Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hanan Ali Henidi | -- | 4227 | 2023-06-15 12:09:41 | | | |
| 2 | Lindsay Dong | Meta information modification | 4227 | 2023-06-16 04:14:12 | | | | |
| 3 | Lindsay Dong | -1 word(s) | 4226 | 2023-06-19 10:15:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems. Encyclopedia. Available online: https://encyclopedia.pub/entry/45642 (accessed on 01 March 2026).
Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as Drug Delivery Systems. Encyclopedia. Available at: https://encyclopedia.pub/entry/45642. Accessed March 01, 2026.
Yusuf, Azeez, Awatif Rashed Z. Almotairy, Hanan Henidi, Ohoud Y. Alshehri, Mohammed S. Aldughaim. "Nanoparticles as Drug Delivery Systems" Encyclopedia, https://encyclopedia.pub/entry/45642 (accessed March 01, 2026).
Yusuf, A., Almotairy, A.R.Z., Henidi, H., Alshehri, O.Y., & Aldughaim, M.S. (2023, June 15). Nanoparticles as Drug Delivery Systems. In Encyclopedia. https://encyclopedia.pub/entry/45642
Yusuf, Azeez, et al. "Nanoparticles as Drug Delivery Systems." Encyclopedia. Web. 15 June, 2023.
Copy Citation
The application of inventions or products from nanotechnology has revolutionised all aspects of everyday life ranging from medical applications to its impact on the food industry. Nanoparticles have made it possible to significantly extend the shelf lives of food product, improve intracellular delivery of hydrophobic drugs and improve the efficacy of specific therapeutics such as anticancer agents.
nanotechnology
nanoparticles
drug delivery systems
nanomedicine
1. Nanotechnology
Nanotechnology is the intentional engineering and manipulation of particulate matter into a physical state of between 1 nm and 100 nm that can be rearranged or reassembled into nano-systems with improved function [1]. The emergence of nanotechnology and its application have put Ireland for instance, at the forefront of scientific research in the last decade [2]. Nanoparticles are the ultimate result of the technological modification of matter, and depending on their sizes, they are a few degrees larger than an atom consequence of the molecular processing of matter. As they possess enhanced characteristics such as auto-reactive stability and self-reassembly, they are easily adaptable and can be modified to achieve a specific characteristic or intended properties such as high surface area when compared to conventional substances [3][4].
Nanotechnology, as a relatively new branch of science, has gained attention in the last two decades and is rapidly expanding from the academic arena into the industry. Due to the possible advancements that can be achieved by nanotechnology, it has been estimated that nanotechnology will impact the global economy by about three trillion dollars by 2020 [5], making the field highly viable economically speaking. This could be attributed to the unique physicochemical properties of nanoparticles at the interface of chemistry, medicine, physics, and engineering.
The field of nanotechnology is one of the fastest-growing areas of scientific research and development, with significant advances being made in a range of applications. Currently, the state of the art in nanotechnology covers a wide range of areas, including electronics, energy, materials science, biomedicine, and more. In electronics, researchers are exploring the use of nanoscale transistors and other components to create smaller, faster, and more energy-efficient devices. In energy, nanotechnology is being used to develop new materials and devices for solar energy conversion, energy storage, and more. In biomedicine, nanotechnology is being used to develop new diagnostic tools, therapies, and tissue engineering strategies. Overall, the current state of the art in nanotechnology reflects a highly dynamic and rapidly evolving field, with many exciting new developments and applications yet to come.
Nanoparticles and nanomaterials are increasingly being explored for their potential applications in medicine. One of the most promising areas of application is drug delivery, where nanoparticles can be used as carriers to deliver drugs to specific cells or tissues in the body. Nanoparticles can be engineered to have specific surface properties that allow them to selectively target diseased cells while avoiding healthy ones, which can increase efficacy and reduce the side effects of drugs [6]. Additionally, nanoparticles can be designed to release their cargo in a controlled manner, allowing for sustained drug delivery over time [7]. Nanoparticles can also be used for diagnostic purposes, such as contrast agents in medical imaging, or the detection of specific biomolecules in biological samples [8]. In regenerative medicine, nanomaterials can be used as scaffolds for tissue engineering or as carriers for growth factors and other signaling molecules that promote tissue repair and regeneration. While the field of nanomedicine is still in its early stages, these and other potential applications hold great promise for improving the diagnosis and treatment of a wide range of medical conditions.
2. Industrial Application of Nanotechnology
2.1. Food Industry
With the increasing awareness and demand for healthy food products, research has been devoted to devising tools for improving food shelf life and nutrient absorption. Nanotechnology as an enabling technology has been widely employed in achieving these fits in recent years for food preservation and delivery of nutraceuticals [6][7]. Nanoparticles are added to packaging materials to act as barrier molecules or as antibacterial agents and have displayed great promise [7]. One of the more widely utilised nanoparticle additives for this purpose is that of silver nanoparticle (AgNP) primarily due to silver’s innate antibacterial properties. AgNP can be added to food products in form of an edible biodegradable casing for food products, such as fruits, meat, and poultry, or included as an active ingredient in the polymeric matrix of the packaging material [8]. In fact, some studies have investigated the preservative effect of AgNP-containing packaging on asparagus [9], poultry meat [10], orange juice [11], and strawberries [12], all of which improved shelf life by inhibiting the activities of pathogens such as E. coli, S. aureus, moulds, and yeasts. In addition to AgNP, Zinc oxide (ZnO2) and titanium dioxide (TiO2) are effective against a wide variety of food pathogens such as S. aureus, Salmonella typhi, and Klebsiella pneumoniae [13]. Their used in the preservation of food items such as orange juice, strawberries, and liquid egg albumen, as documented [7]. In addition, TiO2 and ZnO2 have both been used as food additives for their whitening and UV-protective properties, respectively [13].
