brilliant red FG, reactive red 120, etc., are very reactive towards the nucleophilic sites of the fabrics. They can make strong covalent bonds with the fabric’s nucleophilic site and are applied to cotton, silk and rayon. (d) Dispersive dyes, such as dispersol, samaron, etc., are sparingly soluble and disperse in water. They attach to the fabric by hydrogen bonding and are useful for nylon, acrylic and polyester [8].
There are many man-made food and juice colorants that are commonly used in the food industry, each with their own associated potential toxicity, and the toxicity associated with each one can vary depending on the specific chemical compound [13] and the dosage [14]. Some synthetic dyes, such as Red 40, Yellow 5, and Yellow 6, have been linked to adverse health effects, such as hyperactivity in children, allergic reactions, and, in animal studies, cancer. As a result, some countries have banned or restricted the use of these dyes in food products, but many are still illegally used in those countries [15]. Some of the most common food and juice colorants that are illegally added to our food in most parts of the world, along with their uses and associated toxicity, are presented in Table 1.
Table 1. Various food dyes, their uses and associated toxicity.
| S. No | Name of the Dye | Synonyms | Chemical Structure | Uses | Toxic Effects |
|---|---|---|---|---|---|
| 1 | Allura Red AC | Red 40 | 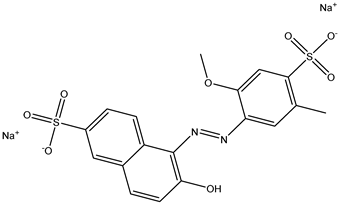 |
used in candy, baked goods, cereals, and other processed foods [16][17]. | causes hyperactivity in children, DNA damage in animal studies, and may be carcinogenic [14][18]. |
| 2 | Tartrazine | Yellow 5 | 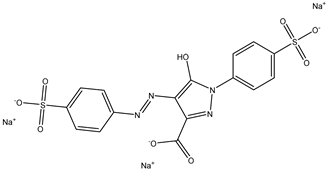 |
used in processed foods, soft drinks, snacks, and candies [19]. | associated with allergic reactions, asthma, hyperactivity in children, kidney tumors in animal studies [20]. |
| 3 | Brilliant Blue FCF | Blue 1 | 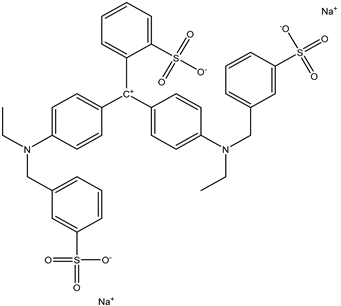 |
used in sports drinks, candies, baked goods, and other processed foods [21]. | causes hyperactivity in children, and it may be carcinogenic [22]. |
| 4 | Carmine | Red 4 | 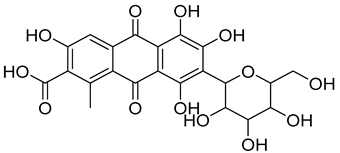 |
used in many food products, including juices, ice cream, and baked goods [23]. | associated with allergic reactions and can cause severe allergic reactions in some individuals [24]. |
| 5 | Fast green FCF | Green 3 | 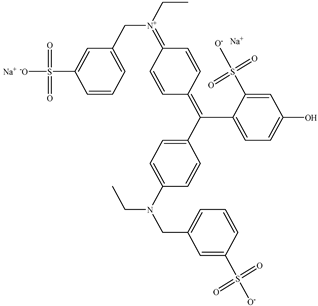 |
used in processed foods, including candy, ice cream, and baked goods [25]. | affects bone marrow chromosomes in animal studies [26]. |
| 6 | Metanil yellow | Acid yellow 36 | 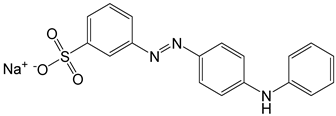 |
used in a lot of basic food stuffs, sauces, spicy products, ice creams, cold drinks, and juices [21]. | mutagenic effects [27], causes damage to gastric mucosa, and often tends to be carcinogenic [28]. |
2. Methods Used for the Degradation of Food Dyes
Various methods have been commonly employed for the degradation of toxic food dyes. The most common methods include photocatalytic degradation of dye incorporating NPs that use UV light to break down the dye molecules into smaller and less harmful compounds, most often water and carbon dioxide [29]. Another method used for degradation of dyes is biodegradation. This method involves the use of fungi and certain strains of bacteria that can break the dye molecules into smaller, harmless compounds [30]. Ozonation is one of the other most used methods for this purpose. It involves using ozone as an oxidizing agent to breakdown the dye molecules. Ozone is a strong oxidizing agent that can oxidize many organic compounds, including food dyes, into smaller, environmentally friendly compounds [31]. Chemical methods have also been employed for the degradation of dyes. This involves the use of certain chemicals, such as hydrogen peroxide or sodium hypochlorite, to break down the dye molecule [32]. Adsorption can also be used to adsorb the dye molecules from the water and food sources using certain adsorbents, such as activated carbon and hydrogels, after which the dye can be removed by physical means [33].
