Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jean-luc Teddy,gerard Wautier | -- | 1823 | 2023-06-15 15:19:00 | | | |
| 2 | Fanny Huang | -1 word(s) | 1822 | 2023-06-19 08:02:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wautier, J.; Wautier, M. Cytokine Production in Human Pathology. Encyclopedia. Available online: https://encyclopedia.pub/entry/45663 (accessed on 08 February 2026).
Wautier J, Wautier M. Cytokine Production in Human Pathology. Encyclopedia. Available at: https://encyclopedia.pub/entry/45663. Accessed February 08, 2026.
Wautier, Jean-Luc, Marie-Paule Wautier. "Cytokine Production in Human Pathology" Encyclopedia, https://encyclopedia.pub/entry/45663 (accessed February 08, 2026).
Wautier, J., & Wautier, M. (2023, June 15). Cytokine Production in Human Pathology. In Encyclopedia. https://encyclopedia.pub/entry/45663
Wautier, Jean-Luc and Marie-Paule Wautier. "Cytokine Production in Human Pathology." Encyclopedia. Web. 15 June, 2023.
Copy Citation
Cytokines can perform a dual role, being growth promotors or inhibitors and having pro- and anti-inflammatory properties. The complex interactions between cytokines, vascular cells and immune cells are responsible for dramatic conditions and lead to the concept of cytokine storm observed during sepsis, multi-organ failure and in some cases of COVID-19 infection. Cytokines such as interferon and hematopoietic growth factor have been used as therapy. Alternatively, the inhibition of cytokine functions has been largely developed using anti-interleukin or anti-TNF monoclonal antibodies in the treatment of sepsis or chronic inflammation.
inflammation
cytokines
1. Cytokines
Cytokines are essential mediators in the inflammatory process (Figure 1). Two cytokines, interleukin-1 (IL-1) and tumor necrosis factor (TNF), which have been recognized for decades, have been shown to coordinate the initiation and cascade of reactions [1]. They participate in the increase in vascular permeability and leukocyte production, stimulation and activation. Instead of being isolated molecules, they are now grouped into families of proteins: the IL-1, IL-6, TNF, TGF and INF families.