Nano-encapsulation is a well-established technique used in retaining and enhancing the release of functional nutrients and flavour in food items. Typically, these encapsulations are carbohydrate-based delivery systems made from starch, cellulose, chitosan, and dextrin that have been modified [14]. For example, phosphatidylcholine-based liposomes have been employed in the delivery of vitamin C, and this encapsulation is found to be more effective at maintaining the bioavailability of the nutrient likely through controlled release of the content when compared with free supplements administered orally [15]. Chitosan nanoparticles, in particular, have been shown to improve the stability and bioavailability of bioactive compounds in foods, such as curcumin and resveratrol [16]. Polymer-based nanoparticles, such as chitosan and poly-(lactic-co-glycolic acid) (PLGA), have been investigated for their ability to encapsulate and deliver bioactive compounds, such as antioxidants and vitamins, in food products [17].
2.2. Cosmetic Industry
There is considerable usage of nanotechnology in the cosmetic industry with cosmetic manufacturers now including nanomaterials in their products for a variety of reasons. In the lucrative sunscreen industry, nanoparticles of zinc oxide and titanium dioxide are routinely added to sunscreen by virtue of their sizes, and they act as efficient filters of UV radiation without serious health hazards [18] or unsightly “white streaking” when the cream is applied due to the reduction in particle size. Liposomes prepared from varying lipid formulations of synthetic or natural lipids are also widely used in cosmetics such as ethosomes and transferosomes that are used to improve transdermal delivery of active cosmetic ingredients. The primary justification for the inclusion of liposomes in cosmetics is to enhance the transdermal delivery of cosmetic ingredients based on the ability of the liposomal lipid bilayer to fuse with cell membranes and alter the membrane fluidity for easy entry and delivery of liposomal content [19]. In addition, AgNPs are important ingredients in many cosmetic products as effective antibacterial agents such as in bathing products as active antibacterial ingredients, and because of AgNP activity against different yeast strains, they are also present in different dental products such as mouthwash and toothpaste [20][21].
2.3. Nanomedicine
Nanotechnology was first conceptualised in medicine by Dr. Richard P. Feynman in the late 1950s, while describing the creation of molecular machines with atomic precision that can be used in engineering and medicine. He described the use of molecular mechanical machines that are capable of carrying out surgery or those that can permanently reside in the body for functional assistance of damaged organs [22]. Nanotechnology has strongly influenced the field of medicine, influencing how diseases are treated, particularly with the use of advanced drug delivery systems from both natural and synthetic compounds.
Many nanoparticles are thought to have improved pharmacokinetic properties due to their physical nature and reduced size; they can target specific cells for selective action dependent on the particle type. These particles can easily penetrate target cells and accumulate into subcellular structures to modify cellular processes, which may be beneficial in the treatment of lifelong diseases such as diabetes, cancer, and kidney diseases [23]. As such, many of these nanoparticles have already been approved by the Federal Drug Administration in the United States for clinical use. Nanoparticles that are popularly used in research for therapeutic purposes include encapsulated mRNA (siRNA) or DNA (in gene therapy), inorganic metal and metal complexes, or chemotherapeutic agents with pharmacologic abilities [24][25].
Iron oxide and silica-based nanoparticles have been used to develop multifunctional imaging platforms such as MRI/optical dual-modal imaging, which possess several advantages over existing positron emission tomography (PET) and computed tomography (CT), both of which have radiation-related concerns [26][27][28]. Iron oxide is a magneto-responsive metal that is also biocompatible due to its degradable nature within biological systems. This in addition to its optical properties makes it a good imaging material for MRI. Iron oxide nanoparticles have been widely used as contrast agents for MRI. They are superparamagnetic in nature and can enhance the contrast in MRI images by altering the magnetic relaxation times of tissues [29]. This property has been used in clinical imaging for various applications, including cancer detection, inflammation imaging, and atherosclerosis imaging. Similarly, Silica-based nanoparticles have also been used as X-ray contrast agents for CT imaging [30].