Of the methods discussed so far for the degradation of dyes, photocatalytic degradation using NPs possesses several advantages over other methods, including:
-
High efficiency: As the NPs possess a large surface-area-to-volume ratio, they offer many active sites within a small area and can thus increase the degradation efficiency even at a smaller concentration of the dye [34].
-
Selectivity: Photocatalytic degradation using NPs is a selective process that targets specific compounds and leaves the other compounds in the food matrix unharmed. This is because the photocatalysts are activated by light on a specific wavelength which can be tailored to specific dyes [35].
-
Environmental friendliness: The most important advantage of using photocatalysis over the other is the environmental friendliness of this method as it would most commonly result in the production of water and carbon dioxide as the end products which are not harmful to the environment, and can thus reduce the toxicity of the dyes to a greater extent [36].
-
Versatility: photocatalytic degradation can be used to degrade many toxic organic compounds, including those that are resistant to other methods; therefore, its properties are highly versatile [37].
-
Cost effectiveness: This method is relatively low cost as compared to the other as it does not involve the use of any expensive material or compound. All that is required is NPs, such as ZnO or TiO2, and exposure to UV light that can be obtained by exposure to the sunlight [38].
Overall, photocatalytic degradation using NPs is a promising technique for the degradation of toxic food dyes offers several advantages over other methods. However, the efficacy of the method may depend on several factors, such as the type and concentration of the dye, nature of the food matrix, and desired level of degradation. Some of the most common methods used for the degradation of dyes are shown in Figure 1.
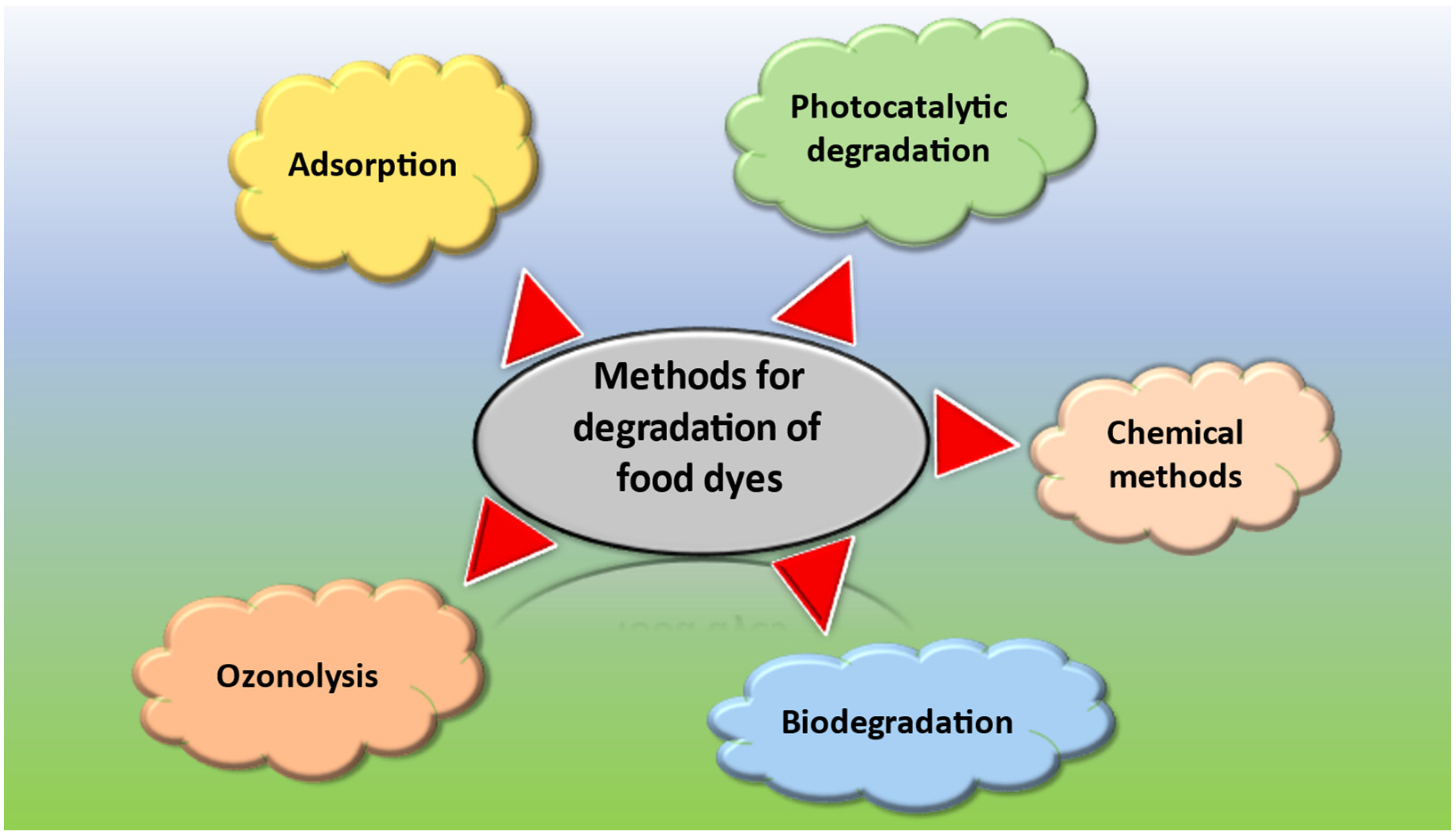
Figure 1. Common methods used for the degradation of food dyes.
2.1. Various Approaches for Synthesis of Photocatalytic NPs