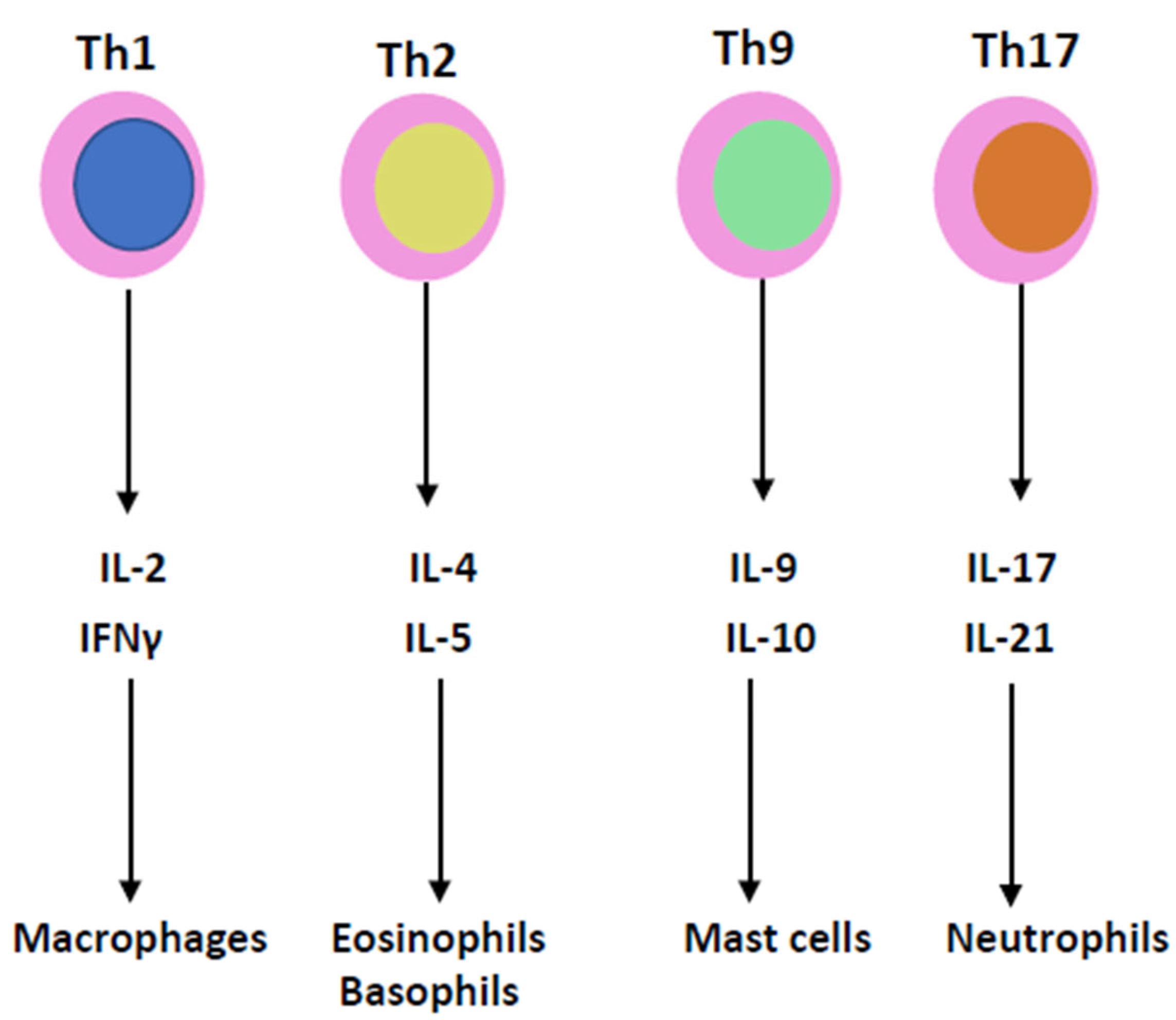
Figure 1. T lymphocyte helpers of T cell subgroups 1, 2, 9 and 17 produce IL-2, IL-4, IL-9, IL-17, IFNγ, IL-5, IL-10 and IL-21, which are involved in the pathophysiology of the cytokine storm.
1.1. IL-1 and IL-6 Families
To try to permit better access to the function of cytokines, a new way of grouping the cytokines into IL-1 and IL-6 families has been proposed in addition to the grouping of other major cytokines, TNF, IFNγ and TGFβ.
The IL-1 family has different ligands with agonist activity (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, IL-36γ), receptor antagonists (IL-1Ra, IL-36Ra, IL-38) and an anti-inflammatory cytokine, IL-37 [2]. Several IL-1 family members can be produced by apoptotic cells and may induce sterile inflammation. IL-38 reduces IL-6 production [3].
The IL-6 family of cytokines includes IL-6, IL-11, leukemia inhibitory factor (LIF), oncostatin M (OSM), cardiotropin-1 (CT-1), ciliary inhibitory factor (CNF), neuropoietin (NPN), IL-27 and IL-31. Interleukin-6 (IL-6) is produced by monocytes, endothelial cells and fibroblasts but is also present in mesangial cells, keratinocytes and T and B lymphocytes. Il-6 is a 21–28 kDa glycosylated protein with a four-helix structure. It is involved in inflammation and infection but also in the homeostatic process. As in several homeostatic systems, there is a balance between promoters and inhibitors. The stimulatory activity of IL-6 is mediated by the IL-6 receptor, which consists of two subunits, IL-6R and gp130 (Figure 2). After gp130 dimerization, gp130-associated Jak1 kinase is activated and leads to intracellular transmission, including jak/stat, ERk and P13k [4][5].