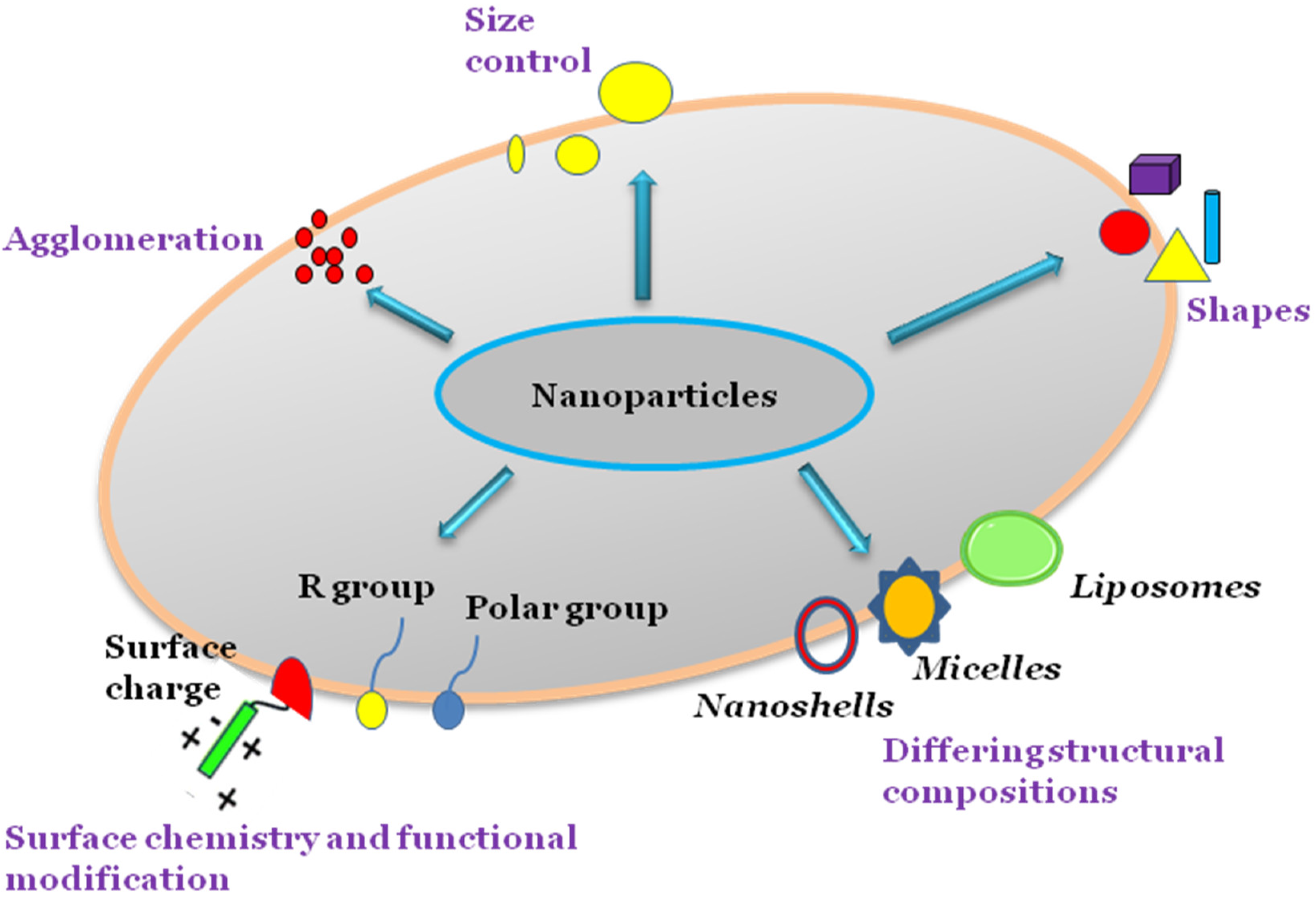
3. Physiochemical Properties of Nanoparticles in Medicine
Nanoparticles have various properties that facilitate enhanced pharmacologic behaviour when compared with larger molecules. As such, significant efforts are being made in research modifying the nanoparticle size, shape, surface area, and surface chemistry to maximise their benefits for medical purposes.
Different nanoparticles such as gold nanoshells, liposomes, and micelles are synthesised in various ways, and the sizes and shapes of these nanoparticles can be controlled during the synthesis process based on the intended functionality. Nanoparticles can agglomerate into larger-sized particles during synthesis, which may enhance or indeed suppress the nanoparticle cytotoxicity depending on composition. The surface chemistry of nanoparticles can be modified by adding reactive groups or molecules such as antibodies to surfaces in targeted drug delivery systems (Figure 1).

Figure 1. Physicochemical properties of nanoparticles.
3.1. Size and Surface Area
As stated, nanoparticles are small particles with sizes ranging between 1 nm and 100 nm, giving them a high surface area to volume ratio. By virtue of this property, nanoparticles have a high surface area of interaction per mass unit compared with more bulky particles, making some particles that are otherwise inert such as gold, to be reactive in the nanometer range [31]. A nanoparticle’s small size that is controllable also allows them to easily infiltrate body tissues and fluids, which are otherwise hindered when in the bulk form. In essence, the size and surface area of these particles contribute to the rate at which these nanoparticles are endocytosed, distributed, retained, and eliminated within biological systems [32]. As nanoparticles do not simply diffuse through the cell membrane, the extensive research into nanoparticles movements into normal and cancer cell lines has shown that they are internalised by endocytotic means in a size-dependent fashion [33][34]. Nanoparticles < 200 nm are known to be internalised by clathrin-coated vesicles, while larger nanoparticles, usually 500 nm, are known to be internalised by caveolae-mediated endocytosis [35]. In immune cells such as macrophages however, nanoparticles are prone to phagocytosis, and indeed research has shown that nanoparticles less than 500 nm in size enter immune cells through the phagocytotic pathway, while particles with larger particle sizes of between 2 and 3 µm, approximately around the size of bacteria cells, exhibit maximal phagocytotic uptake. Smaller nanoparticles such as liposomes can now be engineered for maximal uptake by mammalian cells based on their size [36].

3.2. Surface Chemistry
The surface chemistry of nanoparticles such as charge or attached chemical groups is an important factor that determines their reactivity and ultimately can control their function. Many nanoparticles have been modified to change their surface chemistry to suit specific purposes. Rod-shaped gold nanoparticles (AuNPs) and DNA, because of their charge, cannot easily permeate or enter the cell. Both the AuNP and DNA have had their surfaces modified by coating them with lipid layers, while DNA has also been electrostatically conjugated to cationic liposomes to facilitate their transport into the cell, which resulted in improved uptake [36][37][38][39]. As liposomes and micelles have lipid layers that can interact and fuse with the cell membrane through hydrophobic interactions resulting in improved uptake, they can be used to deliver higher concentrations of nanoparticles intracellularly. Silicon nanoparticles (SiNPs) are important semiconductors that are used in optoelectronics, but their hydrophobicity hinders their application in biomedicine such as applications of internal imaging of tissues, since the biological system is aqueous and SiNPs are not stable in aqueous environments.