NPs are generally synthesized using two most common approaches that includes top-down and bottom-up approach. Single atoms and molecules are used in the bottom-up process to build structures. As compared to those in macro formations, the covalent forces that bind these atoms together are far greater. NPs are created first, and are then assembled to create a final structure with the desired properties. Using a top-down method, nanostructures are created by physically or chemically shrinking the size of the starting material. Some of the methods utilized in the top-down approach include cutting, carving, molding, laser ablation, electroplating, hydrothermal treatment, and nano lithography [39]. Almost any element in the periodic table can be used in nanotechnology, depending on the intended use, which can range from nanomedicine to nano sensors. Designing materials with control over size, structure, and composition at the nanoscale level results in materials with enhanced properties [39]. Physical approaches for producing NPs have some significant drawbacks, including limited productivity, high prices, and significant energy consumption to reach synthesis conditions, such as high pressure and temperature [40]. Wet chemical processes are gaining popularity as a means of overcoming the constraints of physical approaches for the creation of NPs. A common technique entails creating NPs in a liquid reaction media while specific reducing chemicals, such as hydrazine and potassium bitartrate, are present. To prevent nanoparticle aggregation, several stabilizing agents are added to the reaction mixture, such as polyvinyl pyrrolidone. Chemical processes may have certain drawbacks despite their low cost and high output, including the production of hazardous byproducts and pollution from precursor solvents [41]. Thus, the need for innovative, nontoxic, high yielding, and environmentally acceptable methods of metallic nanoparticle manufacturing is increasing. Recent years have seen a huge increase in interest in the biological approach in this area. Due to their accessibility in nature, biological resources, such as plants, microorganisms, algae, fungi, etc., could be employed to synthesize NPs [41]. Various methods that have been used so far for the synthesis of NPs are shown in Figure 2.