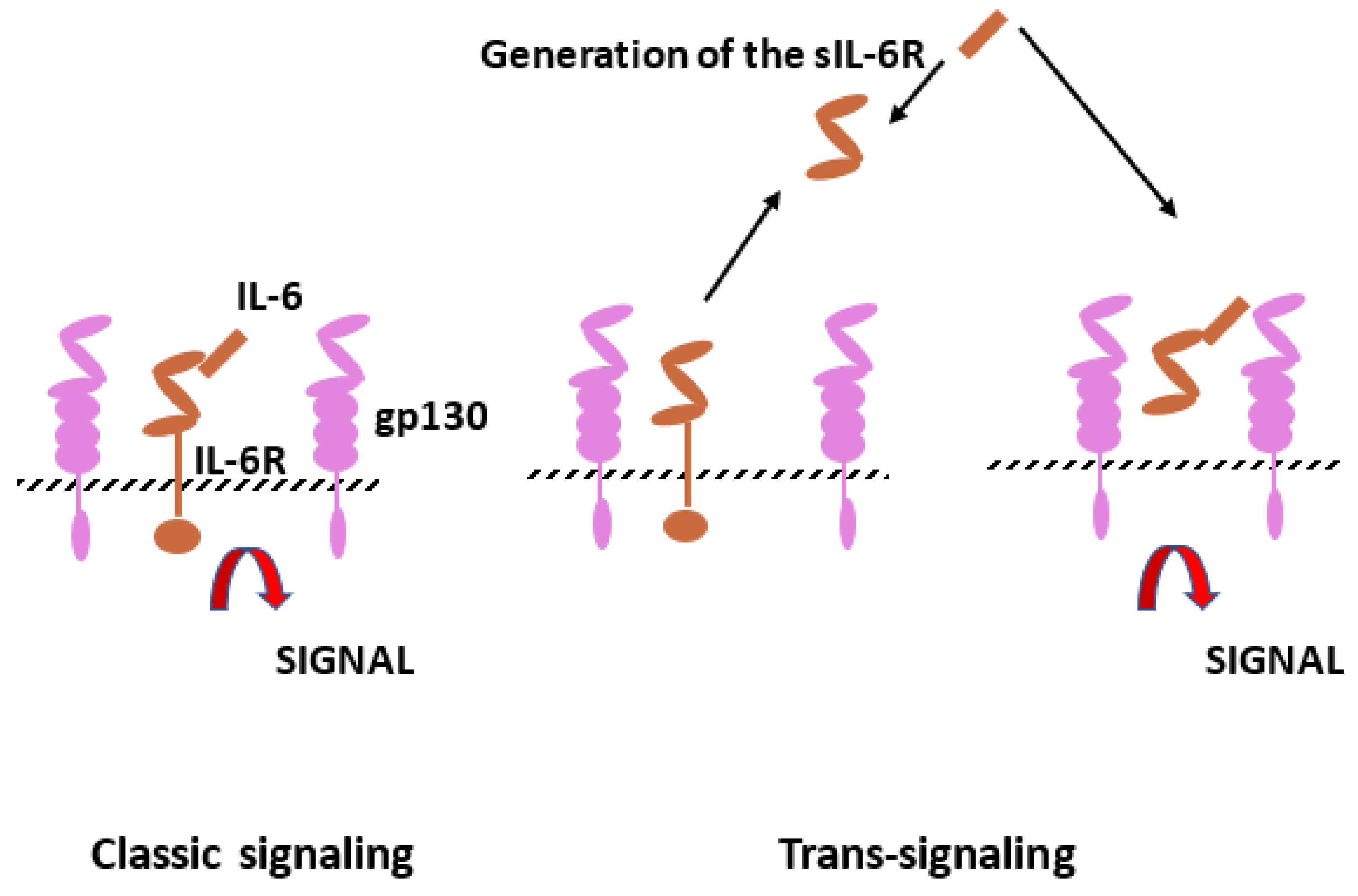
Figure 2. Interleukin-6 (IL-6) signaling pathways. Il-6 can bind to IL-6 membrane bound receptor (IL-6R), which corresponds to the classic signaling but also to soluble IL-6R (sIL-6R) resulting from limited proteolysis by ADAM proteases. Cells with gp130 can be stimulated by the complex IL-6 and sIL-6R (trans-signaling).
Il-6 appears to have a significant effect on immunoglobulins via B cells directly or through the CD4+ T cell helper properties. In patients with systemic lupus erythematosus (SLE), IL-6 potentiates the activity of autoreactive B cells [6][7].
1.2. IL-1 and IL-6 Inhibitors
IL-1 and IL-6 are considered as cytokines which play a major role in inflammatory chronic conditions such as rheumatoid arthritis [8], SLE [9] and polyneuritis [10] but also in acute septic shock and COVID-19 infection. This concept is at the origin of the use of monoclonal antibody therapy using antibodies directed against the cytokines or the receptors. Neutralization of IL-1β activities results in a rapid reduction in local inflammation and a decrease in inflammatory disease severity. IL-1 receptor antagonist (IL-1Ra) binds to IL-R1 and prevents IL-1α and IL-1β activities [11].
