
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ludovico Carbone | -- | 2156 | 2023-06-14 18:24:44 | | | |
| 2 | Jessie Wu | + 24 word(s) | 2180 | 2023-06-15 05:03:11 | | |
Video Upload Options
Despite its decreasing incidence, gastric cancer remains an important global healthcare problem due to its overall high prevalence and high mortality rate. Since the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) and Fédération Nationale des Centres de Lutte contre le Cancer (FNLCC)/ Fédération Francophone de Cancérologie Digestive (FFCD) trials, the neoadjuvant chemotherapy has been recommended throughout Europe in gastric cancer. Potential benefits of preoperative treatments include a higher rate of R0 resection achieved by downstaging the primary tumor, a likely effect on micrometastases and isolated tumor cells in the lymph nodes, and, as a result, improved cancer-related survival. Nevertheless, distortion of anatomical planes of dissection, interstitial fibrosis, and sclerotic tissue changes may increase surgical difficulty. The collection of at least twenty-five lymph nodes after neoadjuvant therapy would seem to ensure removal of undetectable node metastasis and reduce the likelihood of locoregional recurrence.
1. D2 Lymphadenectomy
1.1. Adequate Disease Staging Due to the Increased Number of Nodes Retrieved
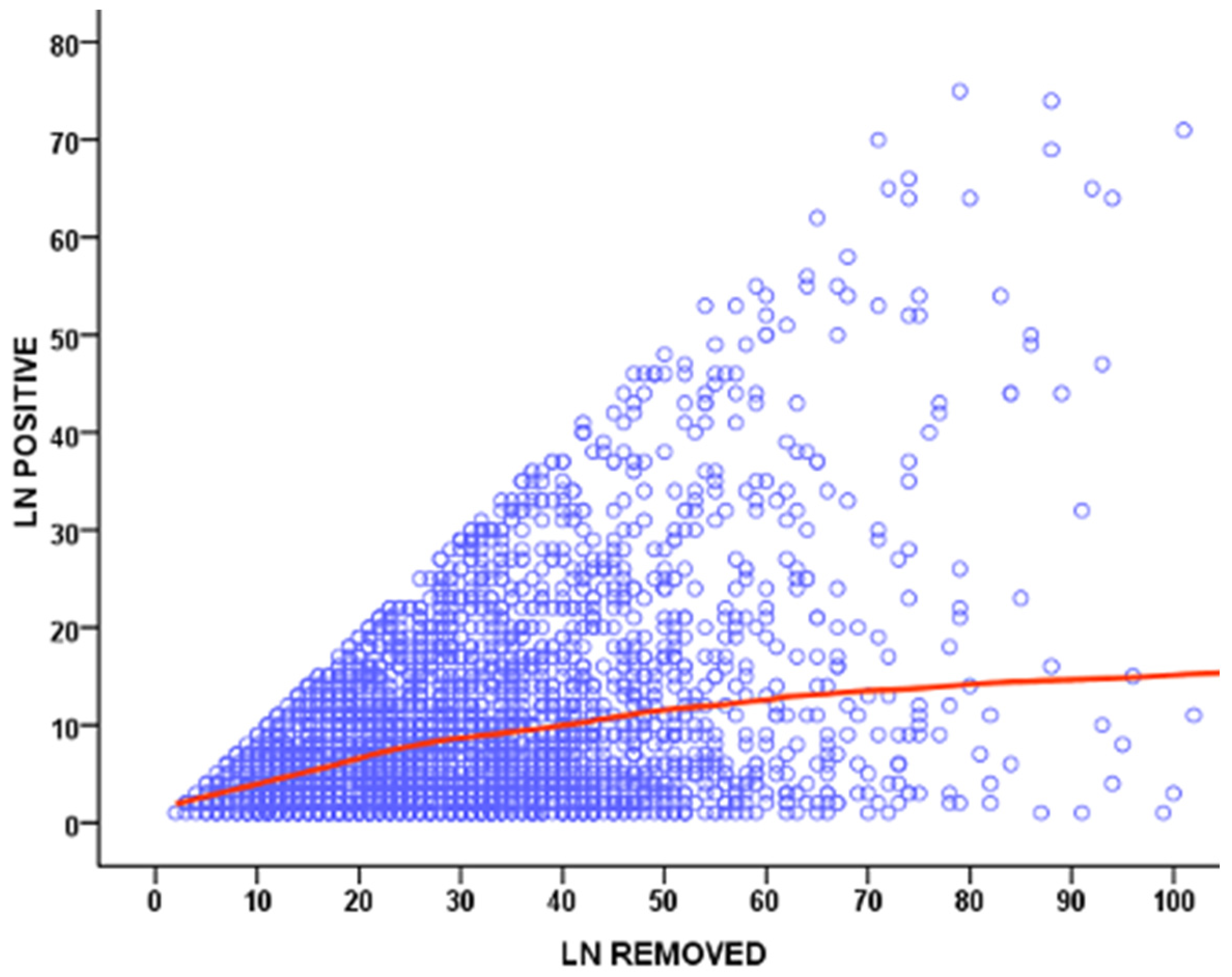
1.2. Removal of Potentially Metastatic Lymph Nodes, Resulting in Increased Surgical Radicality
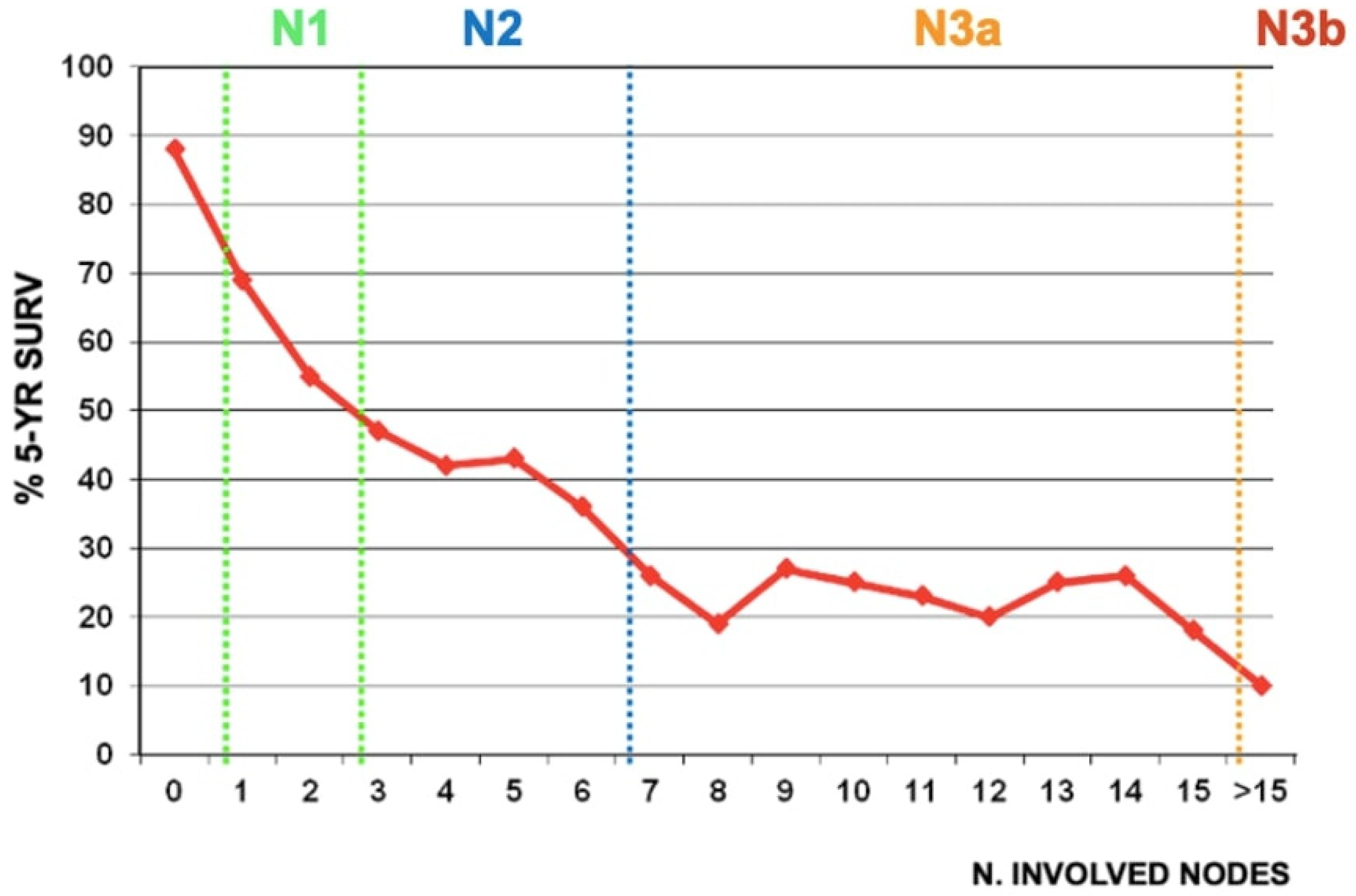
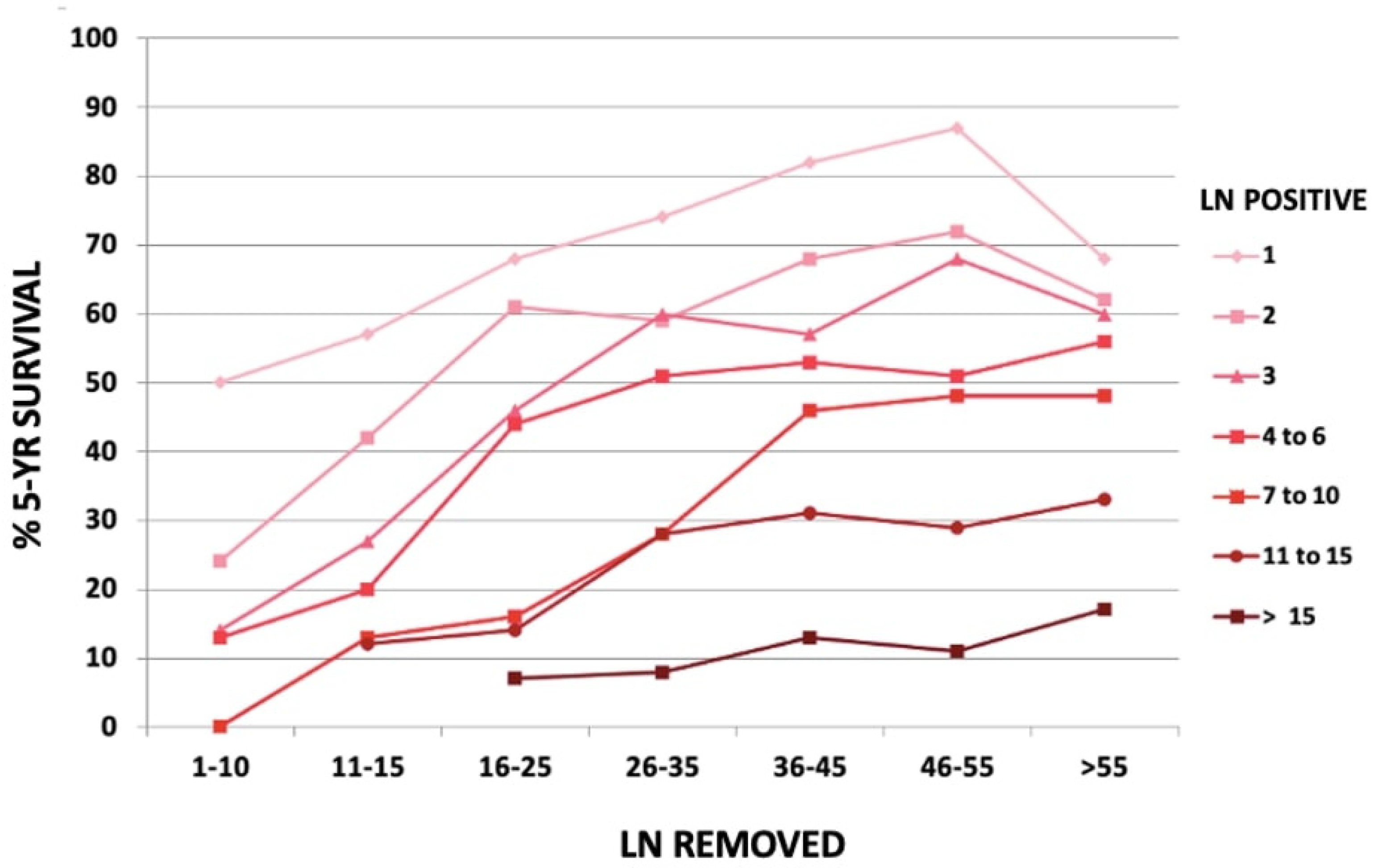
1.3. Decrease in Gastric Cancer Recurrence, Especially Locoregional
1.4. Prognostic Benefit by Potential Improvement in Long-Term Survival
-
D2 lymphadenectomy is the standard surgical treatment with curative intent for advanced gastric cancer.