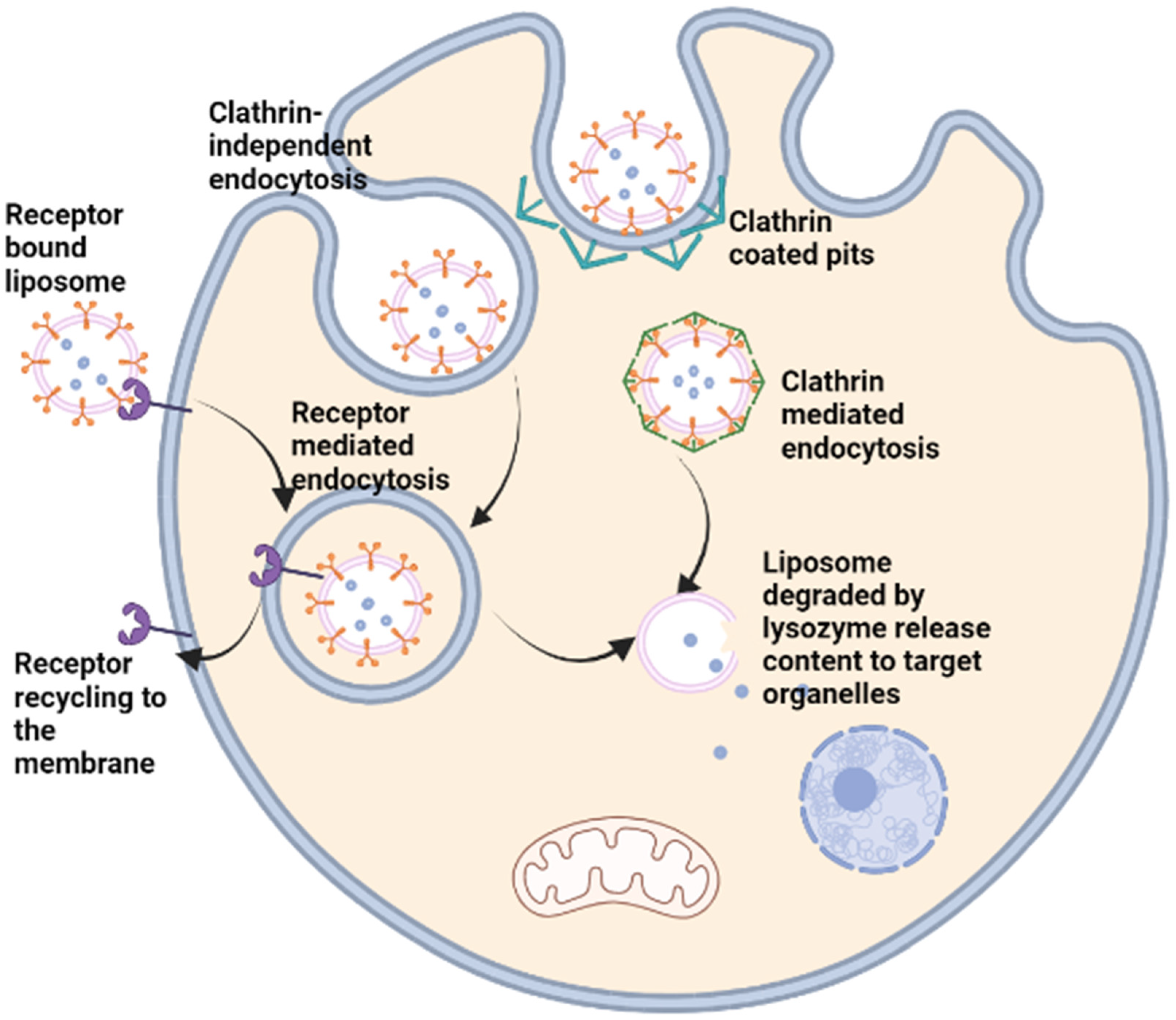
Liposomes are made up of phospholipids that mimic the lipid bilayer of the plasma membrane. The phospholipids component of the liposomes is amphiphilic with a polar head and a hydrophobic tail (Figure 2). The polar head is comprised of a phosphate group and glycerol both containing oxygen that can form hydrogen bonds in an aqueous environment. The hydrophobic tail on the other hand is made up of long-chain fatty acid, which aligns with the hydrophobic tail of another adjacent phospholipid, creating a hydrophobic core that can hold non-polar hydrophobic drugs in the bilayer so formed. The compatibility of liposomal surface chemistry with that of plasma membrane allows the adsorption of the liposome to the cell membrane where the liposome is internalised via receptor-mediated endocytosis or through fusion with the plasma membrane inducing membrane invagination and internalisation [40].

Figure 2. Liposomal modification for drug delivery.
3.3. Shape
As stated previously, nanomaterials have tunable sizes, but their shape is also controllable during their synthesis. The shapes of nanoparticles can be altered during the last synthesis stage and typically involves nucleation of the nanoparticles from seed. The nucleation process involves the fusion of nanoparticle nuclei known as the seeds forming a template on which the nanoparticle crystals grow. Just like the size, the shape of a nanoparticle is paramount to its biological function and reactivity. Generally, nanoparticles that are round or spherical in shape are easily endocytosed in comparison to rod or tube-shaped nanoparticles [41]. This is because the shape affects endocytosis, which interferes with the way the membrane wraps over the nano-construct during contact. As such, the reduced endocytosis of nano-rods or other shapes is most likely due to the inability of the cell to initiate the necessary actin-dependent membrane kinetics required for endocytosis. This reason may explain why most nanoparticles with pharmacologic properties are spherical in nature. On the contrary, there are reports from new studies on nanoparticles of different shapes with potential applications in drug delivery.
4. Nanoparticle Cytotoxicity
With the advent of nanotechnology and its growing application in almost all facets of everyday living, comes the concern on possible hazards resulting from increased human exposure. Significant research into the toxic effect or toxicity of nanoparticle exposure gave rise to the field of nanotoxicology. In recent years, this field has identified that the properties of nanoparticles that confer them with suitable pharmacologic behaviour are also responsible for their toxicity [42].
Several studies have investigated the toxicity of different nanoparticles using different cell lines and experimental conditions. For instance, toxicity of carbon nanotubes has been shown to affect the diversity of soil bacteria, [43], inhibit the growth of Daphnia magna, Chlorella vulgaris, and Oryzias latipes [44], and result in oxidative stress, membrane damage and inflammation in human A549 lung carcinoma cell line [45]. Different findings have shown that the mechanism of nanoparticle size-dependent cytotoxicity is due to their ability to infiltrate body tissues and subsequently enter cells to modify crucial cellular functions, one of which is to rupture the membrane of subcellular structures and induce the overproduction of reactive oxygen species (ROS) [46]. The presence of elevated levels of ROS induces oxidative stress that affects the normal physiological processes of the cell subsequently resulting in DNA damage, dysregulation of cell signaling, and ultimately cell death.
