Retinoids are a class of natural and synthetic compounds derived from vitamin A. They are involved in several biological processes like embryogenesis, reproduction, vision, growth, inflammation, differentiation, proliferation, and apoptosis. In light of their important functions, retinoids have been widely investigated for their therapeutic applications. Thus far, their use for the treatment of several types of cancer and skin disorders has been reported. However, these therapeutic agents present several limitations for their widespread clinical translatability, i.e., poor solubility and chemical instability in water, sensitivity to light, heat, and oxygen, and low bioavailability. These characteristics result in internalization into target cells and tissues only at low concentration and, consequently, at an unsatisfactory therapeutic dose.
1. Introduction
Retinoids are a family of compounds related to vitamin A (retinol), essential for the life of all chordates. They are signaling molecules that, after binding to the nuclear retinoic acid receptors (RARs) and retinoic X receptors (RXRs), activate genetic networks involved in important biological and physiological processes, such as cell proliferation, cell differentiation, apoptosis, and fetal development
[1][2][3][1,2,3]. The term “
retinoids” was introduced in 1976 by Sporn and colleagues
[4], and in 1981, the IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) defined retinoids: (i) compounds composed of four isoprenoid units joined head-to-tail; (ii) derived from a monocyclic molecule; (iii) containing five carbon-carbon double bonds and a functional terminal group at the end of the acyclic portion. According to this definition, the retinoids family includes the natural forms of vitamin A and their synthetic derivatives (
[1] and
http://publications.iupac.org) (accessed on 15 March 2021). To date, more than 1500 different related compounds have been discovered and tested. Retinoids have raised interest within the scientific community thanks to their beneficial effects in vision
[5], skin disorders (acne, psoriasis, and keratinization disorders)
[6], and cancer
[7][8][9][7,8,9]. Specifically, in the oncology field, retinoids attracted researchers’ attention due to their known anti-tumor properties. In particular, they were demonstrated to be effective in inducing differentiation and/or apoptosis of tumor cells, as well as cell growth inhibition
[10][11][12][13][10,11,12,13]. Moreover, they also showed chemo-preventive effects in experimental animal models of chemically-induced cancer
[14]. The most frequently tested vitamin A derivatives in cancer medicine are represented by all-trans retinoic acid (ATRA, tretinoin), 9-cis retinoic acid (9-cis-RA, alitretinoin), and 13-cis retinoic acid (13-cis-RA, isotretinoin)
[7]. Clinically, the most encouraging therapeutic results were obtained by the use of ATRA and 13-cis-RA following bone marrow transplantation in patients affected by acute promyelocytic leukemia and high-risk neuroblastoma (NB), respectively
[15][16][17][15,16,17]. In contrast, the initial enthusiasm derived from several pre-clinical studies faded after their clinical application because of toxicity-driven limitations. Indeed, long-term and therapeutic-dosage administrations of natural retinoids caused liver toxicity, dry skin and irritation, bone damage, lipid alterations, and teratogenicity
[7][18][19][20][7,18,19,20]. Further, retinoids are sensitive to oxygen, heat, and light and present poor solubility in water, all characteristics that reduce their bioavailability drastically and, consequently, their therapeutic efficacy
[18]. Finally, they are characterized by a short lifetime due to the degradation by the cytochrome P450-dependent monooxygenase system
[18]. Consequently, the development of less toxic and more bioavailable vitamin A-related compounds became necessary.
Developed at the beginning to treat skin disorders, it was then investigated for its potential as a novel anti-cancer therapeutic. 4-HPR represented, indeed, one of the most promising drugs because of its favorable toxicological profile, characterized by minimal systemic toxicity, good tolerability, and high anti-tumor efficacy
[21][22][24,25]. Due to its preferential accumulation in the mammary gland, it initially seemed very efficacious against breast cancer
[23][26]. Successively, in vitro and pre-clinical experiments first and clinical trials later demonstrated that fenretinide was active against several types of cancers, including bladder, lung, ovary, prostate, melanoma, and NB
[10][11][24][10,11,27]. Evidence shows that 4-HPR exerts anti-tumor effects on both premalignant cells by inhibiting the carcinogenesis process, and on transformed cells, by activating apoptosis, making fenretinide a promising compound for clinical application, both as a chemo-preventive agent and an anti-cancer drug
[21][25][24,28].
A different strategy to reduce side-effects related to free retinoids administration while increasing their bioavailability and maximizing their therapeutic index is also represented by the design and development of appropriate Drug Delivery Systems. With this aim, several formulations loaded with retinoids have been developed
[18][26][18,29].
