
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fabio Pastorino | -- | 2121 | 2023-06-15 13:40:46 | | | |
| 2 | Jessie Wu | -1 word(s) | 2120 | 2023-06-16 05:02:32 | | |
Video Upload Options
Retinoids are a class of natural and synthetic compounds derived from vitamin A. They are involved in several biological processes like embryogenesis, reproduction, vision, growth, inflammation, differentiation, proliferation, and apoptosis. In light of their important functions, retinoids have been widely investigated for their therapeutic applications. Thus far, their use for the treatment of several types of cancer and skin disorders has been reported. However, these therapeutic agents present several limitations for their widespread clinical translatability, i.e., poor solubility and chemical instability in water, sensitivity to light, heat, and oxygen, and low bioavailability. These characteristics result in internalization into target cells and tissues only at low concentration and, consequently, at an unsatisfactory therapeutic dose.
1. Introduction
2. Drug Delivery Systems for Cancer Therapy
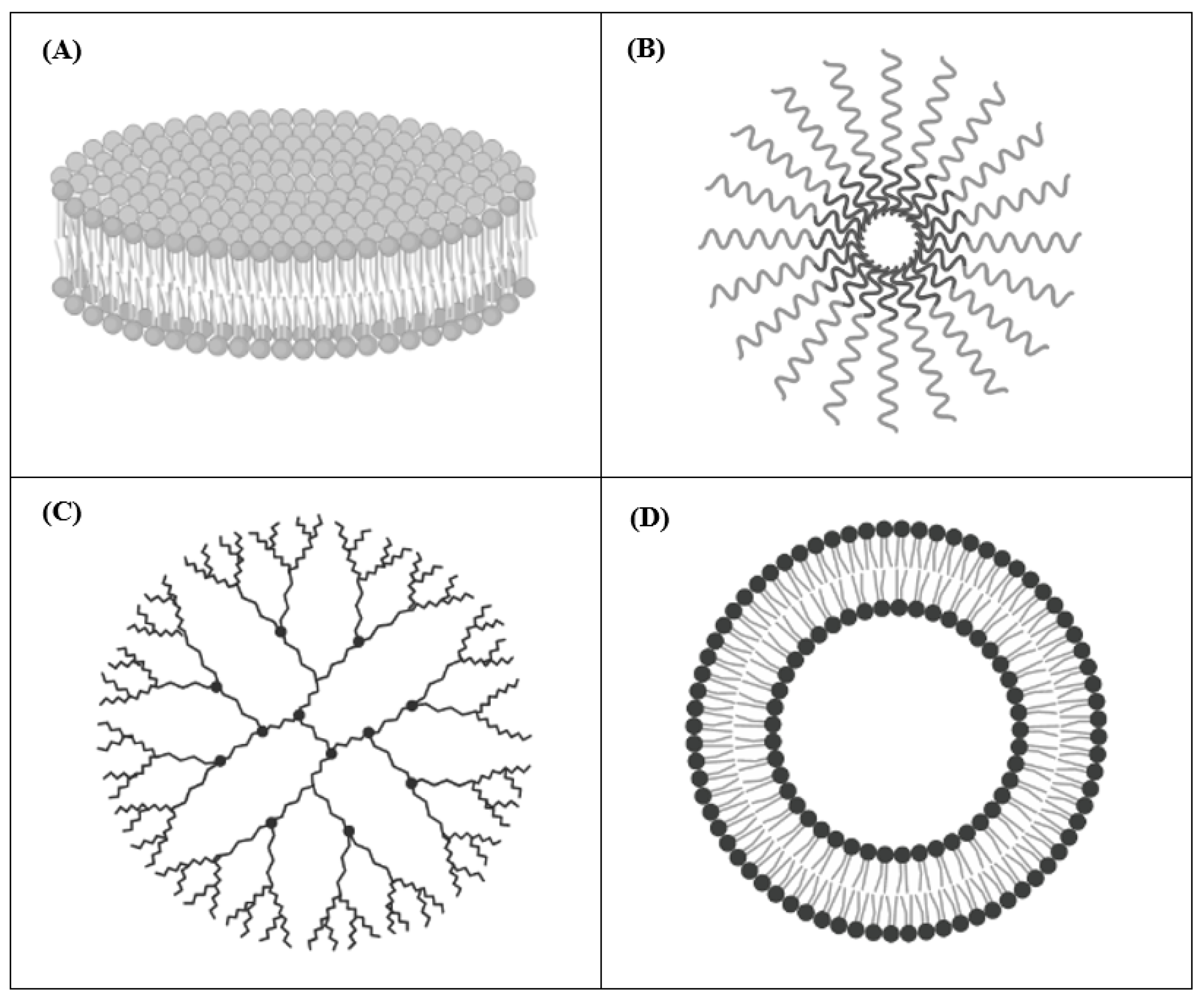
2.1. Nanodisks (NDs)
2.2. Polymeric Micelles
2.3. Dendrimers
2.4. Liposomes
References
- Blomhoff, R.; Blomhoff, H.K. Overview of retinoid metabolism and function. J. Neurobiol. 2006, 66, 606–630.