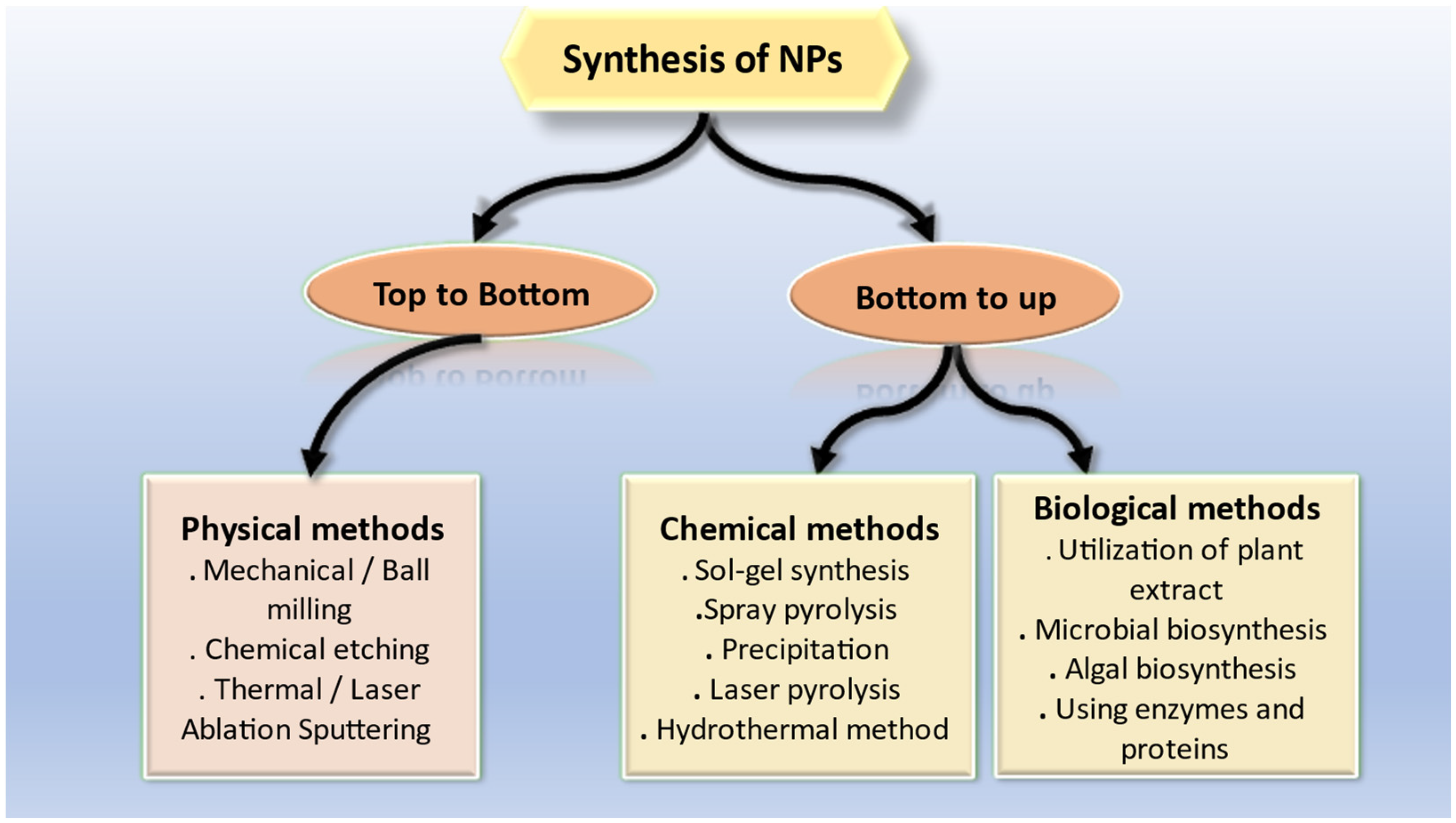
Figure 2. Various approaches for synthesis of NPs.
2.2. Green Synthesis and Characterization of Photocatalytic NPs
Green-synthesized NPs are nanoparticles that are synthesized using environmentally friendly methods, such as using plant extracts or other natural sources. Green synthesis of NPs involves the use of environmentally friendly methods to produce NPs without the use of hazardous chemicals. This approach has gained increasing attention in recent years due to its potential for producing biocompatible and non-toxic materials that can be used in a wide range of applications, including photocatalysis [42].
Green synthesis of NPs for photocatalytic applications typically involves the use of plant extracts, microorganisms, or other natural sources as reducing and stabilizing agents [43]. These methods offer several advantages over traditional synthesis methods, such as the use of toxic chemicals, high temperatures, and high pressures. Green synthesis methods are also relatively simple and cost-effective, making them ideal for large-scale production. This method also offers several advantages over other green synthesis methods, such as microorganism-mediated synthesis or physical methods. Plant-mediated synthesis of NPs has been reported to be effective for the synthesis of a wide range of metallic NPs, including silver [44], gold [45], platinum [46], and copper [47]. For instance, nanoparticles synthesized using plant extracts can have a higher degree of crystallinity, smaller particle size, and higher surface area compared to those synthesized using traditional methods [48]. These properties can enhance the photocatalytic activity of the NPs, leading to more efficient degradation of food and juice toxins. Green synthesis of NPs using plant extract mainly include collection of the plant material (root, stem, or leaves) followed by air drying, which usually takes 5–6 days. Then the dried parts are thoroughly grinded, and the weighed quantity is boiled in water, usually at 70–80 °C, for 3–4 h. After filtration, the plant extract is obtained. A salt solution and plant extract are allowed to mix together, which usually results in the synthesis of NPs, either by the precipitation method or the combustion method. The various phytochemicals present in the plant extract serve as reducing, stabilizing and capping agents [49].
