Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Danladi Chiroma Husaini | -- | 3487 | 2023-06-15 15:39:53 | | | |
| 2 | Jessie Wu | Meta information modification | 3487 | 2023-06-16 05:21:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Frazzoli, C.; Grasso, G.; Husaini, D.C.; Ajibo, D.N.; Orish, F.C.; Orisakwe, O. Herbal Plants for Epidemics and Pandemics. Encyclopedia. Available online: https://encyclopedia.pub/entry/45669 (accessed on 07 February 2026).
Frazzoli C, Grasso G, Husaini DC, Ajibo DN, Orish FC, Orisakwe O. Herbal Plants for Epidemics and Pandemics. Encyclopedia. Available at: https://encyclopedia.pub/entry/45669. Accessed February 07, 2026.
Frazzoli, Chiara, Gerardo Grasso, Danladi Chiroma Husaini, Doris Nnenna Ajibo, Fortune Chiemelie Orish, Orish Orisakwe. "Herbal Plants for Epidemics and Pandemics" Encyclopedia, https://encyclopedia.pub/entry/45669 (accessed February 07, 2026).
Frazzoli, C., Grasso, G., Husaini, D.C., Ajibo, D.N., Orish, F.C., & Orisakwe, O. (2023, June 15). Herbal Plants for Epidemics and Pandemics. In Encyclopedia. https://encyclopedia.pub/entry/45669
Frazzoli, Chiara, et al. "Herbal Plants for Epidemics and Pandemics." Encyclopedia. Web. 15 June, 2023.
Copy Citation
With over 6 million coronavirus pandemic deaths, the African continent reported the lowest death rate despite having a high disease burden. The African community’s resilience to the pandemic has been attributed to climate and weather conditions, herd immunity, repeated exposure to infectious organisms that help stimulate the immune system, and a disproportionately large youth population. In addition, functional foods, herbal remedies, and dietary supplements contain micronutrients and bioactive compounds that can help boost the immune system.
pandemics
epidemics
immune system
immune boosters
bioactive compounds
fermented foods
herbal remedies
medicinal plants
Africa
1. Garcinia kola Heckel (Fam. Clusiaceae) [Bitter Cola]
Every part of bitter kola (Garcinia kola Heckel) is traditionally used in Africa to treat typhoid fever, bronchitis, bacterial infections, malignant tumors, skin infections, tuberculosis, gastritis, colds, and jaundice [1][2]. The plant is indigenous to Africa and pharmacologically evaluated in animal studies to have antiviral, antiasthma, antioxidant, antidiabetic, antihypertensive, antibacterial, antiasthma, and hepatoprotective activities [3][4][5]. Although G. kola has not been scientifically documented for use in pandemics, its traditional usage and bioactive constituents make the plant a candidate for use in pandemics and epidemics in Africa [1]. For instance, in recent reviews, G. kola has been suggested as a potential and promising medicinal plant for the treatment of coronaviruses due to its antiviral and antioxidant activities [6][7][8]. Similarly, G. kola, the active constituent of G. kola, has been reported to be active against polioviruses, measles virus, yellow fever virus, influenza, and herpes simplex virus-1 [9][10]. G. kola’s immunorestorative and immunomodulatory activities make it a significant plant in diseases causing immunodeficiencies, such as COVID-19 and AIDS [11]. The bioactive compounds found in G. kola include alkaloids, phenols, saponins, sterols, tannins, garciniflavanone, kolanone, garcinoic acid, kolaflavanone, and kolaviron [1][5][12]. The seed is also rich in phosphorous and potassium [12]. Kolavoviron has significant anti-inflammatory and antioxidant activity. It was reported to be effective against viruses via immunomodulating activities, metal chelating, and as a potent radical scavenger [9][13][14]. Furthermore, kolavoviron modulate oxidative stress via stimulation of phase 2 detoxification of enzymes, proving its chemopreventive effects. This action mitigates the expression of COX-2 and iNOS at the molecular level, downregulating AP-1 DNA and NF-kB binding, and mitigating oxidative damage to biomolecules [13][15][16]. Other derivatives of G. kola, such as guttiferones and garcinol, have been reported to inhibit the cytopathic effects of HIV [17]. Toxicological studies in rats and mice showed G. kola to be safe [18]. The long history of the seed’s usage and the safe toxicological profile make G. kola a suitable candidate for clinical trials in pandemics.
2. Artemisia afra Jacq. (Fam. Asteraceae) [African Wormwood]
Dihydroxybishopsolicepolide, scopoletin, acacetin, flavonoids, and yomogiartemin are the common phytochemicals identified and described in Artemisia [19][20]. The shrub is abundant in Northern, Eastern, and Southern Africa. It has been reported for use traditionally in treating influenza, respiratory infections, cough, malaria, diabetes, and fever [21]. The plant’s bioactive ingredients have been reported to have potent antioxidant activity through scavenging hydrogen peroxide and hydroxyl ions and modulating reactive oxygen species, thus, making Artemisia a protective agent that strengthens the antioxidant defense mechanism [22]. Furthermore, the phytochemicals in Artemisia and their derivatives provided selective cytotoxicity in randomized double-blinded subjects treated for colorectal cancer [23]. Slezakova and Ruda-Kucerova (2017) [24] further reported Artemisia’s promising potential in hepatocellular carcinoma, lung cancer, and breast cancer.
Artemisia also has significant antiviral activities against influenza virus A, human herpes viruses 1 and 2, hepatitis B and C, and HIV-1 viruses [25][26][27]. Artemisia’s general mechanism of action blocks the host-cell–type and metabolic requirements for viral replication by inhibiting the central regulatory activity of viral-infected cells [25][27][28].
Artemisia is an African indigenous traditional medicinal plant for treating diseases associated with pandemics and epidemics. Its antioxidant, anti-inflammatory, antiviral activities, low toxicity, and safety make Artemisia a potential drug candidate for the prevention and treatment of diseases in pandemics and epidemics on the continent of Africa [21][29].
3. Piper guineense (Fam. Piperaceae) [African Black Pepper]
One of the most valuable African plant species widely applied in traditional medicine is P. guineense. The plant has potent antioxidant properties with strong antibacterial, anticancer, and antiviral properties [30]. An ethnopharmacological survey showed that the plant is used in traditional African settings for sexually transmitted diseases [31]. Piperine, piperlongumine, ligans, monoterpenes, terpenoids, sterols, sesquiterpenes, and volatile oils are some of the bioactive compounds in P. guineense [32]. These alkaloids are scaffolds for discovering new drugs since they contain antimicrobial pharmacological properties [33][34]. For instance, piperine, a potent antibacterial agent, inhibits the efflux pump in Staphyloccocus aureus, making it a potential phytochemical for multidrug-resistant bacteria [35].
Furthermore, a recent study suggested that a combination of piperine and rifampicin improved rifampicin’s effectiveness, making piperine an agent that can reduce the adverse effects of rifampin when used in clinical therapy [36]. Apart from piper’s reported effectiveness as an anti-inflammatory and antiproliferative, the bioactive compound was also reported to demonstrate protection against chronic diseases based on clinical studies [26]. Furthermore, piperlongumine is effective as an anticancer, antifungal, antihelminth, and in treating many neglected tropical diseases in Africa, making P. guineense a valuable plant used in pandemics [26]. The effectiveness of piperlongumine in neurodegenerative diseases is due to its ability to inhibit or reduce the synthesis of prostaglandins E2, nitric oxide, cyclooxygenases-2, nuclear factor kappa B, interleukin-6, and tumor necrosis factor-alpha 9 [37][38]. Osho et al. (2016) reported the antiviral activity of P. guineense’s methanolic extract in broiler chickens infected with Newcastle disease virus (NDV) [39]. The numerous underlying mechanisms of action and multitargeting potentials of P. guineense are still being studied, even though the benefits have existed in Africa for a long time [38].