2. Drug Delivery Systems for Cancer Therapy
Drug Delivery Systems (DDSs) refer to formulations able to transport and deliver active molecules/drugs to cell/tissue targets in order to achieve a specific and hopefully increased therapeutic effect compared to the free drug while minimizing its potential side effects. The use of DDSs in cancer therapy lies in the possibility of increasing the therapeutic index of the encapsulated drugs by delivering them to tumor cells through both passive and active targeting. Passive targeting of tumor cells exploits the so-called “Enhanced Permeability and Retention” effect (EPR)
[27][30]. It is well known that the newly formed blood vessels of solid tumors present altered permeability, rendering them more permeable compared to those of healthy tissues
[27][30]. In these circumstances, the leaky blood vessels allow for the non-selective extravasation of macromolecules (larger than 40 kDa) and small particles (ranging from 50 to 500 nm) into the tumor stroma, finally leading to tumor cells killing
[27][28][30,31]. However, the passive targeting capability of DDSs only leads to a modest increased delivery of the encapsulated drug to the target site, and it is strictly dependent on different factors such as size and circulation time of the carrier, as well as on tumor biology features, such as vascularity of the tumor and leakiness of the vessels
[28][31]. On the other hand, the passive targeting capability of DDSs can be further optimized by coupling moieties (e.g., monoclonal antibodies, peptides etc.) on their external surface, with the aim to specifically recognize and target tumor-associated antigens
[18][28][29][30][31][18,31,32,33,34].
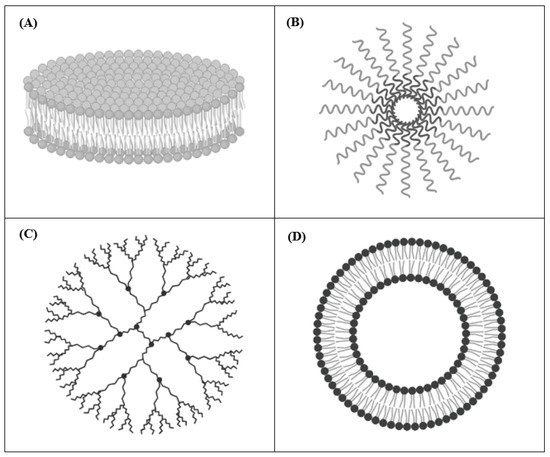
The most relevant DDSs used in pre-clinical studies of cancer therapy are nanodisks (NDs), polymeric micelles, dendrimers, and liposomes (
Figure 1). In these formulations, retinoic acids can be either entrapped into the inner core or mixed with the outer surface
[18]. Below, some examples of each mentioned retinoids carrying DDSs are presented.
Figure 1.
Generic structure of nanodisks (
A
), polymeric micelles (
B
), dendrimers (
C
), and liposomes (
D
).
2.1. Nanodisks (NDs)
NDs are self-assembled nanoscale carriers composed of a phospholipid bilayer surrounded by amphipathic apolipoproteins that stabilize the structure, serving as a scaffold
[32][33][35,36] (
Figure 1A). This composition allows the encapsulation and the delivery of hydrophobic molecules, such as amphotericin B and ATRA
[32][34][35,37]. NDs have the advantages of being very small in size (8–20 nm in diameter) and fully soluble in water
[33][36]. Singh and colleagues used NDs to encapsulate ATRA for treating cell culture models of mantle cell lymphoma (MCL). Compared to free ATRA, they demonstrated that ATRA-NDs were more effective in inducing MCL cells apoptosis and G1 cell cycle arrest in vitro
[35][38]. Then, they optimized the formulation by adding the single chain variable antibody fragment against CD20 on the surface in order to improve the selective targeting of CD20-positive MCL cells. In this case, NDs were loaded with either ATRA (ATRA-NDs) or curcumin (curcumin-NDs) and the combination therapy was able to induce higher tumor apoptosis compared to each single treatment
[35][36][38,39]. Importantly, these anti-CD20 NDs, although developed for treating MCL cells, may be useful for any other CD20-expressing tumors
[36][39]. In another study, Buehler et al. engineered vault nanoparticles in order to encapsulate ATRA, using a vault-binding lipoprotein complex that creates a lipid bilayer NDs
[37][40]. Testing hepatocellular carcinoma cell viability after ATRA-vaults treatment, they demonstrated that ATRA-NDs caused increased tumor cells killing compared to that obtained by free ATRA
[37][40].
2.2. Polymeric Micelles
Polymeric Micelles are composed of amphiphilic polymers, which self-associate when added to an aqueous solvent. After self-assembly in the aqueous environment, the hydrophilic polymers (e.g., poly(ethylene glycol), chitosan, dextran, and hyaluronic acids) face the aqueous medium forming a hydrophilic shell, while the hydrophobic ones (e.g., poly(lactide) (PLA), poly(caprolactone) (PCL), poly(lactide-co-glycolide) (PLGA), polyesters, poly(amino acids), and lipids) form the hydrophobic core (
Figure 1B). Similar to NDs, Polymeric Micelles can be employed to encapsulate hydrophobic drugs
[38][41]. The anti-cancer agents can be conjugated to the distal ends of polymer to prepare pharmacologically active polymeric systems that enhance solubility and stability of the conjugates, providing an opportunity for combined drug delivery
[38][41]. Specifically, an efficient intracellular drug delivery system is represented by the use of biocompatible polymeric micelles (BPMs), which allow the administration of retinoic acid (RA), protecting RA from metabolic deactivation while reducing RA-mediated toxicity
[39][42]. For instance, the apoptotic effects induced by RA, either free or encapsulated into BPMs, were compared on colon cancer cell lines. When loaded into BPMs, RA led to a stronger effect with respect to the free administration, also despite the lower dose used
[39][42]. Furthermore, Orienti et al. developed a nano-micellar formulation entrapping 4-HPR into the inner core, called bionanofenretinide (Bio-nFeR)
[40][43]. This system increased fenretinide bioavailability, showing anti-tumor activity against lung, colon, and melanoma cancer stem cells, both in vitro and in tumor xenografts. Interestingly, Bio-nFeR showed lower toxicity when compared to NCI-FeR, an oral formulation of 4-HPR, consisting of soft gelatin capsules, actually available at the National Cancer Institute, and administered in clinical trials
[40][43].