Characterization of NPs is an essential step in evaluating their photocatalytic properties. Common techniques used for nanoparticle characterization include UV-Visible spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and Fourier transform infrared spectroscopy (FTIR) and XPS.
Green-synthesized NPs can provide insights into the optimal photocatalyst properties for effective toxin degradation. The use of green synthesis methods can also enhance the sustainability and eco-friendliness of the overall process [50]. Therefore, studying the green synthesis and characterization of NPs for this application is crucial for the development of efficient and sustainable photocatalytic degradation methods for food and juice toxins.
For example, spherical-shaped silver NPs were successfully synthesized using Viburnum opulus fruit extract [51]. Another group of researchers also synthesized spherical-shaped silver NPs using the leaf extract of Kalanchoe brasiliensis [44]. A schematic representation for the green synthesis of nanoparticles is shown in Figure 3.
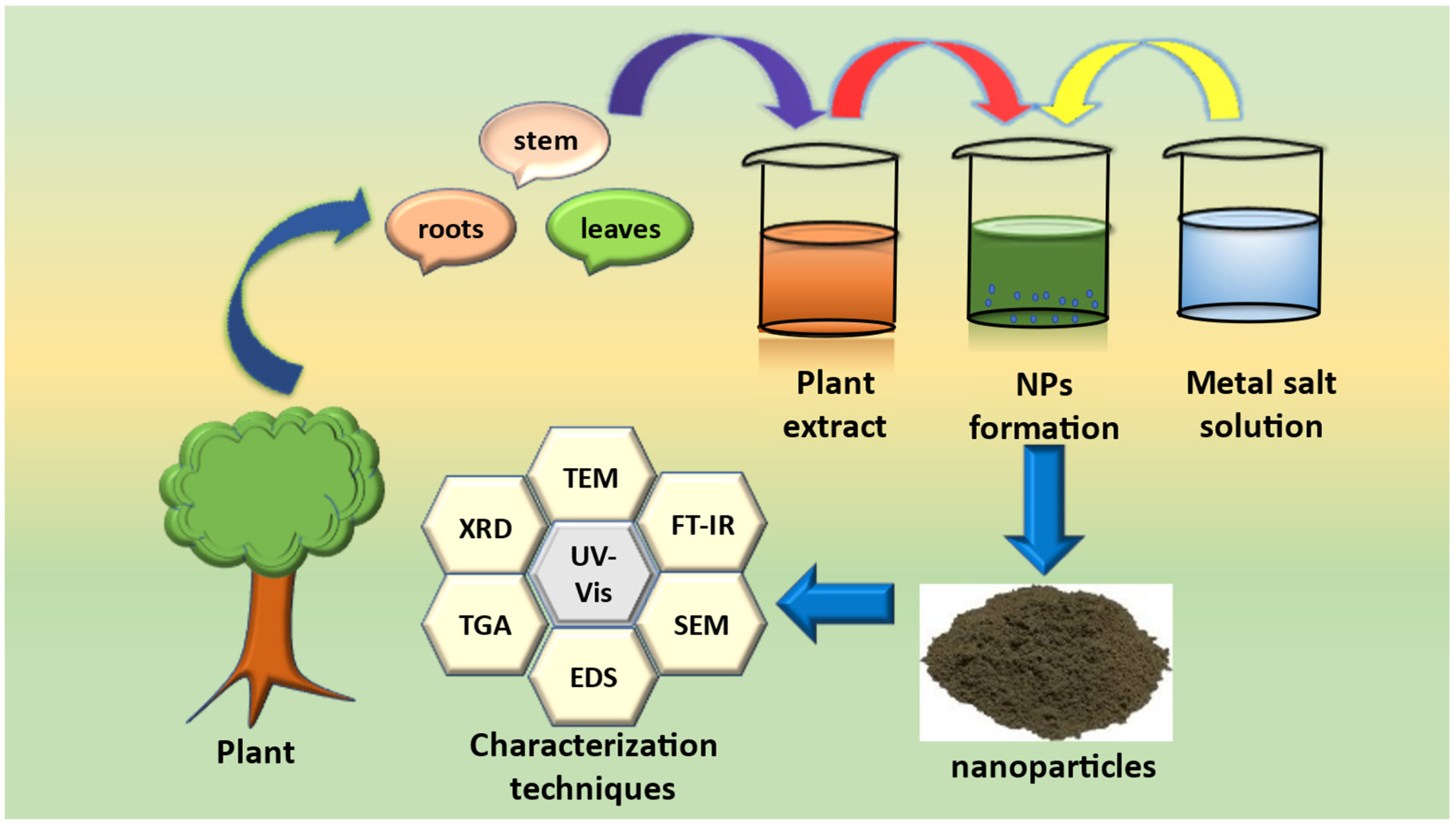
Figure 3. Schematic representation for green synthesis of nanoparticles.