4. Achyranthes Aspera Linn. (Fam. Amaranthaceae)
The origin of Achyranthes aspera is in Africa, even though some literature indicates that the plant is also to be native to South Asia [40]. The plant has been associated with its usefulness in pandemics due to its significant antioxidant and immune-boosting abilities and its antiviral effects [40][41]. The antiviral potential of a methanolic extract of Achyranthes aspera was evaluated against herpes simplex virus type 1 and type 2. A. aspera demonstrated good anti-Herpes simplex virus activity [41]. In addition to its usefulness in pandemics, the entire part of A. aspera has been traditionally used in Africa and other parts of Asia for dysentery, arthritis, malaria, hemorrhoids, fever, pain, and diarrhea [40]. In addition, the plant is diuretic, anti-inflammatory, antiasthmatic, and valuable for pneumonia [42][43][44].
Triacontanol, eugenol, ecdysterone, and betaine are the main bioactive compounds in A. aspera. Furthermore, the plant’s antioxidant and immune-boosting activities are enhanced by ascorbic acid [44][45][46][47]. The ascorbic acid in A. aspera significantly boosts immunity and alleviates inflammation in the SARS-coronavirus by conferring antioxidant properties [8]. The chemopreventive effects of A. aspera have also been reported in a few studies. Saponin fractions of A. aspera considerably reduce early antigen activation elicited by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate in Raji cells by Epstein-Barr virus. [48]. Furthermore, A. aspera induced apoptosis through a mitochondrial-mediated pathway [49].
5. Allium sativum L. (Fam. Liliaceae) [Garlic]
Garlic (Allium sativum L., Fam. Liliaceae), though historically believed to originate from West China, is presently cultivated and used globally as a spice, immune booster, and a remedy during pandemics. Garlic is the first remedy to prevent and treat pandemics, such as influenza, typhus, cholera, and dysentery [50]. Garlic was fed to pyramid builders to boost immunity and was reported as a nutritional supplement in ancient inscriptions in Egyptian pyramid plates. In addition, garlic provided the builders with vitamins, balance, and the energy to pull the heavy plates used in building the pyramids [51].
Traditionally, garlic has been used in Africa to manage bacterial, parasitic, viral, and other infectious diseases [52][53][54]. Other uses included gynecological diseases, toothaches, snake bites, arthritis, and hypertension [52][53][55][56]. In general, garlic extracts are effective as antioxidants, anticancer, and antimicrobials and tend to reduce the risk of cardiovascular events [50][57][58]. Experimental animal and clinical studies have provided evidence of garlic’s effectiveness in managing the common flu, diabetes, hypertension, arthritis, and cancer prevention [58][59].
Over a hundred bioactive compounds are found in garlic, with allicin (thiosulfate) being the most active and responsible for garlic taste and smell [60]. Other sulfur-containing bioactive substances found in garlic include diallyl trisulfate, ajoenes, diallyl disulfide (sulfides), 2-vinyl-(4H)-1,3-dithiin, 3-vinyl-(4H)-1,2-dithiin (vinyldithiins), and alliin, which, constitutes majorly cysteine sulfoxide [61][62][63].
Garlic is used in pandemics due to its antioxidant, antiviral, and antibacterial activities [4]. Reduction in the synthesis of oxygen-free radical species has been reported with garlic when it is frequently consumed, promoting antioxidant activity [63][64]. Similarly, garlic extracts have been reported to reduce glutathione peroxides and superoxide dismutase in rats’ hepatic tissues [65][66]. In addition, high radical scavenging activity with different sulfur-containing substances in garlic, phenols, and flavonoids has been demonstrated with garlic extracts [67]. Other antioxidant activities of the bioactive compounds in garlic include a decrease in the synthesis of reactive nitrogen and oxygen species by diallyl sulfide through the enzymatic suppression of cytochrome P450-2E1, leading to hepatoprotection [63][68]; inhibition and H2O2-induced DNA damage with saponins extracted from garlic [69]; and the inhibition of NADPH oxidase 1 through the prevention of reactive oxygen species with alliin [70].
In a recent review, Batiha et al. (2020) [63] reported that garlic extract is effective against diverse viruses, such as vesicular stomatitis virus, human rhinovirus type 2, influenza virus type 3, human cytomegalovirus, influenza B type virus, and herpes simplex 1 and 2 [71]. The inhibition of adhesive interaction and fusion of leukocytes with ajoenes; inhibiting the synthesis of thiol enzymes by allicin; and the enhancement of natural killer-cell activity through the destruction of cells infected by viruses’ diallyl trisulfide on human cytomegalovirus are a few mechanisms of garlic’s action [63][71]. Finally, allicin has a wide range of activities against many bacterial organisms, including K. aerogenes, E. faecalis, S. enterica, E. coli, S. pyogenes, S. mutans, Mycobacteria, Shigella, P. vulgaris, and P. aeruginosa [53][65][72][73][74][75]. The ability of garlic to inhibit NF-kB and modulate cytokine expression makes it an immunomodulatory and effective plant for pandemics in Africa. Finally, prostaglandin-E2, COX-2, and nitric oxide production inhibition lead to a significant reduction in the synthesis of inflammatory interferon γ, interleukin-6, and TNF- α with garlic [75].
6. Moringa oleifera Lam. (Fam. Moringaceae)
Moringa oleifera is commonly called the miracle tree in Africa because of its numerous traditional and pharmacological activities against various diseases. The plant’s multipurpose nutritional and health benefits have been widely reported in Africa and most parts of the world [56][76][77][78]. The plant has been traditionally used in Africa for food, livestock feed, nutrition, and medicine [79]. Recently, Moringa has been used in biofuel production, cosmetics, and water purification [77][80]. The phytochemicals responsible for Moringa’s biological effects include isothiocyanate, phenolic acids, polyphenols, sterols, alkaloids, terpenes, flavonoids, and flavanol glycosides [76][77][78][79]. Some ethnopharmacological activities of moringa include antioxidant, parasitic diseases, antituberculosis, anticancer, antidiabetic, anti-inflammatory, sexually transmitted infections, typhoid fever, cardioprotective, neuroprotective, antihypertensive, and hepatoprotective effects [78]. Even though moringa significantly decreased triglyceride, cholesterol, and glucose levels in rats; the plant is safe and has a high therapeutic index [81]. Moringa leaves are rich in beta carotene, minerals, and proteins, essential compounds lacking in most populations found in developing nations. The ability of Moringa to boost the immune system and help the body fight infections has been reported. The plant showed some significant activities against various viruses, including HIV, and has been reported to be used in managing AIDS and diseases related to AIDS infections [82][83]. In addition, Moringa is effective against the influenza A virus, new castle disease virus, herpes simplex virus, Epstein-Barr virus, hepatitis B virus, and foot-and-mouth disease virus in cloven-footed animals [84]. Protection of infected host cells from cytopathic effect; decrease in the levels of cytokines; inhibition of the expression and nuclear transfer of cellular proteins transcription factor EB leading to a weakening of the autophagy infected cells; and the inhibition of viral replication in host cells are some of the reported antiviral mechanisms of action of moringa [85]. These biologic activities made Moringa an African medicinal plant used to prevent and treat pandemics and diseases related to pandemics in the African continent.
7. Zingiber officinale R. (Fam Zingiberaceae) [Ginger]
Zingiber officinale R. (Fam Zingiberaceae) is presently widely cultivated in most African countries for the prevention and treatment of common influenza, cough, sore throats, arthritis, lung diseases, peptic ulcer disease, hypertension, and infectious diseases, such as bacterial and viral infections. Although Ginger was initially used as a food spice and for medicinal plants in China, the use of ginger in Africa for the prevention and treatment of diseases has been documented [86]. Presently over 100 species of ginger are cultivated worldwide; however, Z. officinale is the most cultivated and used as an ingredient for food and medicinal plants [87][88].