A meta-analysis of the first published trials in patients with COVID-19 treated by anti-IL-6 agents showed no statistically significant reduction in intensive care unit transfer. In other studies, including the RECOVERY study, patients with COVID-19 treated with IL-6 antagonists (e.g., tocilizumab) had a lower mortality rate at 28 days [12].Interleukin-6 inhibition has been tested in patients who may develop atherosclerosis. A novel anti-IL-6 monoclonal antibody (ziltivekimab) was tested in patients with chronic kidney diseases who were at a high risk of developing atherosclerosis. In the recent RESCUE (Reduction in Inflammation in Patients with Advanced Chronic Renal Disease Utilizing Antibody-Mediated IL-6 Inhibition) trial, a significant reduction in high-sensitivity C-reactive protein (hsCRP) was observed. IL-1 inhibition lowers cardiovascular event rates. Anti-IL-6 strategies can reduce inflammation and atherothrombosis [13].
1.3. TNF Family
Tumor necrosis factor is also named cachectin since it produces weight loss in mice. Two cytotoxic factors isolated from macrophages and lymphocytes were named TNF and lymphotoxin, then TNFα and TNFβ. Based on sequence homology, a group of 19 members is considered to belong to the TNF superfamily, having various roles in immunology, inflammation, cell proliferation, angiogenesis and oncogenesis. The TNF members have pro-inflammatory activities, but most of the members have beneficial effects. TNFα stimulates B cell proliferation and differentiation and plays a role in cardiovascular, neurologic, autoimmune and metabolic disorders. The superfamily members are TNFα, TNFβ, lymphotoxin β, CD40L, FasL, CD30L, 4-1BBL, CD27L, OX40L, TNF-related apoptosis-inducing ligand (TRAIL), LIGHT, the receptor activator of NF-κB ligand (RANKL), TNF-related weak inducer of apoptosis (TWEAK), a proliferation-inducing ligand (APRIL), B-cell-activating factor (BAFF), vascular endothelial growth inhibitor (VEGI), ectodysplasin A (EDA), EDA-A1, EDA-A2 and glucocorticoid-induced TNF-related receptor ligand (GITRL) [14]. The members of the TNF family interact with distinct receptors, and the expression of receptors varies between cell types and can transmit a dual signal. TNFα induces several types of signals, including NF-κB, extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (p38MAPK kinase) and c-Jun N-terminal kinase (JNK) [15].The pro-inflammatory activity of TNF is dependent upon NF-κB-regulated proteins (IL-6, IL-8, IL-18), iNOS, COX-2 and soluble lectin-like oxidized low-density lipoprotein (sLOX) [16].
1.4. TNF Inhibitors
Since TNF family members have been implicated in the pathophysiology of several diseases, they are becoming a target for drug development. Various antagonists are used in the treatment of rheumatoid arthritis, psoriasis, Crohn’s disease and ankylosing spondylarthritis [17].
Several trials targeting the TNF family have been started (phases 1–3) in relation to Crohn’s disease, kidney cancer, colorectal cancer and rheumatoid arthritis. A more complete list was published in [14]. TNF inhibitors have been extensively developed in the last decade and are used in the treatment of inflammatory and autoimmune disorders. Several monoclonal antibodies have been prepared: adalimumab, certolizumab and infliximab. They have been tested in various pathologies and are now currently applied in the treatment of rheumatoid arthritis (RA), Crohn’s disease and amyotrophy lateral sclerosis (ALS). Adalimumab and infliximab are also indicated in the treatment of psoriasis and psoriatic arthritis (PA). Alternatively, etanercept, a receptor derivative (RD), is also an approved treatment for RA, PA and ALS.
1.5. Transforming Growth Factor Family
The TGFβ family comprises members which are involved in growth and cell differentiation; they are encoded by 33 genes in mammals [18]. TGFβ is a bifunctional regulator which stimulates or inhibits cell proliferation. The thirty-three known human proteins of the TGFβ family include TGFβ isoforms, activins, nodal and bone morphogenetic proteins (BMPs) and growth and differentiation factors (GDFs). A limited number of receptors named Smad proteins transmit the intracellular signal (Figure 3).