References
- Brudzyńska, P.; Sionkowska, A.; Grisel, M. Plant-Derived Colorants for Food, Cosmetic and Textile Industries: A Review. Materials 2021, 14, 3484.
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety Assessment of Personal Care Products/Cosmetics and Their Ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259.
- Padervand, M.; Rhimi, B.; Wang, C. One-Pot Synthesis of Novel Ternary Fe3N/Fe2O3/C3N4 Photocatalyst for Efficient Removal of Rhodamine B and CO2 Reduction. J. Alloys Compd. 2021, 852, 156955.
- Chequer, F.D.; De Oliveira, G.R.; Ferraz, E.A.; Cardoso, J.C.; Zanoni, M.B.; de Oliveira, D.P. Textile Dyes: Dyeing Process and Environmental Impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176.
- Khan, A.D.; Alam, M.N. Cosmetics and Their Associated Adverse Effects: A Review. J. Appl. Pharm. Sci. Res. 2019, 2, 1–6.
- Sakin Omer, O.; Hussein, M.A.; Hussein, B.H.M.; Mgaidi, A. Adsorption Thermodynamics of Cationic Dyes (Methylene Blue and Crystal Violet) to a Natural Clay Mineral from Aqueous Solution between 293.15 and 323.15 K. Arab. J. Chem. 2018, 11, 615–623.
- Senthil Kumar, P.; Varjani, S.J.; Suganya, S. Treatment of Dye Wastewater Using an Ultrasonic Aided Nanoparticle Stacked Activated Carbon: Kinetic and Isotherm Modelling. Bioresour. Technol. 2018, 250, 716–722.
- Haleem, A.; Shafiq, A.; Chen, S.Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081.
- Pimol, P.; Khanidtha, M.; Prasert, P. Influence of Particle Size and Salinity on Adsorption of Basic Dyes by Agricultural Waste: Dried Seagrape (Caulerpa lentillifera). J. Environ. Sci. 2008, 20, 760–768.
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A Review on Classifications, Recent Synthesis and Applications of Textile Dyes. Inorg. Chem. Commun. 2020, 115, 107891.
- Arami, M.; Limaee, N.Y.; Mahmoodi, N.M. Evaluation of the Adsorption Kinetics and Equilibrium for the Potential Removal of Acid Dyes Using a Biosorbent. Chem. Eng. J. 2008, 139, 2–10.
- Safarik, I.; Mullerova, S.; Pospiskova, K. Semiquantitative Determination of Food Acid Dyes by Magnetic Textile Solid Phase Extraction Followed by Image Analysis. Food Chem. 2019, 274, 215–219.
- Eisenbrand, G.; Pool-Zobel, B.; Baker, V.; Balls, M.; Blaauboer, B.; Boobis, A.; Carere, A.; Kevekordes, S.; Lhuguenot, J.-C.; Pieters, R. Methods of in Vitro Toxicology. Food Chem. Toxicol. 2002, 40, 193–236.
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health Safety Issues of Synthetic Food Colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922.
- Kobun, R.; Siddiquee, S.; Shaarani, S.M. Rapid Detection of Allura Red (E129) Based on Chitosan/Nanoparticles/MWCNTs Modified Gold Electrode in Food Products. Trans. Sci. Technol. 2015, 2, 56–64.
- Wu, D.; Yan, J.; Wang, J.; Wang, Q.; Li, H. Characterisation of Interaction between Food Colourant Allura Red AC and Human Serum Albumin: Multispectroscopic Analyses and Docking Simulations. Food Chem. 2015, 170, 423–429.
- Tsuda, S.; Murakami, M.; Matsusaka, N.; Kano, K.; Taniguchi, K.; Sasaki, Y.F. DNA Damage Induced by Red Food Dyes Orally Administered to Pregnant and Male Mice. Toxicol. Sci. 2001, 61, 92–99.
- Zhong, Y.; Shi, T.; Liu, Z.; Cheng, S.; Huang, Y.; Tao, X.; Liao, G.; Tang, Z. Ultrasensitive Non-Enzymatic Glucose Sensors Based on Different Copper Oxide Nanostructures by in-Situ Growth. Sens. Actuators B Chem. 2016, 236, 326–333.
- Journal, T.H.E.; Tropical, O.F.; Science, L. Survey on the use of synthetic food colors in food samples procured from. J. Trop. Life Sci. 2013, 3, 1–7.