The US Department of Agriculture (2013) identified steroids, phenols, and alkaloids as the bioactive compounds found in ginger with therapeutic activities. Other active compounds include gingerols, zingerone, zingiberol, paradols, and shogaols [89][90][91][92]. Ginger has been widely reported to have antiarthritic, anticancer, antioxidant, anti-rhinoviral, antimicrobial, and antiglycemic pharmacological activities [90][93][94]. Shogaol and gingerol have significant pharmacological activity compared to other bioactive compounds found in ginger [95].
Ginger’s antiviral, antioxidant, and antibacterial effects make it a viable medicinal herb and spice used to prevent and manage pandemics in Africa [96]. Chang et al. (2013) demonstrated that fresh ginger acts by blocking viral attachments and internalization and is effective against human respiratory syncytial virus-induced plaque formation on airway epithelium [97]. Similarly, Rathinavel et al. (2020) reported that 6-gingerol, a phytocompound from Z. officinale, showed high binding affinity against SARS COVID-19, including spike proteins, RNA binding, and viral proteases proving antiviral activity, making 6-gingerol a promising bioactive compound for COVID-19. Good ADME pharmacokinetic properties and excellent drug-likeness parameters with zero rule violations were demonstrated by 6-gingerol [98]. Generally, the Zingiberaceae plant family (Zingiber officinale, Curcuma longa, and Aframomum melegueta) is effective against a wide variety of viruses, such as Enterovirus 71, Japanese encephalitis virus, Epstein-Barr virus, Herpes simplex virus types 1 and 2, influenza A virus, human immunodeficiency viruses, coronavirus SARS-CoV-1, rhinovirus, chikungunya virus, and respiratory syncytial virus. In addition, these herbs are significant immune system boosters and excellent sources of nutrition [96].
Furthermore, besides possessing antiviral activities, Z. officinale has significant antioxidant activities. Zingerone, for instance, suppresses lipid peroxidation and scavenges hydroxyl ions and peroxides [99][100][101]. Similarly, 6-gingerol effectively protects against ultraviolet B-induced skin disorders in rats by inhibiting the induction of NF-kappa translocation, proteins, and cyclooxygenase-2 mRNA [102]. Z. officinale terpenoids significantly rendered endometrial cancer cells ineffective by promoting the stimulation of p53 [94]. In addition, the combination of 10-gingerol, 8-gingerol, 6-gingerol, and 6-shogaol prevented the rapid multiplication of PC-3 prostate cancer cells, thereby providing cytoprotective effects [102][103]. Other studies on ginger’s cytoprotective effects reported ginger powder suppressing the synthesis of the COX-1 enzyme associated with intestinal cancers [104]. Similarly, a decrease in the synthesis of metalloproteinase-9 and the colonization of breast cancer cells was demonstrated by 6-shogaol [105]. In addition, 6-gingerol stimulates the generation and formation of new blood vessels, aiding in the prevention of the spread of cancer cells [106]. Preventing the synthesis of prostaglandin synthase or 5-lipoxygenase with gingerol and shogaol effectively inhibits the expression of prostaglandins and leukotrienes [107][108]. Although the rhizome is commonly used, all the parts of ginger are utilized in African traditional medicine [8].
8. Momordica charantia (Fam. Cucurbitaceae) [Bitter Melon]
Bitter melon (Momordica charantia) has been used as a medicinal plant to treat viral and gastrointestinal infections and for ritual purposes in Africa [109]. Bitter melon has been traditionally used to treat diabetes [110]. It contains several bioactive compounds that contribute to its antiviral and antioxidant activities [109][111][112]. The fruit contains minerals, vitamins, phenolic compounds, triterpenes, lipids, proteins, glycosides, steroids, saponins, and flavonoids [111].
M. charantia possessed antiviral activities and was reported to be effective against HIV-1, dengue virus, and hepatitis B [113]. Rebultan (1995) reported that M. charantia significantly increased and normalized CD4 count and CD4/CD8 ratio in HIV-infected persons while reducing recurrent respiratory infections [114].
In addition, M. charantia has been suggested as a promising anticancer agent whose bioactive compounds target cancer cells and are used to correct metabolic aberrations [115]. Autophagy and apoptosis are the mechanisms of action of M. charantia-mediated cell death in colon and breast cancers, respectively [116][117]. The plant’s potential medicinal effects and applications for HIV, diabetes, and cancers made it a good candidate for the treatment of pandemic-related viral diseases and to boost immunity in poor African populations where sophisticated healthcare systems are not available [110][113][116][118]. The variety of steroids and protein compounds isolated from M. charantia, such as kuguacin C and kuguacin E, give the plant its antiviral properties. In a recent review, Jia et al. (2017) summarized the antiviral activity of the Momordica anti-HIV protein of 30 kD (MAP30). The extracted protein from M. charantia inhibits HIV-1 DNA replication in monocytes and selectively kills lymphocytes and macrophage-infected with HIV with minimal toxicity to the cells unaffected by HIV [112]. With a rich collection of phytochemicals and years of successful application in managing and preventing various diseases, M. charantia is a potential source of relief in managing Africa’s present and future epidemics and pandemics.
9. Curcuma longa L. (Fam. Zingiberaceae) [Tumeric]
Several African scientists have reported the use of C. longa in African traditional folk medicine and its use as a food spice [119][120][121][122]. Although the plant is cultivated throughout the entire continent of Africa and other parts of the world, Western and Eastern Africa seem to have wider plant cultivation than the rest of Africa [123]. C. longa has been traditionally reported for use in conjunctivitis, smallpox, and sinusitis [121][122][124]. The plant has antioxidant, anticancer, immunomodulator, and antimicrobial properties [122][124]. These pharmacological potentials and properties of C. longa have been exploited in Africa for pandemics and epidemics [124]. Curcuminoids, quercetin, curcumin, zingiberine, borneol, alpha-phellandrene, and a variety of sesquiterpenes are some of the bioactive phytochemicals found in C. longa [120][122][124]. Aggarwal et al. (2016) reported curcumin as a potent antioxidant by suppressing NF-κB and NF-α, in addition to its antibacterial effect on organisms, such as Vibrio cholera and Klebsiella pneumonia [124]. Furthermore, African C. longa possessed substantial antiviral activities making it a potential plant in pandemics associated with viruses. The plant is effective against HIV-1, H1N1, hepatitis C, parainfluenza virus type 3, H6N1, human papillomavirus, and coxsackievirus B3 [120][122][124]. The ability of curcumin to inhibit viral hemagglutination, suppress viral replication, and down-regulate viral transcription made curcumin a candidate for use in pandemics [124]. The antibacterial, antiviral, anti-inflammatory, and antioxidant activities of C. longa made the plant a valuable and potential therapy in pandemics throughout Africa [125].
The bioactive compounds in medicinal plants, their pharmacologic activity, and their potential to mitigate infectious diseases during epidemics and pandemics are presented in Table 1 and Figure 1.