5. Nanoparticle Drug Delivery Systems (DSSs) in Disease Treatment
Nanoparticles used in drug delivery range from 10 to 1000 nm in size with at least one dimension being below 100 nm in size. The small sizes of nanoparticles as well as their surface chemistry are known to offer pharmaceutically beneficial attributes but may also contribute to their toxic effects as discussed earlier. Smaller nanoparticles enter cells more effectively when compared with larger molecules, but the administration of nanoparticles with a reduced clearance may result in some of the particles being retained within the body. In the case of a more active or cytotoxic nanoparticle being retained rather than a bulk of the drug being eliminated during the first pass effect, this may result in harmful effects on the targeted site due to unwanted retention. Systemic administration of cytotoxic drugs may cause the drugs to exert their cytotoxicity on tissues during the first pass before they reach the intended tissues. Overall, 70% of globally synthesised drugs have poor aqueous solubility and hence poor pharmacokinetic properties in vivo [47]. As a solution to this, nanoparticle drug delivery systems (DSSs) have been developed to achieve targeted and more efficient delivery of the therapeutic substance, which would prevent damage to surrounding organs from the effect of administered drugs that will otherwise arise if the drugs were in the free form.
5.1. Lipid-Based DSSs
DSSs made from lipids vary in formulation and size and mainly consist of two types: namely micelles and liposomes. Micelles are formed through the self-assembly of a monolayer of lipid molecules in an aqueous environment into a nano-vesicle of between 5 and 50 nm [48]. They are used to successfully transport hydrophobic molecules, trapped in the hydrophobic core, at concentrations above their inherent water solubility. This is possible because the hydrophilic phospholipids are exposed to the aqueous environment while the hydrophobic tails form the core that can interact with the drug.
Unlike micelles, liposomes are bilayer nano-vesicles similar to the cell membrane with sizes ranging from 10 nm to several microns. The hydrophilic phospholipids of the outer layer are exposed to the aqueous environment, while that of the inner layer encloses the aqueous core. Consequently, the hydrophobic tails of the bilayer lie above each other and are often used to trap hydrophobic drugs while the aqueous core is used to entrap hydrophilic drugs [40]. Liposomes have been one of the most useful tools in drug delivery in cancer treatment due to their ability to transport both water-soluble and insoluble drugs [49][50][51]. Conventional drugs, which are often small molecular drugs that have poor selectivity for tumour cells, are not retained within the tumour microenvironment as they diffuse back into the circulation system, causing cytotoxic side effects to normal cells. Liposomes, however, can improve the delivery of such drugs to the tumour microenvironment, which have tight junctions with gaps between 100 nm and 800 nm unlike normal epithelial junctions, which are 5 nm to 10 nm, via an enhanced permeability and retention (EPR) effect. Using the EPR effect, liposomes accumulate at the tight junctions of tumour cells and extravasate the blood vessels to the tumour microenvironment for delivery of the encapsulated drugs [52].
Liposomes generally have short half-life, but advancements in drug delivery research such as PEGylation of liposomes has allowed the development of liposomes with increased half-life [53]. In forming DSPE-PEG for instance, PEG is conjugated to phosphatidyl ethanolamine of DSPE via covalent linking of the amide group of DSPE to the carboxyl end of PEG [54]. Through the PEG linker on the liposome surface, several other moieties can be conjugated to the liposome as in targeted drug delivery. For example, click chemistry can be used to couple an azide-functionalized antibody to a Dibenzocyclooctyne-amine (DBCO)-PEG functionalized liposome in an azide–alkyne cycloaddition reaction [55].
Liposomes can be used to transport hydrophobic drugs in the lipid bilayer via hydrophobic interactions with the fatty acid tail of the phospholipids, while hydrophilic molecules, such as DNA or crystalline drugs, can be encapsulated within the aqueous core. Surface modifications are now possible on the surface of the liposomes allowing enhanced bioavailability, as occurred with PEG. Surface coating of drugs via electrostatic or ionic interactions or conjugation of antibodies, chemotherapeutic agents, peptides, and other proteins can prove useful for targeted delivery are routinely done with the aid of different linkers such as avidin-biotin complexes, PEG, or peptide linkers that are chemically conjugated to the phospholipid head and to the drug or protein of choice (Figure 2 and Figure 3).

Figure 3. Targeted delivery and metabolism of liposome encapsulated drug.
The pH of the environment where the particles are delivered can also affect the function of the nanoparticle-based on its surface chemistry, and this phenomenon has been utilised to trigger drug release in the tumour microenvironment that is characterised by acidic pH. For example, carrageenan oligosaccharide-capped AuNP have been recently shown to significantly release epirubicin in an acidic pH inducing cell death in HCT-116 colorectal cancer cells [56]. The surface of nanoparticles can alter their movement within aqueous biological systems and subsequently affect their reactivity or delivery. Such surface properties facilitate their use in a variety of ways such as in biomedical sensors, coatings of medical implants, and drug delivery systems.
5.2. Polymeric DSSs
Polymer-based nanoparticle DSSs are made up of a repeating unit of specific polymers and have been widely investigated for medical purposes in recent years [57][58]. Some of the known polymeric DSSs are PEG, chitosan, poly-(lactic-co-glycolic acid) (PLGA), and polylactic acid (PLA), but PEG, PLGA and PLA are the more widely studied, while chitosan research is beginning to gain more attention due to its biocompatibility, low immunogenicity and low toxicity [59]. Several PEGylated drugs have been approved by FDA for clinical use, making it the most commercialised polymeric DSSs. PLGA and PLA are, however, known to be characterised by an initial burst release of the encapsulated drug (within 24 h) irrespective of the drug localization, and this may result in high delivery of drugs at unwanted sites, reducing drug benefits [60]. This has led to the development of polymeric DSSs with different triggers for the release of entrapped drugs.