- Motta, C.M.; Simoniello, P.; Arena, C.; Capriello, T.; Panzuto, R.; Vitale, E.; Agnisola, C.; Tizzano, M.; Avallone, B.; Ferrandino, I. Effects of Four Food Dyes on Development of Three Model Species, Cucumis sativus, Artemia salina and Danio rerio: Assessment of Potential Risk for the Environment. Environ. Pollut. 2019, 253, 1126–1135.
- Shah, A. A Novel Electrochemical Nanosensor for the Simultaneous Sensing of Two Toxic Food Dyes. ACS Omega 2020, 5, 6187–6193.
- Nayak, D.S.; Shetti, N.P. A Novel Sensor for a Food Dye Erythrosine at Glucose Modified Electrode. Sens. Actuators B Chem. 2016, 230, 140–148.
- Arif, A.; Ahmad, A.; Ahmad, M. Toxicity Assessment of Carmine and Its Interaction with Calf Thymus DNA. J. Biomol. Struct. Dyn. 2021, 39, 5861–5871.
- Guaraldo, T.T.; Zanoni, T.B.; de Torresi, S.I.; Gonçales, V.R.; Zocolo, G.J.; Oliveira, D.P.; Zanoni, M.V.B. On the Application of Nanostructured Electrodes Prepared by Ti/TiO2/WO3 “Template”: A Case Study of Removing Toxicity of Indigo Using Visible Irradiation. Chemosphere 2013, 91, 586–593.
- Asif Ahmed, M.; Al-Khalifa, A.S.; Al-Nouri, D.M.; El-din, M.F.S. Dietary Intake of Artificial Food Color Additives Containing Food Products by School-Going Children. Saudi J. Biol. Sci. 2021, 28, 27–34.
- Giri, A.K.; Banerjee, T.S.; Talukder, G.; Sharma, A. Effects of Dyes (Indigo Carmine, Metanil Yellow, Fast Green FCF) and Nitrite in Vivo on Bone Marrow Chromosomes of Mice. Cancer Lett. 1986, 30, 315–320.
- Srivastava, L.P.; Khanna, S.K.; Singh, G.B.; Murti, C.R.K. In Vitro Studies on the Biotransformation of Metanil Yellow. Environ. Res. 1982, 27, 185–189.
- Gupta, S.; Sundarrajan, M.; Rao, K.V.K. Tumor Promotion by Metanil Yellow and Malachite Green during Rat Hepatocarcinogenesis Is Associated with Dysregulated Expression of Cell Cycle Regulatory Proteins. Teratog. Carcinog. Mutagen. 2003, 312, 301–312.
- Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Senthilkumar, P.; Gnanasekaran, K.; Shanmugavel, M.; Manikandan, E.; Pugazhendhi, A. Synthesis of Ecofriendly Copper Oxide Nanoparticles for Fabrication over Textile Fabrics: Characterization of Antibacterial Activity and Dye Degradation Potential. J. Photochem. Photobiol. B Biol. 2019, 191, 143–149.
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial Degradation of Dyes: An Overview. Bioresour. Technol. 2020, 314, 123728.
- Muniyasamy, A.; Sivaporul, G.; Gopinath, A.; Lakshmanan, R.; Altaee, A.; Achary, A.; Velayudhaperumal Chellam, P. Process Development for the Degradation of Textile Azo Dyes (Mono-, Di-, Poly-) by Advanced Oxidation Process—Ozonation: Experimental & Partial Derivative Modelling Approach. J. Environ. Manag. 2020, 265, 110397.
- Dhawle, R.; Frontistis, Z.; Mantzavinos, D.; Lianos, P. Production of Hydrogen Peroxide with a Photocatalytic Fuel Cell and Its Application to UV/H2O2 Degradation of Dyes. Chem. Eng. J. Adv. 2021, 6, 100109.
- Wang, H.; Li, Z.; Yahyaoui, S.; Hanafy, H.; Seliem, M.K.; Bonilla-Petriciolet, A.; Luiz Dotto, G.; Sellaoui, L.; Li, Q. Effective Adsorption of Dyes on an Activated Carbon Prepared from Carboxymethyl Cellulose: Experiments, Characterization and Advanced Modelling. Chem. Eng. J. 2021, 417, 128116.
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green Synthesis of Silver Nanoparticles Using Cauliflower Waste and Their Multifaceted Applications in Photocatalytic Degradation of Methylene Blue Dye and Hg2+ Biosensing. SN Appl. Sci. 2020, 2, 738.