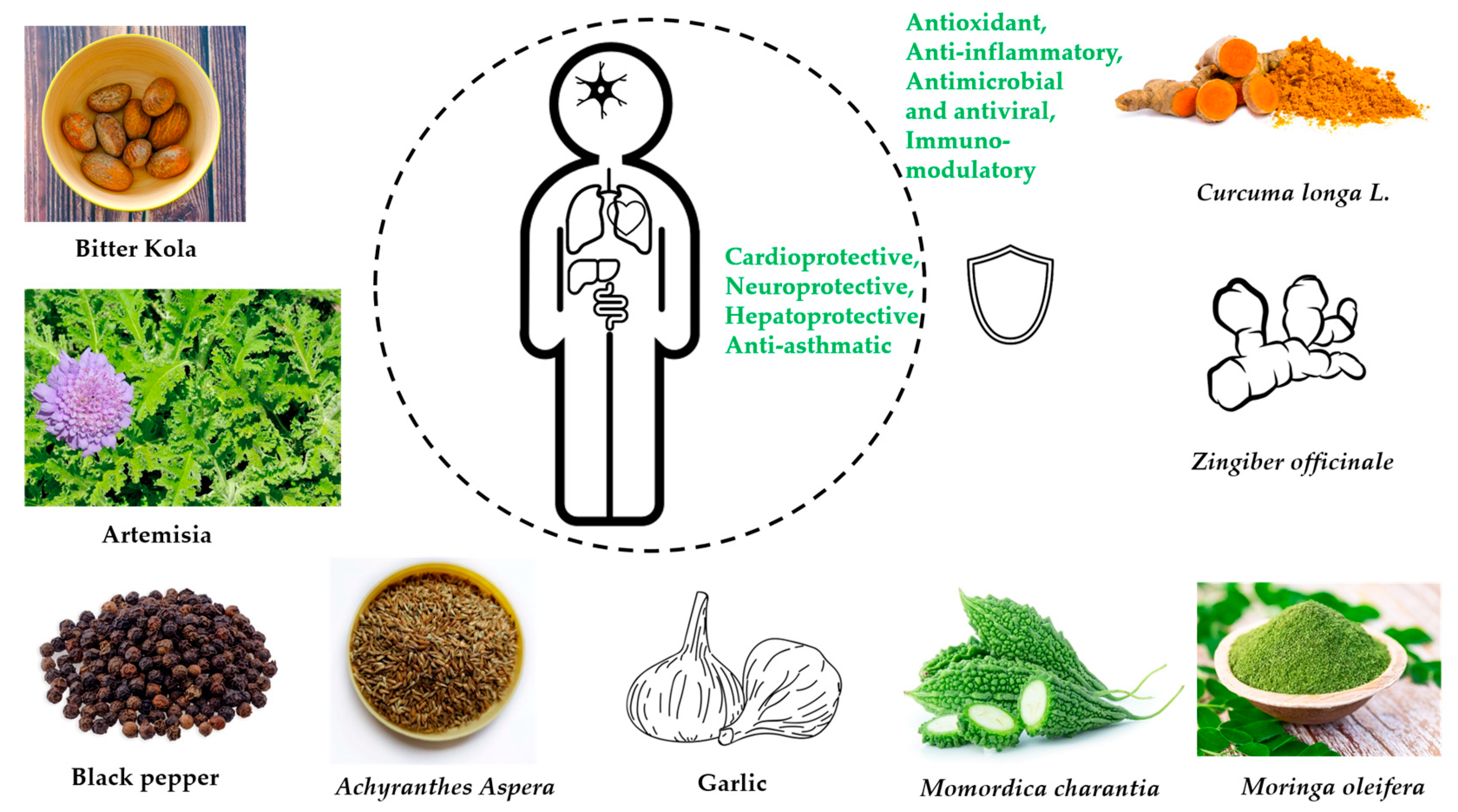
Figure 1. Graphical illustration of main health effects from traditional herbal remedies.
Table 1. African herbs/medicinal plants with immune-boosting and antiviral potentials.
| Herbs/ Medicinal Plants |
Traditional Uses | Bioactive Substances | Pharmacologic Activity | Immunologic Activity | Antiviral Activity | References |
|---|---|---|---|---|---|---|
| Garcinia kola Heckel (Fam. Clusiaceae) [Bitter cola] |
Typhoid fever, bronchitis, bacterial infections, malignant tumors, skin infections, tuberculosis, gastritis, cold, jaundice | Alkaloids, phenols, saponins, sterols, tannins, garciniflavanone, kolanone, garcinoic acid, kolaflavanone, and kolaviron | Antiviral, antiasthma, antioxidant, antidiabetic, antihypertensive, antibacterial, antiasthma, and for hepatoprotective activities | Antioxidant, hepatoprotective, immunomodulatory, metal chelating, potent radical scavenger, modulate oxidative stress |
Polioviruses, measles virus, yellow fever virus, influenza, herpes simplex Virus-1, HIV | [1][2][9][10][13][15][117] |
| Artemisia Afra Jacq. (Fam. Asteraceae) | Influenza, respiratory infections, cough, malaria, diabetes, and fever | Dihydroxybishopsolicepolide, scopoletin, acacetin, flavonoids, yomogiartemin | Cytotoxic, anticancer, antiviral | Antioxidant, anti-inflammatory | Influenza virus A, human herpes viruses 1 and 2, Hepatitis B and C, HIV-1 viruses | [19][20][21][22][25][26][29] |
| Piper guineense (Fam. Piperaceae) [African black pepper] | Sexually transmitted diseases | Piperine, piperlongumine, ligans, monoterpenes, terpenoids, sterols, sesquiterpenes, and volatile oils | Antibacterial, anticancer, antiviral, antiproliferative, antifungal, antihelminth | Antioxidant, anti-inflammatory | Newcastle disease virus | [19][30][36][38] |
| Achyranthes Aspera Linn. (Fam. Amaranthaceae) | Dysentery, arthritis, malaria, hemorrhoids, fever, pain, and diarrhea | Triacontanol, eugenol, ecdysterone, betaine, ascorbic acid | Diuretic, anti-inflammatory, anti-asthmatic, and valuable for pneumonia | Antioxidant, immune boosting, chemopreventative | Herpes simplex virus type 1 (HSV-1, oral herpes) and type 2 (HSV-2, genital herpes). | [8][40][41][42] |
| Allium sativum L. (Fam. Liliaceae) [Garlic] |
Influenza, typhus, cholera, dysentery, toothaches, snake bites, arthritis, and hypertension | Phenols, flavonoids, saponins, allicin (thiosulfate), diallyl trisulfate, ajoenes, diallyl disulfide | Anticancer, antimicrobial, flu, diabetes, hypertension, arthritis, and for the prevention of cancer | Antioxidants, immune booster | Vesicular stomatitis virus, Human rhinovirus type 2, influenza virus type 3, human cytomegalovirus, influenza B type virus, and herpes simplex 1 and 2 | [50][58][59][63][71][126] |
| Moringa oleifera Lam. (Fam. Moringaceae) | Food, livestock feed, nutrition, medicines | isothiocyanate, phenolic acids, polyphenols, sterols, alkaloids, terpene, flavonoids, and flavanol glycosides | Anti-parasitic, antituberculosis anticancer, antiviral, antidiabetic, sexually transmitted infections, typhoid fever, antihypertensive | Anti-inflammatory, cardio-protective, neuro-protective, hepato-protective | Influenza A virus, new castle disease virus, herpes simplex virus, Epstein-Barr virus, hepatitis B virus, and foot-and-mouth disease virus in cloven-footed animals | [76][77][78][79][84][126] |
| Zingiber officinale R. (Fam Zingiberaceae) [Ginger] |
Influenza, cough, sore throats, arthritis, lung diseases, peptic ulcer disease, hypertension, infectious diseases | Steroids, phenols, alkaloids, gingerols, zingerone, zingiberol, paradols, and shogaols | Antiarthritic, anticancer, antioxidant, antirhinoviral, antimicrobial, antiglycemic | Antioxidant, anti-inflammatory | Enterovirus 71, Japanese encephalitis virus, Epstein-Barr virus, herpes simplex virus types 1 and 2, influenza A virus, human immunodeficiency viruses, coronavirus SARS-CoV-1, rhinovirus, chikungunya virus, respiratory syncytial virus | [8][89][90][91][92][93][94][98][102] |
| Momordica charantia (Fam. Cucurbitaceae) [Bitter melon] | Diabetes, treat viral infections, toothache, diarrhea, gastrointestinal infections, ritual purposes | Minerals, vitamins, phenol compounds, triterpene, lipid, protein, glycosides, steroids, saponins, flavonoids | Antiviral, recurrent respiratory tract infections, anthelmintic, anticancer, antidiabetic, abortifacient, contraceptive | Antioxidant | HSV-1 and SINV viruses, HIV-1 | [110][111][112] [114] |
| Curcuma longa L. (Fam. Zingiberaceae) [Tumeric] | Conjunctivitis, smallpox, sinusitis | Curcuminoids, Quercetin, Cuscumin, zingiberine, borneol, alpha phellandrene | Antioxidant, anticancer, immunomodulatory, antimicrobial, antiviral | Antioxidant, immune modulator | HIV-1, H1N1, hepatitis C, parainfluenza virus type-3, H6N1, human papillomavirus, and coxsackievirus B3 | [121][122][123] [125] |
References
- Ekene, E.N. Garcinia Kola: A Review of Its Ethnomedicinal, Chemical and Pharmacological Properties. Int. J. Curr. Res. Rev. 2014, 6, 1.