- Campo-Paysaa, F.; Marlétaz, F.; Laudet, V.; Schubert, M. Retinoic acid signaling in development: Tissue-specific functions and evolutionary origins. Genesis 2008, 46, 640–656.
- Niederreither, K.; Subbarayan, V.; Dollé, P.; Chambon, P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999, 21, 444–448.
- Sporn, M.B.; Dunlop, N.M.; Newton, D.L.; Henderson, W.R. Relationships between structure and activity of retinoids. Nature 1976, 263, 110–113.
- Kiser, P.D.; Golczak, M.; Palczewski, K. Chemistry of the retinoid (visual) cycle. Chem. Rev. 2014, 114, 194–232.
- Beckenbach, L.; Baron, J.M.; Merk, H.F.; Loffler, H.; Amann, P.M. Retinoid treatment of skin diseases. Eur. J. Dermatol. 2015, 25, 384–391.
- Dobrotkova, V.; Chlapek, P.; Mazanek, P.; Sterba, J.; Veselska, R. Traffic lights for retinoids in oncology: Molecular markers of retinoid resistance and sensitivity and their use in the management of cancer differentiation therapy. BMC Cancer 2018, 18, 1059.
- Bollag, W. Retinoids and cancer. Cancer Chemother. Pharmacol. 1979, 3, 207–215.
- Zusi, F.C.; Lorenzi, M.V.; Vivat-Hannah, V. Selective retinoids and rexinoids in cancer therapy and chemoprevention. Drug Discov. Today 2002, 7, 1165–1174.
- Ponzoni, M.; Bocca, P.; Chiesa, V.; Decensi, A.; Pistoia, V.; Raffaghello, L.; Rozzo, C.; Montaldo, P.G. Differential effects of N-(4-hydroxyphenyl)retinamide and retinoic acid on neuroblastoma cells: Apoptosis versus differentiation. Cancer Res. 1995, 55, 853–861.
- Montaldo, P.G.; Pagnan, G.; Pastorino, F.; Chiesa, V.; Raffaghello, L.; Kirchmeier, M.; Allen, T.M.; Ponzoni, M. N-(4-hydroxyphenyl) retinamide is cytotoxic to melanoma cells in vitro through induction of programmed cell death. Int. J. Cancer 1999, 81, 262–267.
- Ferrara, F.F.; Fazi, F.; Bianchini, A.; Padula, F.; Gelmetti, V.; Minucci, S.; Mancini, M.; Pelicci, P.G.; Lo Coco, F.; Nervi, C. Histone deacetylase-targeted treatment restores retinoic acid signaling and differentiation in acute myeloid leukemia. Cancer Res. 2001, 61, 2–7.
- Liu, M.; Iavarone, A.; Freedman, L.P. Transcriptional activation of the human p21(WAF1/CIP1) gene by retinoic acid receptor. Correlation with retinoid induction of U937 cell differentiation. J. Biol. Chem. 1996, 271, 31723–31728.
- Johnson, K.J. The retinoids: Biology, chemistry and medicine (second edition) Edited by M B Sporn, A B Roberts and the late D S Goodman. pp 311. Raven Press, NY. 1993. $250.00 ISBN 0-7817-0082-5. Biochem. Educ. 1994, 22, 56.
- Hu, J.; Liu, Y.F.; Wu, C.F.; Xu, F.; Shen, Z.X.; Zhu, Y.M.; Li, J.M.; Tang, W.; Zhao, W.L.; Wu, W.; et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 2009, 106, 3342–3347.
- Villablanca, J.G.; Khan, A.A.; Avramis, V.I.; Seeger, R.C.; Matthay, K.K.; Ramsay, N.K.; Reynolds, C.P. Phase I trial of 13-cis-retinoic acid in children with neuroblastoma following bone marrow transplantation. J. Clin. Oncol. 1995, 13, 894–901.
- Reynolds, C.P.; Matthay, K.K.; Villablanca, J.G.; Maurer, B.J. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003, 197, 185–192.