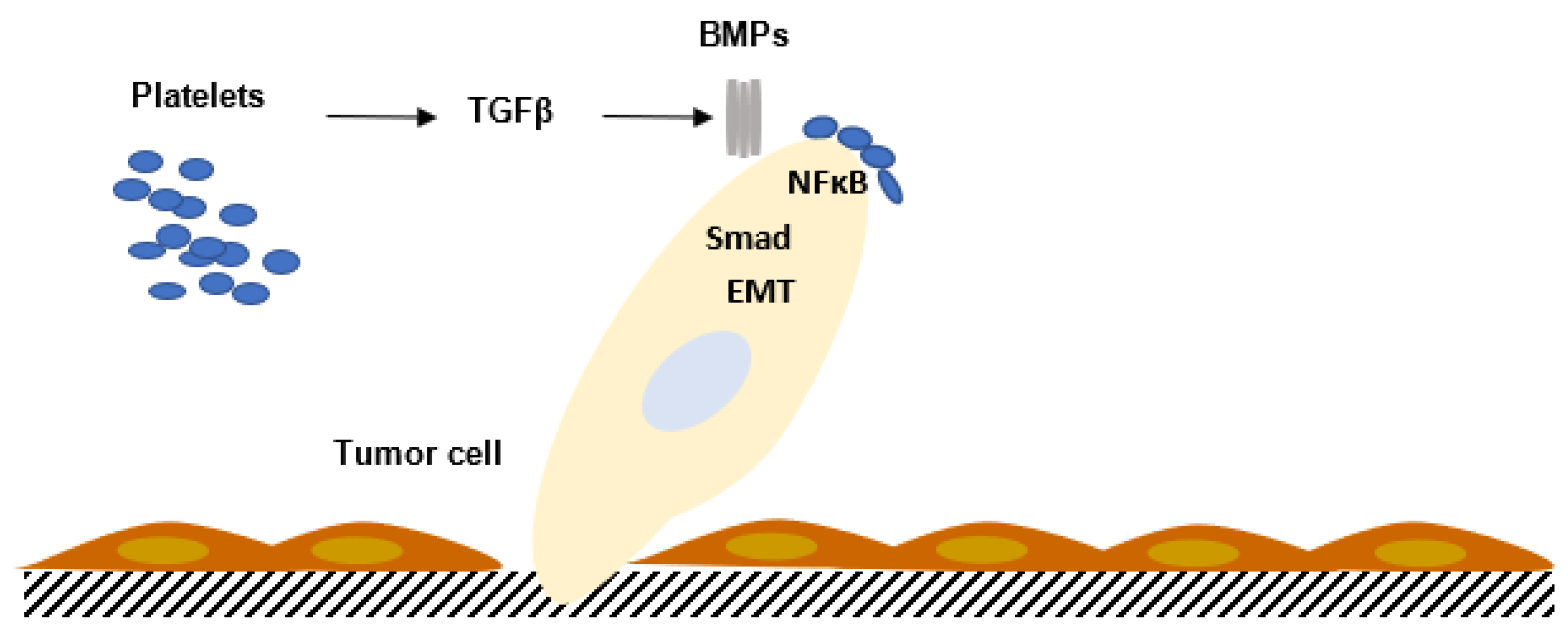
Figure 3. Interaction between platelets and tumor cells activates the TGFβ/SMAD pathway in tumor cells, enhancing tumor cell migration and increasing the metastatic process. The contact between platelets and tumor cells, via TGFβ/SMAD and NFκB, potentiates epithelial–mesenchymal transition (EMT) and metastasis. BMPs: bone morphogenetic proteins.