- Maňourová, A.; Leuner, O.; Tchoundjeu, Z.; Van Damme, P.; Verner, V.; Přibyl, O.; Lojka, B. Medicinal Potential, Utilization and Domestication Status of Bitter Kola (Garcinia kola Heckel) in West and Central Africa. Forests 2019, 10, 124.
- Seanego, C.T.; Ndip, R.N. Identification and Antibacterial Evaluation of Bioactive Compounds from Garcinia kola (Heckel) Seeds. Molecules 2012, 17, 6569–6584.
- Konziase, B. Protective activity of biflavanones from Garcinia kola against Plasmodium infection. J. Ethnopharmacol. 2015, 172, 214–218.
- Kalu, W.; Okafor, P.; Ijeh, I.; Eleazu, C. Effect of kolaviron, a biflavanoid complex from Garcinia kola on some biochemical parameters in experimentally induced benign prostatic hyperplasic rats. Biomed. Pharmacother. 2016, 83, 1436–1443.
- BC, I.C.; Maduka Tochukwu, O.D.; Enyoh, C.E.; JM, I.U. Potential Plants for Treatment and Management of COVID-19 in Nigeria. Acad. J. Chem. 2020, 5, 69–80.
- Oladele, J.O.; Ajayi, E.I.; Oyeleke, O.M.; Oladele, O.T.; Olowookere, B.D.; Adeniyi, B.M.; Oyewole, O.I.; Oladiji, A.T. A systematic review on COVID-19 pandemic with special emphasis on curative potentials of Nigeria based medicinal plants. Heliyon 2020, 6, e04897.
- Adeleye, O.A.; Femi-Oyewo, M.N.; Bamiro, O.A.; Bakre, L.G.; Alabi, A.; Ashidi, J.S.; Balogun-Agbaje, O.A.; Hassan, O.M.; Fakoya, G. Ethnomedicinal herbs in African traditional medicine with potential activity for the prevention, treatment, and management of coronavirus disease 2019. Futur. J. Pharm. Sci. 2021, 7, 1–14.
- Awogbindin, I.O.; Olaleye, D.O.; Farombi, E.O. Kolaviron Improves Morbidity and Suppresses Mortality by Mitigating Oxido-Inflammation in BALB/c Mice Infected with Influenza Virus. Viral Immunol. 2015, 28, 367–377.
- Obi, R.; Olayinka, A.; Adesegun, S. The antiviral activities of Garcinia kola (Heckel.) and Azadirachta indica (A. Juss.) on viruses of public health importance in Nigeria. Int. J. Infect. Dis. 2020, 101, 119.
- Nworu, C.S.; Akah, P.A.; Esimone, C.O.; Okoli, C.O.; Okoye, F.B.C. Immunomodulatory Activities of Kolaviron, a Mixture of Three Related Biflavonoids of Garcinia Kola Heckel. Immunopharmacol. Immunotoxicol. 2008, 30, 317–332.
- Ukaoma, A.A.; Okechukwu, R.I.; Ukaoma, V.O.; Iwuagwu, M. Phytochemical screening and antibacterial properties of Garcinia kola. J. Phytopharm. 2013, 2, 34–38.
- Farombi, E.O. African indigenous plants with chemotherapeutic potentials and biotechnological approach to the production of bioactive prophylactic agents. Afr. J. Biotechnol. 2003, 2, 662–671.
- Adaramoye, O.; Awogbindin, I.; Okusaga, J. Effect of Kolaviron, a Biflavonoid Complex from Garcinia kola Seeds, on Ethanol-Induced Oxidative Stress in Liver of Adult Wistar Rats. J. Med. Food 2009, 12, 584–590.
- Farombi, E.O.; Shrotriya, S.; Surh, Y.-J. Kolaviron Inhibits Dimethyl Nitrosamine-Induced Liver Injury by Suppressing COX-2 and INOS Expression via NF-ΚB and AP-1. Life Sci. 2009, 84, 149–155.
- Farombi, E.O.; Owoeye, O. Antioxidative and Chemopreventive Properties of Vernonia amygdalina and Garcinia biflavonoid. Int. J. Environ. Res. Public Health 2011, 8, 2533–2555.
- Salehi, B.; Kumar, N.V.A.; Şener, B.; Sharifi-Rad, M.; Kılıç, M.; Mahady, G.B.; Vlaisavljevic, S.; Iriti, M.; Kobarfard, F.; Setzer, W.N.; et al. Medicinal Plants Used in the Treatment of Human Immunodeficiency Virus. Int. J. Mol. Sci. 2018, 19, 1459.
- Okoye, T.C.; Uzor, P.F.; Onyeto, C.A.; Okereke, E.K. Safe African Medicinal Plants for Clinical Studies. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 535–555.
- More, G.; Lall, N.; Hussein, A.; Tshikalange, T.E. Antimicrobial Constituents of Artemisia Afra Jacq. Ex Willd. against Periodontal Pathogens. Evid. Based Complement Alternat. Med. 2012, 2012, 252758.
- Moyo, P.; Kunyane, P.; Selepe, M.A.; Eloff, J.N.; Niemand, J.; Louw, A.I.; Maharaj, V.J.; Birkholtz, L.-M. Bioassay-guided isolation and identification of gametocytocidal compounds from Artemisia afra (Asteraceae). Malar. J. 2019, 18, 65.
- Kshirsagar, S.; Rao, R. Antiviral and Immunomodulation Effects of Artemisia. Medicina 2021, 57, 217.
- Du, L.; Chen, J.; Xing, Y.-Q. Eupatilin prevents H2O2-induced oxidative stress and apoptosis in human retinal pigment epithelial cells. Biomed. Pharmacother. 2017, 85, 136–140.
- Krishna, S.; Ganapathi, S.; Ster, I.C.; Saeed, M.E.; Cowan, M.; Finlayson, C.; Kovacsevics, H.; Jansen, H.; Kremsner, P.G.; Efferth, T.; et al. A Randomised, Double Blind, Placebo-Controlled Pilot Study of Oral Artesunate Therapy for Colorectal Cancer. Ebiomedicine 2014, 2, 82–90.
- Slezakova, S.; Ruda-Kucerova, J. Anticancer Activity of Artemisinin and its Derivatives. Anticancer. Res. 2017, 37, 5995–6003.
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.G.; Marschall, M. The Antiviral Activities of Artemisinin and Artesunate. Clin. Infect. Dis. 2008, 47, 804–811.
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364.
- Efferth, T. Beyond malaria: The inhibition of viruses by artemisinin-type compounds. Biotechnol. Adv. 2018, 36, 1730–1737.
- Uzun, T.; Toptas, O. Artesunate: Could Be an Alternative Drug to Chloroquine in COVID-19 Treatment? Chin. Med. 2020, 15, 1–4.
- Chen, W. A potential treatment of COVID-19 with TGF-β blockade. Int. J. Biol. Sci. 2020, 16, 1954–1955.
- Ene-Obong, H.; Onuoha, N.; Aburime, L.; Mbah, O. Chemical composition and antioxidant activities of some indigenous spices consumed in Nigeria. Food Chem. 2018, 238, 58–64.