- Ferreira, R.; Napoli, J.; Enver, T.; Bernardino, L.; Ferreira, L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nat. Commun. 2020, 11, 4265.
- David, M.; Hodak, E.; Lowe, N.J. Adverse effects of retinoids. Med. Toxicol. Advers. Drug Exp. 1988, 3, 273–288.
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Torrisi, R.; Decensi, A. Fenretinide and cancer prevention. Curr. Oncol. Rep. 2000, 2, 263–270.
- Kelloff, G.J.; Crowell, J.A.; Boone, C.W.; Steele, V.E.; Lubet, R.A.; Greenwald, P.; Alberts, D.S.; Covey, J.M.; Doody, L.A.; Knapp, G.G.; et al. Strategy and planning for chemopreventive drug development: Clinical development plans. Chemoprevention Branch and Agent Development Committee. National Cancer Institute. J. Cell Biochem. Suppl. 1994, 20, 55–62.
- Mehta, R.G.; Moon, R.C.; Hawthorne, M.; Formelli, F.; Costa, A. Distribution of fenretinide in the mammary gland of breast cancer patients. Eur. J. Cancer 1991, 27, 138–141.
- Cooper, J.P.; Reynolds, C.P.; Cho, H.; Kang, M.H. Clinical development of fenretinide as an antineoplastic drug: Pharmacology perspectives. Exp. Biol. Med. (Maywood) 2017, 242, 1178–1184.
- Formelli, F.; Barua, A.B.; Olson, J.A. Bioactivities of N-(4-hydroxyphenyl) retinamide and retinoyl beta-glucuronide. FASEB J. 1996, 10, 1014–1024.
- Trapasso, E.; Cosco, D.; Celia, C.; Fresta, M.; Paolino, D. Retinoids: New use by innovative drug-delivery systems. Expert Opin. Drug Deliv. 2009, 6, 465–483.
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135.
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198.
- Pastorino, F.; Marimpietri, D.; Brignole, C.; Di Paolo, D.; Pagnan, G.; Daga, A.; Piccardi, F.; Cilli, M.; Allen, T.M.; Ponzoni, M. Ligand-targeted liposomal therapies of neuroblastoma. Curr. Med. Chem. 2007, 14, 3070–3078.
- Corti, A.; Pastorino, F.; Curnis, F.; Arap, W.; Ponzoni, M.; Pasqualini, R. Targeted Drug Delivery and Penetration Into Solid Tumors. Med. Res. Rev. 2012, 32, 1078–1091.
- Pastorino, F.; Brignole, C.; Di Paolo, D.; Perri, P.; Curnis, F.; Corti, A.; Ponzoni, M. Overcoming Biological Barriers in Neuroblastoma Therapy: The Vascular Targeting Approach with Liposomal Drug Nanocarriers. Small 2019, 15, e1804591.
- Redmond, K.A.; Nguyen, T.S.; Ryan, R.O. All-trans-retinoic acid nanodisks. Int. J. Pharm. 2007, 339, 246–250.
- Ho, B.N.; Pfeffer, C.M.; Singh, A.T.K. Update on Nanotechnology-based Drug Delivery Systems in Cancer Treatment. Anticancer Res. 2017, 37, 5975–5981.
- Ryan, R.O. Nanodisks: Hydrophobic drug delivery vehicles. Expert Opin. Drug Deliv. 2008, 5, 343–351.
- Singh, A.T.; Evens, A.M.; Anderson, R.J.; Beckstead, J.A.; Sankar, N.; Sassano, A.; Bhalla, S.; Yang, S.; Platanias, L.C.; Forte, T.M.; et al. All trans retinoic acid nanodisks enhance retinoic acid receptor mediated apoptosis and cell cycle arrest in mantle cell lymphoma. Br. J. Haematol. 2010, 150, 158–169.
- Stauffer, R.G.; Mohammad, M.; Singh, A.T. Novel Nanoscale Delivery Particles Encapsulated with Anticancer Drugs, All-trans Retinoic Acid or Curcumin, Enhance Apoptosis in Lymphoma Cells Predominantly Expressing CD20 Antigen. Anticancer Res. 2015, 35, 6425–6429.
- Buehler, D.C.; Toso, D.B.; Kickhoefer, V.A.; Zhou, Z.H.; Rome, L.H. Vaults engineered for hydrophobic drug delivery. Small 2011, 7, 1432–1439.
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147.