The precursor polypeptides of TGFβ are composed of three segments. The prosegments vary in length (150 to 450 residues). The prosegments are also called latency-associated peptides (LAPs). Before the binding of TGFβ to the cell surface, the LAPs must be released from the mature peptide. TGFβ LAP and β3 LAP have an integrin recognition peptide, Arg–Gly–Asp (RGD), which can bind several integrins, including αvβ6 and αvβ8. TGFα is related to RGF and binds to the EGF receptor. The anti-proliferative activity of TGFβ has been observed in endothelial cells, epithelial cells, hematopoietic and glial cells. TGFβ induces cyclin-dependent kinase inhibitors and inhibits the expression of mediators that contribute to cell proliferation [19]. On the other hand, TGFβ can stimulate cell growth of chondrocytes, osteoblasts, mesenchymal stem cells and fibroblasts [20]. TGFβ controls the cell differentiation of immune cells, blood cells and neuronal cells [21].
2. Cytokine Production in Human Pathology
2.1. Pathophysiology
The cytokine family and/or receptors have different roles in immune homeostasis and contribute to the pathophysiology of autoimmunity, metabolic and endocrinologic disorders and cardiovascular diseases and cancer.
The pro-inflammatory cytokines IL-1β, IL-6, IL-8, IL-12, IFNγ and TNFα are released at the beginning of the reaction. The cytokines synthetized and secreted by recruited leukocytes are involved according to the stage of reaction and the stimulus: polymorphonuclear cells, macrophages or lymphocytes. The coordinated response to infectious agents which protects the host can become detrimental when chronic. During cancer, proliferation and dissemination of pro-inflammatory cytokines such as IL-1, IFNγ and TNFα may have a deleterious effect.
Interferon gamma is produced in the host defense against infection and may be elevated during autoimmune and inflammatory diseases. IFNγ can also be involved in anti-inflammatory mechanisms, inducing interleukin-1 receptor antagonist and interleukin-18 binding protein, activating apoptosis [22].Anti-inflammatory cytokines may inhibit and limit the excess of inflammatory cytokines. The following molecules, IL-1 receptor antagonist, IL-4, IL-6, IL-10, IL-11 and IL-13, are considered to possess anti-inflammatory properties [23]. IL-35 regulated T cell function by suppressing T helper 1 (Th1) and Th17 pathogenic cells in experimental models [24].
2.2. Inflammatory Anemia
In inflammatory conditions, inflammatory anemia is sometimes difficult to differentiate from iron deficiency. In inflammatory anemia, hepcidin limits iron access to the bone marrow, reducing erythropoiesis. In mouse models, TNFα, IL-1 and IFNγ suppressed erythropoiesis in vitro. Hepcidin, which is secreted by human hepatocytes, regulates intestinal absorption of iron. IL-1 and IL-6 enhance expression by macrophages, but TNF and IL-6 impair erythropoiesis. Hepcidin binds the iron exporter ferroportin, decreasing delivery from macrophages to erythrocyte precursors. Inhibitors of hepcidin production modulate inflammatory anemia [25]. The first target for the treatment of inflammatory anemia is to treat the underlaying disease; if anemia is severe, blood transfusion or treatment by erythropoietin-stimulating agents may be necessary [26].
2.3. Cytokine Storm
Pro-inflammatory and anti-inflammatory cytokines participate in the cytokine storm.Cytokine storm refers to an influenza-like syndrome in sepsis immunotherapies and may contribute to multi-organ dysfunction. There is no general agreement about the definition of cytokine storm or cytokine release [1]. Nearly all patients have fever, fatigue, headache, anoxia, arthralgia and myalgia. Disseminated intravascular coagulation, dyspnea, vasodilatory shock and acute respiratory distress syndrome (ARDS) can be observed in the most severe cases and can lead to death, despite mechanical ventilation.
C-reactive protein (CRP) and markers of inflammation are elevated and correlate with the severity [27]. The cytokine levels of IFNγ, IL-6 and IL-10 are found in chimeric antigen receptor T (CART)-cell-induced cytokine storm [28]. Cytokine storm can result from microbial infection. The use of CART cells in the treatment of CD19+ lymphoma can induce cytokine storm. SARS-CoV-2 infection is characterized by heterogenous symptoms ranging from mild fatigue to multi-organ failure. IL-1, IL-6, IP-10, TNF, IFNγ, MIP and VEGF are elevated in COVID-19 patients.
References
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273.
- Hu, D. Role of Anti-inflammatory Cytokines IL-35 and IL-37 in Asthma. Inflammation 2017, 40, 697–707.
- Mora, J.; Weigert, A. IL-1 family cytokines in cancer immunity—A matter of life and death. Biol. Chem. 2016, 397, 1125–1134.