- Ajibesin, K.; Umoh, U.; Bala, D. The use of medicinal plants to treat sexually transmitted diseases in Nigeria: Ethnomedicinal survey of Niger Delta Region. Int. J. Green Pharm. 2011, 5, 181.
- Agbor, G.A.; Vinson, J.A.; Oben, J.E.; Ngogang, J.Y. In Vitro Antioxidant Activity of Three Piper Species. J. Herb. Pharmacother. 2008, 7, 49–64.
- Umadevi, P.; Deepti, K.; Venugopal, D.V.R. Synthesis, anticancer and antibacterial activities of piperine analogs. Med. Chem. Res. 2013, 22, 5466–5471.
- Mgbeahuruike, E.E.; Fyhrquist, P.; Vuorela, H.; Julkunen-Tiitto, R.; Holm, Y. Alkaloid-Rich Crude Extracts, Fractions and Piperamide Alkaloids of Piper guineense Possess Promising Antibacterial Effects. Antibiotics 2018, 7, 98.
- Philipova, I.; Valcheva, V.; Mihaylova, R.; Mateeva, M.; Doytchinova, I.; Stavrakov, G. Synthetic piperine amide analogs with antimycobacterial activity. Chem. Biol. Drug Des. 2017, 91, 763–768.
- Mgbeahuruike, E.E.; Stålnacke, M.; Vuorela, H.; Holm, Y. Antimicrobial and Synergistic Effects of Commercial Piperine and Piperlongumine in Combination with Conventional Antimicrobials. Antibiotics 2019, 8, 55.
- Kim, N.; Do, J.; Bae, J.; Jin, H.K.; Kim, J.-H.; Inn, K.-S.; Oh, M.S.; Lee, J.K. Piperlongumine Inhibits Neuroinflammation via Regulating NF-ΚB Signaling Pathways in Lipopolysaccharide-Stimulated BV2 Microglia Cells. J. Pharmacol. Sci. 2018, 137, 195–201.
- Choudhary, N.; Singh, V. A Census of P. Longum’s Phytochemicals and Their Network Pharmacological Evaluation for Identifying Novel Drug-like Molecules against Various Diseases, with a Special Focus on Neurological Disorders. PLoS ONE 2018, 13, e0191006.
- Osho, I.B.; Adebayo, I.A.; Ajayi, O.I. Immunological Evaluation of Antiviral Activity of Methanolic Extract of Piper Guineense against Newcastle Disease in Experimentally Infected Broiler Chickens. Int. J. Mol. Vet. Res. 2016, 6, 2.
- Sharma, V.; Chaudhary, U. An Overview on Indigenous Knowledge of Achyranthes Aspera. J. Crit. Rev. 2015, 2, 7–19.
- Mukherjee, H.; Ojha, D.; Bag, P.; Chandel, H.S.; Bhattacharyya, S.; Chatterjee, T.K.; Mukherjee, P.K.; Chakraborti, S.; Chattopadhyay, D. Anti-herpes virus activities of Achyranthes aspera: An Indian ethnomedicine, and its triterpene acid. Microbiol. Res. 2013, 168, 238–244.
- Ajibesin, K.; René, N.; Bala, D.; Essiett, U. Antimicrobial Activities of the Extracts and Fractions of Allanblackia floribunda. Biotechnol. 2008, 7, 129–133.
- Edwin, S.; Jarald, E.E.; Deb, L.; Jain, A.; Kinger, H.; Dutt, K.; Raj, A.A. Wound Healing and Antioxidant Activity of Achyranthes aspera. Pharm. Biol. 2008, 46, 824–828.
- Chakrabarti, R.; Srivastava, P.K.; Verma, N.; Sharma, J. Effect of seeds of Achyranthes aspera on the immune responses and expression of some immune-related genes in carp Catla catla. Fish Shellfish. Immunol. 2014, 41, 64–69.
- Rao, A.; Balachandran, B. Role of Oxidative Stress and Antioxidants in Neurodegenerative Diseases. Nutr. Neurosci. 2002, 5, 291–309.
- Abi Beaulah, G.; Mohamed Sadiq, A.; Jaya Santhi, R. Antioxidant and Antibacterial Activity of Achyranthes Aspera: An in Vitro Study. Ann. Biol. Res. 2011, 2, 662–670.
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211.
- Chakraborty, A.; Brantner, A.; Mukainaka, T.; Nobukuni, Y.; Kuchide, M.; Konoshima, T.; Tokuda, H.; Nishino, H. Cancer chemopreventive activity of Achyranthes aspera leaves on Epstein–Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett. 2002, 177, 1–5.
- Arora, S.; Tandon, S. Achyranthes aspera Root Extracts Induce Human Colon Cancer Cell (COLO-205) Death by Triggering the Mitochondrial Apoptosis Pathway and S Phase Cell Cycle Arrest. Sci. World J. 2014, 2014, 129697.
- Petrovska, B.B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110.
- Garcia, S. Pandemics and Traditional Plant-Based Remedies. A Historical-Botanical Review in the Era of COVID19. Front. Plant Sci. 2020, 11, 571042.
- Otunola, A.G.; Asowata-Ayodele, A.M.; Afolayan, A.J. Assessment of the polyphenolic content, free radical scavenging, anti-inflammatory, and antimicrobial activities of acetone and aqueous extracts of Lippia javanica (Burm.F.) spreng. Pharmacogn. Mag. 2016, 12, 353–362.
- Tijani, K.B.; Alfa, A.A.; Sezor, A.A. Studies on Phytochemical, Nutraceutical Profiles and Potential Medicinal Values of Allium sativum Linn (Lilliaceae) on Bacterial Meningitis. Int. Neuropsychiatr. Dis. J. 2019, 13, 1–15.
- Sulaiman, F.A.; Kazeem, M.O.; Waheed, A.M.; Temowo, S.O.; Azeez, I.O.; Zubair, F.I.; Adeyemi, T.A.; Nyang, A.; Adeyemi, O.S. Antimicrobial and Toxic Potential of Aqueous Extracts of Allium Sativum, Hibiscus Sabdariffa and Zingiber Officinale in Wistar Rats. J. Taibah Univ. Sci. 2014, 8, 315–322.
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14.
- Mphuthi, D.D.; Husaini, D.C. Traditional medicinal plants used by hypertensive patients in Belize: A qualitative evaluation of beliefs and practices. Bull. Natl. Res. Cent. 2022, 46, 107.
- Colín-González, A.L.; Santana, R.A.; Silva-Islas, C.A.; Chánez-Cárdenas, M.E.; Santamaría, A.; Maldonado, P.D. The Antioxidant Mechanisms Underlying the Aged Garlic Extract- and S-Allylcysteine-Induced Protection. Oxidative Med. Cell. Longev. 2012, 2012, 907162.
- Aviello, G.; Abenavoli, L.; Borrelli, F.; Capasso, R.; Izzo, A.A.; Lembo, F.; Romano, B.; Capasso, F. Garlic: Empiricism or Science? Nat. Prod. Commun. 2009, 4, 1934578X0900401231.
- Badal, D.S.; Dwivedi, A.K.; Kumar, V.; Singh, S.; Prakash, A.; Verma, S.; Kumar, J. Effect of Organic Manures and Inorganic Fertilizers on Growth, Yield and Its Attributing Traits in Garlic (Allium Sativum L.). J. Pharmacogn. Phytochem. 2019, 8, 587–590.
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322.
- Al-Snafi, A.E. Pharmacological Effects of Allium Species Grown in Iraq. An Overview. Int. J. Pharm. Health Care Res. 2013, 1, 132–147.
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Evidence-Based Complement. Altern. Med. 2017, 2017, 7821095.
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, G.L.; Elewa, Y.H.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium Sativum L.): A Review. Nutrients 2020, 12, 872.