2.3. Dendrimers
Dendrimers are polymeric molecules composed of multiple repetitive branches arising radially from a central core. The terminal groups of every branch provide modifiable functionalities. The number of repeated branching units determines the generation of the dendrimer
[41][42][44,45] (
Figure 1C). Dendrimers are widely used as carriers for the delivery of several therapeutics compounds, including retinoids
[43][46]. They present several advantageous features such as high water solubility, monodispersity, biocompatibility, and low immunogenicity
[44][47]. Moreover, pH-sensitive formulations have been developed in order to be stable at physiological pH and to dissociate in the acid environment of the endosomal and lysosomal tumor compartments, resulting in an enhanced cellular uptake into target cells. For instance, Wang et al. synthesized pH-sensitive nanoparticles based on poly(amidoamine) (PAMAM) dendrimers encapsulating ATRA. They tested the formulation in vitro on human hepatocellular liver carcinoma cells, demonstrating its ability to arrest tumor cell proliferation and increase tumor cell death, compared to free ATRA
[45][48]. Yalçın et al. loaded gemcitabine together with ATRA into PAMAM dendrimer-coated magnetic nanoparticles (DcMNPs) in order to simultaneously target gemcitabine-resistant pancreatic cancer cells and pancreatic stellate cells (PSC), stromal cells that support tumorigenesis, and form a fibrotic barrier against therapeutic agents
[46][47][49,50]. They firstly proved that the DcMNPs were successfully internalized by pancreatic cancer cell lines and by primary human PSC. Then, overcoming pancreatic cancer cell’s resistance to gemcitabine, showed that the increased gemcitabine- and ATRA-loaded DcMNPs accumulation into tumor cells and tumor stroma caused a significant cell death compared to that obtained by ATRA or gemcitabine administered separately
[46][49].
2.4. Liposomes
Liposomes are spherical-shaped vesicles composed of a hydrophilic aqueous space surrounded by one or more phospholipid bilayers, making them similar to the cell membrane structure
[48][49][51,52] (
Figure 1D). They can entrap both hydrophobic and hydrophilic compounds. The ability of liposomes to encapsulate “drugs” characterized by different solubility in water and to specifically target organs, tissues, and cells makes them attractive candidates for drug delivery
[50][53]. They can be classified on the basis of: (i) size; (ii) lipid composition; (iii) surface modification. Due to their good features, such as biocompatibility, biodegradability, and low toxicity, liposomes are the first DDSs that have been translated to clinical application
[51][52][53][54,55,56]. Further, they are the most frequently used formulations for drugs encapsulation and, at present, several liposomal formulations have been approved by the FDA, and different products are available for clinical application (e.g., Doxil
®, Ambisome
®, DepoDur™, DaunoXome
®, etc.
[51][52][53][54,55,56]). Moreover, liposomes are the only nanosystems used in clinical trials for the delivery of retinoids in solid cancer (
https://clinicaltrials.gov/) (accessed on 15 March 2021). To date, pre-clinical evaluations of retinoids-encapsulating liposomes have been testing against several types of cancer, including lung, thyroid, and liver cancers, as well as on neuroectodermal-derived tumors such as melanoma and NB
[54][55][56][57][58][59][57,58,59,60,61,62]. In particular, the anti-tumor effects of cationic liposomes encapsulating ATRA were also tested in pre-clinical animal models of lung cancer
[54][57]. Interestingly, in this
res
earch atudy aimed at investigating ATRA-driven reactivation of the tumor suppressor protein retinoic acid receptor beta (RAR-β), it was shown that, compared to free ATRA, the treatment with ATRA-loaded liposomes led to an enhanced RAR-β expression, thus becoming a useful molecular target therapy for lung cancer
[54][57]. In another study, with the aim to reduce ATRA photo-degradation during administration as a free drug, and consequently to increase its anti-cancer activity, a different liposomal formulation was developed
[55][58]. The authors demonstrated that the liposomes protected ATRA and increased its anti-proliferative properties due to the improvement of its cellular uptake, becoming a useful formulation for the treatment of anaplastic thyroid carcinoma
[55][58]. Moreover, Kawakami and colleagues demonstrated that ATRA incorporated into cationic liposomes was efficiently internalized into ATRA-resistant human lung cancer cells in vitro
[56][59]. Specifically, the interaction between the positive charges of the liposomes and the negative charges of the tumor cell membranes allowed the specific internalization of ATRA, thus overcoming tumor cell resistance and producing pro-apoptotic and cytotoxic effects
[56][59].