5.3. Peptide Nanoparticle DSSs
Linear and cyclic peptides that are either synthesised or derived from existing fragments of naturally occurring proteins are also important contributors to the nanoparticle DSSs that are currently available. Peptides are often used as the targets for cell surface receptors since most proteins that bind to such receptors do so via a specific fragment in their peptide sequence. These coupled with their ease of synthesis and low immunogenicity makes peptides a useful tool as potential DSSs. Several peptides have been used alone or indeed as part of a surface modification to other nanoparticles for improved drug delivery.
5.4. Inorganic Nanoparticle-Based DSSs
Inorganic nanoparticles have been widely studied for their potential use in drug delivery systems due to their unique properties, such as small size, biocompatibility, and stability. Inorganic nanoparticles, such as dendrimers, and inorganic nanocarriers such as silica, magnetic, and gold nanocarriers, can be used to encapsulate and deliver drugs to specific target sites in the body.
Dendrimers are branched, nanoscale polymers that have attracted significant attention as DSSs due to their unique properties, such as small size, high surface-to-volume ratio, and tunable surface functionality [61]. One of the main advantages of dendrimers as DSSs is their ability to encapsulate a large amount of drug in their interior or on their surface. This allows for controlled and sustained release of the drug, improving its therapeutic efficacy and reducing its side effects. Dendrimers can also be functionalized with targeting moieties, such as antibodies or peptides, which can improve their specificity for a specific target site in the body, such as a tumour cell [62]. Another advantage of dendrimers is their biocompatibility, which is due to their nontoxic and biodegradable nature. This makes them a promising platform for the delivery of a wide range of drugs, including small molecules, proteins, and nucleic acids.
5.5. Nanoparticle Delivery Systems and Suppression of Drug-Associated Toxicity
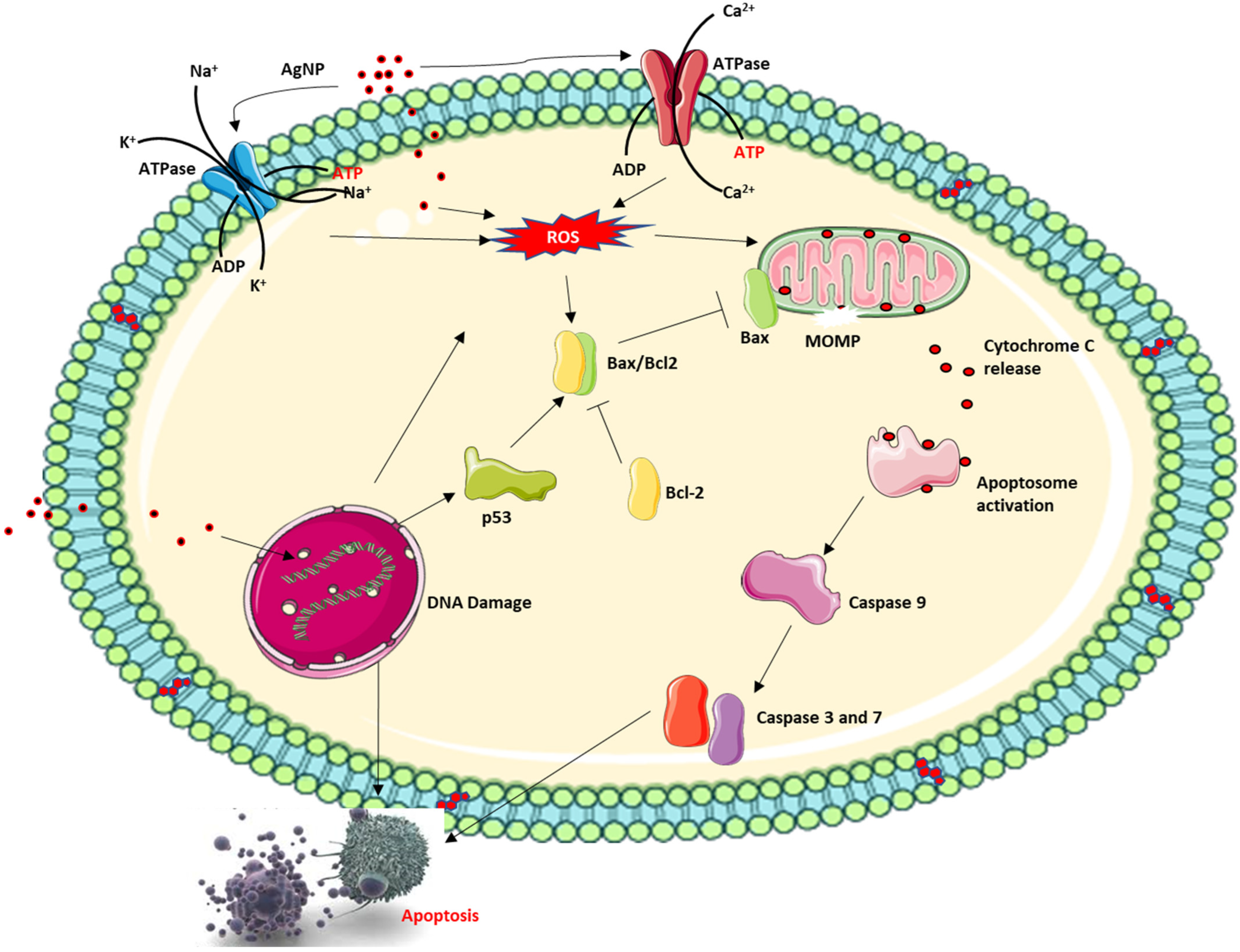
Nanoparticles extensively researched for their medical applications include AgNPs, AuNPs, silicon/silicon oxides and iron oxide nanoparticles. Of these, AgNPs have been extensively researched for medical applications and in fact, AgNP is the most commercialised nanoparticle at present as an active ingredient in an everyday consumable product driven by nanotechnology [63], especially in high concentration. These diverse applications of AgNPs stems from its antibacterial activities and indeed several mechanisms of action have been proposed for their cytotoxic effect. In addition to this, recent investigations have now shifted to investigating the anticancer properties of AgNPs with interesting results. AgNPs have been shown to interact with the DNA inducing DNA damage. AgNPs can also induce ROS which further causes DNA single and double-strand breaks in addition to DNA adducts due to the oxidation of certain nucleotides like guanine to 8-oxo-2-deoxyguanosine, which can base pair with deoxyadenosine resulting in mutation [64][65][66] (Figure 4). This, in addition to the permeabilisation of the mitochondria membrane, can lead to the activation of caspase-dependent cell death. However, the overall effect of both anticancer and antibacterial applications of AgNP possesses increased toxicity risk due to increased and repeated human exposure to the free silver ion (Ag+) released into the local environment by the nanoparticle. Ag+ released from AgNPs has been documented to cause several side effects such as skin irritation and discolouration, hepatotoxicity, kidney damage, DNA damage, and epithelia cell damage [67].