- Asdaq, S.M.B.; Inamdar, M.N. Pharmacodynamic and Pharmacokinetic Interactions of Propranolol with Garlic (Allium sativum) in Rats. Evidence-Based Complement. Altern. Med. 2011, 2011, 824042.
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.W.; Campopiano, D.J. Garlic Revisited: Antimicrobial Activity of Allicin-Containing Garlic Extracts against Burkholderia cepacia Complex. PLoS ONE 2014, 9, e112726.
- Shokrzadeh, M.; Ebadi, A.G. Antibacterial Effect of Garlic (Allium Sativum L.) on Staphylococcus Aureus. Pak. J. Biol. Sci. 2006, 9, 1577–1579.
- Jang, H.-J.; Lee, H.-J.; Yoon, D.-K.; Ji, D.-S.; Kim, J.-H.; Lee, C.-H. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 2017, 27, 219–225.
- Abdel-Daim, M.M.; Shaheen, H.M.; Abushouk, A.I.; Toraih, E.A.; Fawzy, M.S.; Alansari, W.S.; Aleya, L.; Bungau, S. Thymoquinone and diallyl sulfide protect against fipronil-induced oxidative injury in rats. Environ. Sci. Pollut. Res. 2018, 25, 23909–23916.
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246.
- Chen, Y.; Sun, J.; Dou, C.; Li, N.; Kang, F.; Wang, Y.; Cao, Z.; Yang, X.; Dong, S. Alliin Attenuated RANKL-Induced Osteoclastogenesis by Scavenging Reactive Oxygen Species through Inhibiting Nox1. Int. J. Mol. Sci. 2016, 17, 1516.
- Gruhlke, M.C.H.; Nicco, C.; Batteux, F.; Slusarenko, A.J. The Effects of Allicin, a Reactive Sulfur Species from Garlic, on a Selection of Mammalian Cell Lines. Antioxidants 2016, 6, 1.
- Ross, Z.M.; O’Gara, E.A.; Hill, D.J.; Sleightholme, H.V.; Maslin, D.J. Antimicrobial Properties of Garlic Oil against Human Enteric Bacteria: Evaluation of Methodologies and Comparisons with Garlic Oil Sulfides and Garlic Powder. Appl. Environ. Microbiol. 2001, 67, 475–480.
- Cutler, R.R.; Wilson, P. Antibacterial Activity of a New, Stable, Aqueous Extract of Allicin against Methicillin-Resistant Staphylococcus Aureus. Br. J. Biomed. Sci. 2004, 61, 71–74.
- Kuda, T.; Iwai, A.; Yano, T. Effect of red pepper Capsicum annuum var. conoides and garlic Allium sativum on plasma lipid levels and cecal microflora in mice fed beef tallow. Food Chem. Toxicol. 2004, 42, 1695–1700.
- Kumar, P.; Yadav, J.; Jain, M.; Yadav, P.; Goel, A.; Yadava, P.K. Bactericidal Efficacy of Allium sativum (garlic) Against Multidrug Resistant Vibrio cholerae O1 Epidemic Strains. Def. Sci. J. 2016, 66, 479–484.
- Razis, A.F.A.; Ibrahim, M.D.; Kntayya, S.B. Health Benefits of Moringa oleifera. Asian Pac. J. Cancer Prev. 2014, 15, 8571–8576.
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835.
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, Nutritional, and Therapeutic Significance of Moringa Oleifera Lam. Phytother. Res. 2019, 33, 2870–2903.
- Mashamaite, C.V.; Pieterse, P.J.; Mothapo, P.N.; Phiri, E.E. Moringa oleifera in South Africa: A review on its production, growing conditions and consumption as a food source. S. Afr. J. Sci. 2021, 117, 1–7.
- Nouman, W.; Siddiqui, M.T.; BASRA, S.; AHMED, M.; FAROOQ, H.; ZUBAIR, M.; GULL, T. Biomass Production and Nutritional Quality of Moringa Oleifera as a Field Crop. Turk. J. Agric. For. 2013, 37, 410–419.
- El-Hak, H.N.; Moustafa, A.R.A.; Mansour, S.R. Toxic effect of Moringa peregrina seeds on histological and biochemical analyses of adult male Albino rats. Toxicol. Rep. 2017, 5, 38–45.
- Kasolo, J.N.; Bimenya, G.S.; Ojok, L.; Ochieng, J.; Ogwal-Okeng, J.W. Phytochemicals and Uses of Moringa Oleifera Leaves in Ugandan Rural Communities. J. Med. Plant Res. 2010, 4, 753–757.
- Popoola, J.O.; Obembe, O.O. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013, 150, 682–691.
- Biswas, D.; Nandy, S.; Mukherjee, A.; Pandey, D.; Dey, A. Moringa oleifera Lam. and derived phytochemicals as promising antiviral agents: A review. S. Afr. J. Bot. 2019, 129, 272–282.
- Xiong, Y.; Rajoka, M.S.R.; Mehwish, H.M.; Zhang, M.; Liang, N.; Li, C.; He, Z. Virucidal activity of Moringa A from Moringa oleifera seeds against Influenza A Viruses by regulating TFEB. Int. Immunopharmacol. 2021, 95, 107561.
- Otunola, G.A.; Afolayan, A.J. Antidiabetic Effect of Combined Spices of Allium Sativum, Zingiber Officinale and Capsicum Frutescens in Alloxan-Induced Diabetic Rats. Front. Life Sci. 2015, 8, 314–323.
- Ravindran, P.N.; Babu, K.N. Ginger: The Genus Zingiber; CRC press: Boca Raton, FL, USA, 2016.
- Sowley, E.N.K.; Kankam, F. Harnessing the Therapeutic Properties of Ginger (Zingiber officinale Roscoe) for the Management of Plant Diseases. In Ginger Cultivation and Its Antimicrobial and Pharmacological Potentials; IntechOpen: London, UK, 2019.
- Dhanik, J.; Arya, N.; Nand, V. A Review on Zingiber Officinale. J. Pharmacogn. Phytochem. 2017, 6, 174–184.
- Taoheed, A.A.; Tolulope, A.A.; Saidu, A.B.; Odewumi, O.G.; Sunday, R.M.; Usman, M. Phytochemical Properties, Proximate and Mineral Composition of Curcuma longa Linn. and Zingiber officinale Rosc.: A Comparative Study. J. Sci. Res. Rep. 2017, 13, 1–7.
- Sombie, E.N.; Tibiri, A.; N’Do, J.Y.-P.; Traore, T.K.; Ouedraogo, N.; Hilou, A.; Guissou, P.I.; Nacoulma, O.G. Ethnobotanical study and antioxidant activity of anti-hepatitis plants extracts of the COMOE province, Burkina Faso. Int. J. Biol. Chem. Sci. 2018, 12, 1308.
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185.
- Kumar, N.V.; Murthy, P.S.; Manjunatha, J.; Bettadaiah, B. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their derivatives. Food Chem. 2014, 159, 451–457.
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340.
- Suekawa, M.; Ishige, A.; Yuasa, K.; Sudo, K.; Aburada, M.; Hosoya, E. Pharmacological studies on Ginger. I. Pharmacological actions of pungent constituents, (6)-gingerol and (6)-shogaol. J. Pharmacobio-Dyn. 1984, 7, 836–848.
- Mbadiko, C.M.; Inkoto, C.L.; Gbolo, B.Z.; Lengbiye, E.M.; Kilembe, J.T.; Matondo, A.; Mwanangombo, D.T.; Ngoyi, E.M.; Bongo, G.N.; Falanga, C.M.; et al. A Mini Review on the Phytochemistry, Toxicology and Antiviral Activity of Some Medically Interesting Zingiberaceae Species. J. Complement. Altern. Med Res. 2020, 9, 44–56.
- Chang, J.S.; Wang, K.C.; Yeh, C.F.; Shieh, D.E.; Chiang, L.C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 145, 146–151.