- Valerii, M.C.; Benaglia, M.; Caggiano, C.; Papi, A.; Strillacci, A.; Lazzarini, G.; Campieri, M.; Gionchetti, P.; Rizzello, F.; Spisni, E. Drug delivery by polymeric micelles: An in vitro and in vivo study to deliver lipophilic substances to colonocytes and selectively target inflamed colon. Nanomedicine 2013, 9, 675–685.
- Orienti, I.; Salvati, V.; Sette, G.; Zucchetti, M.; Bongiorno-Borbone, L.; Peschiaroli, A.; Zolla, L.; Francescangeli, F.; Ferrari, M.; Matteo, C.; et al. A novel oral micellar fenretinide formulation with enhanced bioavailability and antitumour activity against multiple tumours from cancer stem cells. J. Exp. Clin. Cancer Res. 2019, 38, 373.
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526.
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401.
- Tekade, R.K.; Dutta, T.; Tyagi, A.; Bharti, A.C.; Das, B.C.; Jain, N.K. Surface-engineered dendrimers for dual drug delivery: A receptor up-regulation and enhanced cancer targeting strategy. J. Drug Target. 2008, 16, 758–772.
- Pan, J.; Attia, S.A.; Filipczak, N.; Torchilin, V.P. 10—Dendrimers for drug delivery purposes. In Nanoengineered Biomaterials for Advanced Drug Delivery; Mozafari, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 201–242.
- Wang, Y.; Wang, H.; Lv, X.; Liu, C.; Qi, L.; Song, X.; Yu, A. Enhancement of all-trans retinoic acid-induced differentiation by pH-sensitive nanoparticles for solid tumor cells. Macromol. Biosci. 2014, 14, 369–379.
- Yalcin, S.; Erkan, M.; Unsoy, G.; Parsian, M.; Kleeff, J.; Gunduz, U. Effect of gemcitabine and retinoic acid loaded PAMAM dendrimer-coated magnetic nanoparticles on pancreatic cancer and stellate cell lines. Biomed. Pharmacother. 2014, 68, 737–743.
- Allam, A.; Thomsen, A.R.; Gothwal, M.; Saha, D.; Maurer, J.; Brunner, T.B. Pancreatic stellate cells in pancreatic cancer: In focus. Pancreatology 2017, 17, 514–522.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102.
- Kopeckova, K.; Eckschlager, T.; Sirc, J.; Hobzova, R.; Plch, J.; Hrabeta, J.; Michalek, J. Nanodrugs used in cancer therapy. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2019, 163, 122–131.
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34.
- Barenholz, Y. Doxil (R)—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134.
- Gabizon, A.A.; Patil, Y.; La-Beck, N.M. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist. Updates 2016, 29, 90–106.
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12.
- Viswanathan, S.; Berlin Grace, V.M.; Danisha, J.P. Enhancement of tumor suppressor RAR-β protein expression by cationic liposomal-ATRA treatment in benzo(a)pyrene-induced lung cancer mice model. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 415–426.
- Cristiano, M.C.; Cosco, D.; Celia, C.; Tudose, A.; Mare, R.; Paolino, D.; Fresta, M. Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells. Colloids Surf. B Biointerfaces 2017, 150, 408–416.
- Kawakami, S.; Suzuki, S.; Yamashita, F.; Hashida, M. Induction of apoptosis in A549 human lung cancer cells by all-trans retinoic acid incorporated in DOTAP/cholesterol liposomes. J. Control. Release 2006, 110, 514–521.
- Simile, M.M.; Pagnan, G.; Pastorino, F.; Brignole, C.; De Miglio, M.R.; Muroni, M.R.; Asara, G.; Frau, M.; Seddaiu, M.A.; Calvisi, D.F.; et al. Chemopreventive N-(4-hydroxyphenyl)retinamide (fenretinide) targets deregulated NF-B and Mat1A genes in the early stages of rat liver carcinogenesis. Carcinogenesis 2005, 26, 417–427.
- Pagnan, G.; Montaldo, P.G.; Pastorino, F.; Raffaghello, L.; Kirchmeier, M.; Allen, T.M.; Ponzoni, M. GD2-mediated melanoma cell targeting and cytotoxicity of liposome-entrapped fenretinide. Int. J. Cancer 1999, 81, 268–274.
- Raffaghello, L.; Pagnan, G.; Pastorino, F.; Cosimo, E.; Brignole, C.; Marimpietri, D.; Montaldo, P.G.; Gambini, C.; Allen, T.M.; Bogenmann, E.; et al. In vitro and in vivo antitumor activity of liposomal Fenretinide targeted to human neuroblastoma. Int. J. Cancer 2003, 104, 559–567.