- Navia-Mendoza, J.M.; Filho, O.A.E.; Zambrano-Intriago, L.A.; Maddela, N.R.; Duarte, M.M.M.B.; Quiroz-Fernández, L.S.; Baquerizo-Crespo, R.J.; Rodríguez-Díaz, J.M. Advances in the Application of Nanocatalysts in Photocatalytic Processes for the Treatment of Food Dyes: A Review. Sustainability 2021, 13, 11676.
- Nazim, M.; Khan, A.A.P.; Asiri, A.M.; Kim, J.H. Exploring Rapid Photocatalytic Degradation of Organic Pollutants with Porous CuO Nanosheets: Synthesis, Dye Removal, and Kinetic Studies at Room Temperature. ACS Omega 2021, 6, 2601–2612.
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725.
- Adekunle, A.S.; Oyekunle, J.A.O.; Durosinmi, L.M.; Oluwafemi, O.S.; Olayanju, D.S.; Akinola, A.S.; Obisesan, O.R.; Akinyele, O.F.; Ajayeoba, T.A. Potential of Cobalt and Cobalt Oxide Nanoparticles as Nanocatalyst towards Dyes Degradation in Wastewater. Nano-Struct. Nano-Objects 2020, 21, 100405.
- Hashmi, S.S.; Shah, M.; Muhammad, W.; Ahmad, A.; Ullah, M.A.; Nadeem, M.; Abbasi, B.H. Potentials of Phyto-Fabricated Nanoparticles as Ecofriendly Agents for Photocatalytic Degradation of Toxic Dyes and Waste Water Treatment, Risk Assessment and Probable Mechanism. J. Indian Chem. Soc. 2021, 98, 100019.
- Nadeem, M.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Hashmi, S.S.; Ahmad, W.; Zahir, A. The Current Trends in the Green Syntheses of Titanium Oxide Nanoparticles and Their Applications. Green Chem. Lett. Rev. 2018, 11, 492–502.
- Abbasi, B.H.; Shah, M.; Hashmi, S.S.; Nazir, M.; Naz, S.; Ahmad, W.; Khan, I.U.; Hano, C. Green Bio-Assisted Synthesis, Characterization and Biological Evaluation of Biocompatible Zno Nps Synthesized from Different Tissues of Milk Thistle (Silybum marianum). Nanomaterials 2019, 9, 1171.
- Parveen, K.; Banse, V.; Ledwani, L. Green Synthesis of Nanoparticles: Their Advantages and Disadvantages. AIP Conf. Proc. 2016, 1724, 020048.
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374.
- Eswaran, S.G.; Afridi, P.S. Effective Multi Toxic Dyes Degradation Using Bio-Fabricated Silver Nanoparticles as a Green Catalyst. Appl. Biochem. Biotechnol. 2023, 195, 3872–3887.
- Geetha, R.; Ashokkumar, T.; Tamilselvan, S.; Govindaraju, K.; Sadiq, M.; Singaravelu, G. Green Synthesis of Gold Nanoparticles and Their Anticancer Activity. Cancer Nanotechnol. 2013, 4, 91–98.
- Kumar, P.V.; Jelastin Kala, S.M.; Prakash, K.S. Green Synthesis Derived Pt-Nanoparticles Using Xanthium strumarium Leaf Extract and Their Biological Studies. J. Environ. Chem. Eng. 2019, 7, 103146.
- Jayarambabu, N.; Akshaykranth, A.; Venkatappa Rao, T.; Venkateswara Rao, K.; Rakesh Kumar, R. Green Synthesis of Cu Nanoparticles Using Curcuma longa Extract and Their Application in Antimicrobial Activity. Mater. Lett. 2020, 259, 126813.
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Maaza, M.; Ayeshamariam, A.; Kennedy, L.J. Green Synthesis of NiO Nanoparticles Using Moringa oleifera Extract and Their Biomedical Applications: Cytotoxicity Effect of Nanoparticles against HT-29 Cancer Cells. J. Photochem. Photobiol. B Biol. 2016, 164, 352–360.
- Fatmarin, Z.; Dani Nandiyanto, A.B. Economic Evaluation of Zinc Oxide Nanoparticle Production through Green Synthesis Method Using Cassia fistula Plant Extract. Int. J. Energ. 2020, 5, 18–24.
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A Novel One-Pot “green” Synthesis of Stable Silver Nanoparticles Using Soluble Starch. Carbohydr. Res. 2006, 341, 2012–2018.
- David, L.; Moldovan, B. Green Synthesis of Biogenic Silver Nanoparticles for E Ffi Cient Catalytic Removal of Harmful Organic Dyes. Nanomaterials 2020, 10, 202.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
16 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