- Rathinavel, T.; Palanisamy, M.; Srinivasan, P.; Subramanian, A.; Thangaswamy, S. Phytochemical 6-Gingerol -A promising Drug of choice for COVID-19. Int. J. Adv. Sci. Eng. 2020, 6, 1482–1489.
- Kabuto, H.; Nishizawa, M.; Tada, M.; Higashio, C.; Shishibori, T.; Kohno, M. Zingerone Prevents 6-Hydroxydopamine-induced Dopamine Depression in Mouse Striatum and Increases Superoxide Scavenging Activity in Serum. Neurochem. Res. 2005, 30, 325–332.
- Asnani, V.; Verma, R.J. Antioxidative Effect of Rhizome of Zingiber Officinale on Paraben Induced Lipid Peroxidation: An in Vitro Study. Acta Pol. Pharm. 2007, 64, 35–37.
- Kim, J.-K.; Kim, Y.; Na, K.-M.; Surh, Y.-J.; Kim, T.-Y. (6)-Gingerol Prevents UVB-Induced ROS Production and COX-2 Expression in Vitro and in Vivo. Free Radic. Res. 2007, 41, 603–614.
- Liu, Y.; Whelan, R.; Pattnaik, B.; Ludwig, K.; Subudhi, E.; Rowland, H.; Claussen, N.; Zucker, N.; Uppal, S.; Kushner, D.M.; et al. Terpenoids from Zingiber officinale (Ginger) Induce Apoptosis in Endometrial Cancer Cells through the Activation of p53. PLoS ONE 2012, 7, e53178.
- Brahmbhatt, M.; Gundala, S.R.; Asif, G.; Shamsi, A.S.; Aneja, R. Ginger Phytochemicals Exhibit Synergy to Inhibit Prostate Cancer Cell Proliferation. Nutr. Cancer 2013, 65, 263–272.
- Jiang, Y.; Turgeon, D.K.; Wright, B.D.; Sidahmed, E.; Ruffin, M.T.; Brenner, D.E.; Sen, A.; Zick, S.M. Effect of ginger root on cyclooxygenase-1 and 15-hydroxyprostaglandin dehydrogenase expression in colonic mucosa of humans at normal and increased risk for colorectal cancer. Eur. J. Cancer Prev. 2013, 22, 455–460.
- Ling, H.; Yang, H.; Tan, S.-H.; Chui, W.-K.; Chew, E.-H. 6-Shogaol, an Active Constituent of Ginger, Inhibits Breast Cancer Cell Invasion by Reducing Matrix Metalloproteinase-9 Expression via Blockade of Nuclear Factor-ΚB Activation. Br. J. Pharmacol. 2010, 161, 1763–1777.
- Kim, E.-C.; Min, J.-K.; Kim, T.-Y.; Lee, S.-J.; Yang, H.-O.; Han, S.; Kim, Y.-M.; Kwon, Y.-G. (6)-Gingerol, a Pungent Ingredient of Ginger, Inhibits Angiogenesis in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 2005, 335, 300–308.
- Flynn, D.L.; Rafferty, M.F.; Boctor, A.M. Inhibition of 5-hydroxy-eicosatetraenoic acid (5-HETE) formation in intact human neutrophils by naturally-occurring diarylheptanoids: Inhibitory activities of curcuminoids and yakuchinones. Prostaglandins Leukot. Med. 1986, 22, 357–360.
- Kiuchi, F.; Iwakami, S.; Shibuya, M.; Hanaoka, F.; Sankawa, U. Inhibition of Prostaglandin and Leukotriene Biosynthesis by Gingerols and Diarylheptanoids. Chem. Pharm. Bull. 1992, 40, 387–391.
- Beloin, N.; Gbeassor, M.; Akpagana, K.; Hudson, J.; de Soussa, K.; Koumaglo, K.; Arnason, J.T. Ethnomedicinal Uses of Momordica Charantia (Cucurbitaceae) in Togo and Relation to Its Phytochemistry and Biological Activity. J. Ethnopharmacol. 2005, 96, 49–55.
- Bakare, R.I.; Magbagbeola, O.A.; Akinwande, A.I.; Okunowo, O.W. Nutritional and Chemical Evaluation of Momordica Charantia. J. Med. Plants Res. 2010, 4, 2189–2193.
- Joseph, B.; Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102.
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555.
- Bourinbaiar, A.S.; Lee-Huang, S. The Activity of Plant-Derived Antiretroviral Proteins MAP30 and GAP31 against Herpes Simplex Virus Infectionin Vitro. Biochem. Biophys. Res. Commun. 1996, 219, 923–929.
- Rebultan, S.P. Bitter melon therapy: An experimental treatment of HIV infection. Aids Asia Voice Asian Solidar. Against AIDS 1995, 2, 6–7.
- Raina, K.; Kumar, D.; Agarwal, R. Promise of bitter melon ( Momordica charantia ) bioactives in cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 116–129.
- Ray, R.B.; Raychoudhuri, A.; Steele, R.; Nerurkar, P. Bitter Melon (Momordica Charantia) Extract Inhibits Breast Cancer Cell Proliferation by Modulating Cell Cycle Regulatory Genes and Promotes ApoptosisBitter Melon Extract Inhibits Breast Cancer Cell Growth. Cancer Res. 2010, 70, 1925–1931.
- Kwatra, D.; Subramaniam, D.; Ramamoorthy, P.; Standing, D.; Moran, E.; Velayutham, R.; Mitra, A.; Umar, S.; Anant, S. Methanolic Extracts of Bitter Melon Inhibit Colon Cancer Stem Cells by Affecting Energy Homeostasis and Autophagy. Evidence-Based Complement. Altern. Med. 2013, 2013, 702869.
- Fang, E.F.; Froetscher, L.; Scheibye-Knudsen, M.; Bohr, V.A.; Wong, J.H.; Ng, T.B. Emerging Antitumor Activities of the Bitter Melon (Momordica charantia). Curr. Protein Pept. Sci. 2019, 20, 296–301.
- Akinpelu, C.A.; Adebayo, O.S.; Adewale, O.M.; Adebisi-Adelani, O.O. An Analysis of Turmeric Utilisation Pattern in Ekiti State, Nigeria. Niger. J. Horticult. Sci. 2012, 17, 68–72.
- Mahomoodally, M.F. Traditional Medicines in Africa: An Appraisal of Ten Potent African Medicinal Plants. Evid.-Based Complement. Altern. Med. 2013, 2013, 617459.
- Nwaekpe, J.O.; Anyaegbunam, H.N.; Okoye, B.C.; Asumugha, G.N. Promotion of Turmeric for the Food/Pharmaceutical Industry in Nigeria. Am. J. Exp. Agric. 2015, 8, 335–341.
- Omosa, L.K.; Midiwo, J.O.; Kuete, V. Curcuma Longa. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 425–435.
- Iwu, M.M. African Medicinal Plants; CRC Press: College Park, MD, USA, 1993.
- Aggarwal, M.L.; Chacko, K.M.; Kuruvilla, B.T. Systematic and comprehensive investigation of the toxicity of curcuminoid-essential oil complex: A bioavailable turmeric formulation. Mol. Med. Rep. 2015, 13, 592–604.
- Uchejeso, O.M.; Chinaza, I.R.; Goodluck, O.A.; Rinpan, J.I. Some Igbo Indigenous Plants with Anti-COVID-19 Properties. In Alternative Medicine-Update; IntechOpen: London, UK, 2020.
- Nwokocha, C.R.; Ozolua, I.R.; Owu, D.U.; Nwokocha, I.M.; Ugwu, A.C. Antihypertensive properties of Allium sativum (garlic) on normotensive and two kidney one clip hypertensive rats. Niger. J. Physiol. Sci. 2011, 26, 213–218.
More
Information
Subjects:
Integrative & Complementary Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
986
Revisions:
2 times
(View History)
Update Date:
16 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