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415.
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888.
- Naka, T.; Nishimoto, N.; Kishimoto, T. The paradigm of IL-6: From basic science to medicine. Arthritis Res. 2002, 4 (Suppl. 3), S233–S242.
- Dienz, O.; Eaton, S.M.; Bond, J.P.; Neveu, W.; Moquin, D.; Noubade, R.; Briso, E.M.; Charland, C.; Leonard, W.J.; Ciliberto, G.; et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 2009, 206, 69–78.
- Liote, F.; Boval-Boizard, B.; Weill, D.; Kuntz, D.; Wautier, J.L. Blood monocyte activation in rheumatoid arthritis: Increased monocyte adhesiveness, integrin expression, and cytokine release. Clin. Exp. Immunol. 1996, 106, 13–19.
- Su, D.L.; Lu, Z.M.; Shen, M.N.; Li, X.; Sun, L.Y. Roles of pro- and anti-inflammatory cytokines in the pathogenesis of SLE. J. Biomed. Biotechnol. 2012, 2012, 347141.
- Lindenlaub, T.; Sommer, C. Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta Neuropathol. 2003, 105, 593–602.
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732.
- Zizzo, G.; Tamburello, A.; Castelnovo, L.; Laria, A.; Mumoli, N.; Faggioli, P.M.; Stefani, I.; Mazzone, A. Immunotherapy of COVID-19: Inside and Beyond IL-6 Signalling. Front. Immunol. 2022, 13, 795315.
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746.
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665.
- Berk, B.C.; Abe, J.I.; Min, W.; Surapisitchat, J.; Yan, C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann. N. Y. Acad. Sci. 2001, 947, 93–109; discussion 109–110.
- Natoli, G.; Costanzo, A.; Moretti, F.; Fulco, M.; Balsano, C.; Levrero, M. Tumor necrosis factor (TNF) receptor 1 signaling downstream of TNF receptor-associated factor 2. Nuclear factor kappaB (NFkappaB)-inducing kinase requirement for activation of activating protein 1 and NFkappaB but not of c-Jun N-terminal kinase/stress-activated protein kinase. J. Biol. Chem. 1997, 272, 26079–26082.
- Boyce, E.G.; Halilovic, J.; Stan-Ugbene, O. Golimumab: Review of the efficacy and tolerability of a recently approved tumor necrosis factor-alpha inhibitor. Clin. Ther. 2010, 32, 1681–1703.
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873.
- Moses, H.L.; Yang, E.Y.; Pietenpol, J.A. TGF-beta stimulation and inhibition of cell proliferation: New mechanistic insights. Cell 1990, 63, 245–247.
- Jian, H.; Shen, X.; Liu, I.; Semenov, M.; He, X.; Wang, X.F. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 2006, 20, 666–674.
- Krieglstein, K.; Zheng, F.; Unsicker, K.; Alzheimer, C. More than being protective: Functional roles for TGF-beta/activin signaling pathways at central synapses. Trends Neurosci. 2011, 34, 421–429.
- Muhl, H.; Pfeilschifter, J. Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int. Immunopharmacol. 2003, 3, 1247–1255.
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172.
- Chen, C.; Xu, H.; Peng, Y.; Luo, H.; Huang, G.X.; Wu, X.J.; Dai, Y.C.; Luo, H.L.; Zhang, J.A.; Zheng, B.Y.; et al. Elevation in the counts of IL-35-producing B cells infiltrating into lung tissue in mycobacterial infection is associated with the downregulation of Th1/Th17 and upregulation of Foxp3(+)Treg. Sci. Rep. 2020, 10, 13212.
- Fraenkel, P.G. Anemia of Inflammation. Med. Clin. N. Am. 2016, 101, 285–296.
- Nemeth, E.; Ganz, T. Anemia of inflammation. Hematol. Oncol. Clin. N. Am. 2014, 28, 671–681.
- Lee, D.W.; Gardner, R.A.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.C.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195.
- Grupp, S.A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D.L.; Rheingold, S.R.; Teachey, D.T.; Chew, A.; Hauck, B.; Wright, J.F.; et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013, 368, 1509–1518.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
822
Revisions:
2 times
(View History)
Update Date:
19 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