Figure 4. Proposed mechanism of action of AgNPs.
AgNPs have a dose and size-dependent effect on cellular cytotoxicity, which influence the dynamic changes within the cell. AgNPs can induce apoptosis via the caspase-dependent mitochondrial cell death pathway facilitating cellular dynamics that can damage the cell barrier, inactivate ATPase activity to cause inactivation of Ca2+ ATPase and Na+/K+ ATPase. This, in addition to single and double-strand breaks that is caused by AgNP-induced DNA damage, can excessively generate and accumulate ROS causing the permeabilisation of the mitochondrial membrane and release of cytochrome C and pro-apoptotic protein into the cytoplasm followed by activation of the caspase cascade, and finally apoptosis.
References
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. An introduction to nanotechnology. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 1–27.
- Doran, J.; Ryan, G. Does nanotechnology research generate an innovation premium over other types of research? Evidence from Ireland. Technol. Soc. 2019, 59, 101183.
- Cheng, Y.J.; Wolkenhauer, M.; Bumbu, G.G.; Gutmann, J.S. A Facile Route to Reassemble Titania Nanoparticles into Ordered Chain-like Networks on Substrate. Macromol. Rapid Commun. 2012, 33, 218–224.
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261.
- Roco, M.C. Overview: Affirmation of Nanotechnology between 2000 and 2030. In Nanotechnology Commercialization: Manufacturing Processes and Products; Wiley: Hoboken, NJ, USA, 2017; pp. 1–23.
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010, 75, R50–R57.
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.-K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018, 26, 1201–1214.
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279.
- An, J.; Zhang, M.; Wang, S.; Tang, J. Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. LWT Food Sci. Technol. 2008, 41, 1100–1107.
- Banach, M.; Tymczyna, L.; Chmielowiec-Korzeniowska, A.; Pulit-Prociak, J. Nanosilver biocidal properties and their application in disinfection of hatchers in poultry processing plants. Bioinorg. Chem. Appl. 2016, 2016, 5214783.
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Effect of nanocomposite packaging containing Ag and ZnO on inactivation of Lactobacillus plantarum in orange juice. Food Control 2011, 22, 408–413.
- Zhang, C.; Li, W.; Zhu, B.; Chen, H.; Chi, H.; Li, L.; Qin, Y.; Xue, J. The Quality Evaluation of Postharvest Strawberries Stored in Nano-Ag Packages at Refrigeration Temperature. Polymers 2018, 10, 894.
- Venkatasubbu, G.D.; Baskar, R.; Anusuya, T.; Seshan, C.A.; Chelliah, R. Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloids Surf. B Biointerfaces 2016, 148, 600–606.
- Fathi, M.; Martín, Á.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39.
- Davis, J.L.; Paris, H.L.; Beals, J.W.; Binns, S.E.; Giordano, G.R.; Scalzo, R.L.; Schweder, M.M.; Blair, E.; Bell, C. Liposomal-encapsulated Ascorbic Acid: Influence on Vitamin C Bioavailability and Capacity to Protect Against Ischemia-Reperfusion Injury. Nutr. Metab. Insights 2016, 9, 25–30.
- Hallan, S.S.; Kaur, V.; Jain, V.; Mishra, N. Development and characterization of polymer lipid hybrid nanoparticles for oral delivery of LMWH. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1631–1639.
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A Review on Polymer and Lipid-Based Nanocarriers and Its Application to Nano-Pharmaceutical and Food-Based Systems. Front. Nutr. 2021, 8, 783831.
- Morganti, P. Use and potential of nanotechnology in cosmetic dermatology. Clin. Cosmet. Investig. Dermatol. CCID 2010, 3, 5.
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282.
- Abadi, M.F.D.; Mehrabian, S.; Asghari, B.; Namvar, A.E.; Ezzatifar, F.; Lari, A.R. Silver nanoparticles as active ingredient used for alcohol-free mouthwash. GMS Hyg. Infect. Control 2013, 8, Doc05.
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32.
- Feynman, R.P. There’s plenty of room at the bottom. Eng. Sci. 1960, 23, 22–36.
- Barua, S.; Mitragotri, S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243.
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharm. 2019, 111, 802–812.
- Sharma, A.R.; Lee, Y.H.; Bat-Ulzii, A.; Bhattacharya, M.; Chakraborty, C.; Lee, S.S. Recent advances of metal-based nanoparticles in nucleic acid delivery for therapeutic applications. J. Nanobiotechnol. 2022, 20, 501.
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689.
- Kim, J.S.; Rieter, W.J.; Taylor, K.M.; An, H.; Lin, W. Self-assembled hybrid nanoparticles for cancer-specific multimodal imaging. J. Am. Chem. Soc. 2007, 129, 8962–8963.
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195.
- Nam, J.; Won, N.; Bang, J.; Jin, H.; Park, J.; Jung, S.; Park, Y.; Kim, S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648.
- Shirshahi, V.; Soltani, M. Solid silica nanoparticles: Applications in molecular imaging. Contrast Media Mol. Imaging 2015, 10, 1–17.
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627.
- Powers, K.W.; Palazuelos, M.; Moudgil, B.M.; Roberts, S.M. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology 2007, 1, 42–51.
- Tsai, C.Y.; Lu, S.L.; Hu, C.W.; Yeh, C.S.; Lee, G.B.; Lei, H.Y. Size-dependent attenuation of TLR9 signaling by gold nanoparticles in macrophages. J. Immunol. 2012, 188, 68–76.
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150.
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169.
- Chithrani, D.B.; Dunne, M.; Stewart, J.; Allen, C.; Jaffray, D.A. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomedicine 2010, 6, 161–169.
- Fillion, P.; Desjardins, A.; Sayasith, K.; Lagace, J. Encapsulation of DNA in negatively charged liposomes and inhibition of bacterial gene expression with fluid liposome-encapsulated antisense oligonucleotides. Biochim. Biophys. Acta 2001, 1515, 44–54.
- Dichello, G.A.; Fukuda, T.; Maekawa, T.; Whitby, R.L.D.; Mikhalovsky, S.V.; Alavijeh, M.; Pannala, A.S.; Sarker, D.K. Preparation of liposomes containing small gold nanoparticles using electrostatic interactions. Eur. J. Pharm. Sci. 2017, 105, 55–63.
- Ewert, K.K.; Kotamraju, V.R.; Majzoub, R.N.; Steffes, V.M.; Wonder, E.A.; Teesalu, T.; Ruoslahti, E.; Safinya, C.R. Synthesis of linear and cyclic peptide-PEG-lipids for stabilization and targeting of cationic liposome-DNA complexes. Bioorg. Med. Chem. Lett. 2016, 26, 1618–1623.
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975.
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934.
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Dar, A.M.; Qasim, K.; Zubair, S. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. BioMed Res. Int. 2014, 2014, 498420.
- Kerfahi, D.; Tripathi, B.M.; Singh, D.; Kim, H.; Lee, S.; Lee, J.; Adams, J.M. Effects of functionalized and raw multi-walled carbon nanotubes on soil bacterial community composition. PLoS ONE 2015, 10, e0123042.
- Sohn, E.K.; Chung, Y.S.; Johari, S.A.; Kim, T.G.; Kim, J.K.; Lee, J.H.; Lee, Y.H.; Kang, S.W.; Yu, I.J. Acute toxicity comparison of single-walled carbon nanotubes in various freshwater organisms. BioMed Res. Int. 2015, 2015, 323090.
- Choi, S.J.; Oh, J.M.; Choy, J.H. Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. J. Inorg. Biochem. 2009, 103, 463–471.
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75.
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316.
- Wang, J.; Mongayt, D.; Torchilin, V.P. Polymeric micelles for delivery of poorly soluble drugs: Preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly(ethylene glycol)-lipid conjugate and positively charged lipids. J. Drug Target 2005, 13, 73–80.
- Rau, K.-M.; Lin, Y.-C.; Chen, Y.-Y.; Chen, J.-S.; Lee, K.-D.; Wang, C.-H.; Chang, H.-K. Pegylated liposomal doxorubicin (Lipo-Dox®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: An open-label, multi-center, non-comparative phase II study. BMC Cancer 2015, 15, 423.
- Casagrande, N.; Celegato, M.; Borghese, C.; Mongiat, M.; Colombatti, A.; Aldinucci, D. Preclinical activity of the liposomal cisplatin lipoplatin in ovarian cancer. Clin. Cancer Res. 2014, 20, 5496–5506.
- Guo, S.; Huang, L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol. Adv. 2014, 32, 778–788.
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528.
- Milla, P.; Dosio, F.; Cattel, L. PEGylation of proteins and liposomes: A powerful and flexible strategy to improve the drug delivery. Curr. Drug Metab. 2012, 13, 105–119.
- Marques-Gallego, P.; de Kroon, A.I. Ligation strategies for targeting liposomal nanocarriers. BioMed Res. Int. 2014, 2014, 129458.
- Gai, M.; Simon, J.; Lieberwirth, I.; Mailänder, V.; Morsbach, S.; Landfester, K. A bio-orthogonal functionalization strategy for site-specific coupling of antibodies on vesicle surfaces after self-assembly. Polym. Chem. 2020, 11, 527–540.
- Chen, X.; Han, W.; Zhao, X.; Tang, W.; Wang, F. Epirubicin-loaded marine carrageenan oligosaccharide capped gold nanoparticle system for pH-triggered anticancer drug release. Sci. Rep. 2019, 9, 6754.
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235.
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710.
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186.
- Bouissou, C.; Rouse, J.J.; Price, R.; van der Walle, C.F. The influence of surfactant on PLGA microsphere glass transition and water sorption: Remodeling the surface morphology to attenuate the burst release. Pharm. Res. 2006, 23, 1295–1305.
- Wang, T.; Rong, F.; Tang, Y.; Li, M.; Feng, T.; Zhou, Q.; Li, P.; Huang, W. Targeted polymer-based antibiotic delivery system: A promising option for treating bacterial infections via macromolecular approaches. Prog. Polym. Sci. 2021, 116, 101389.
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138.
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780.
- Liu, C.; Miyajima, T.; Melangath, G.; Miyai, T.; Vasanth, S.; Deshpande, N.; Kumar, V.; Ong Tone, S.; Gupta, R.; Zhu, S.; et al. Ultraviolet A light induces DNA damage and estrogen-DNA adducts in Fuchs endothelial corneal dystrophy causing females to be more affected. Proc. Natl. Acad. Sci. USA 2020, 117, 573–583.
- Tang, J.; Lu, X.; Chen, B.; Cai, E.; Liu, W.; Jiang, J.; Chen, F.; Shan, X.; Zhang, H. Mechanisms of silver nanoparticles-induced cytotoxicity and apoptosis in rat tracheal epithelial cells. J. Toxicol. Sci. 2019, 44, 155–165.
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 13902.
- León-Silva, S.; Fernández-Luqueño, F.; López-Valdez, F. Silver Nanoparticles (AgNP) in the environment: A review of potential risks on human and environmental health. Water Air Soil Pollut. 2016, 227, 306.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.5K
Revisions:
3 times
(View History)
Update Date:
19 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