-
Adequate D2 enables accurate disease staging, reduces the incidence of locoregional recurrences and contributes to an improved long-term survival.
-
Undetectable node metastases are associated with high rates of locoregional recurrence.
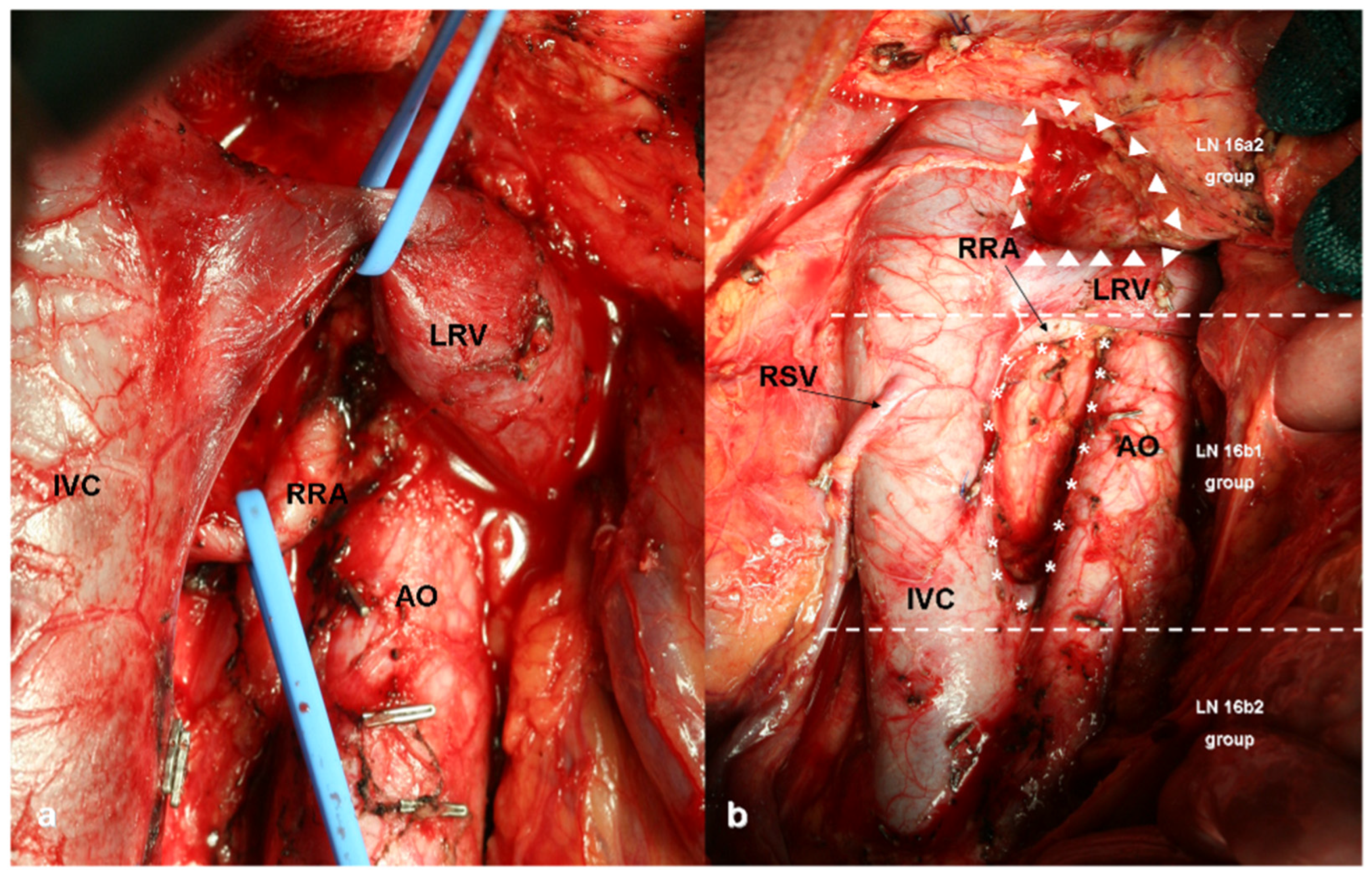
2. D2 plus and D3 Lymphadenectomies
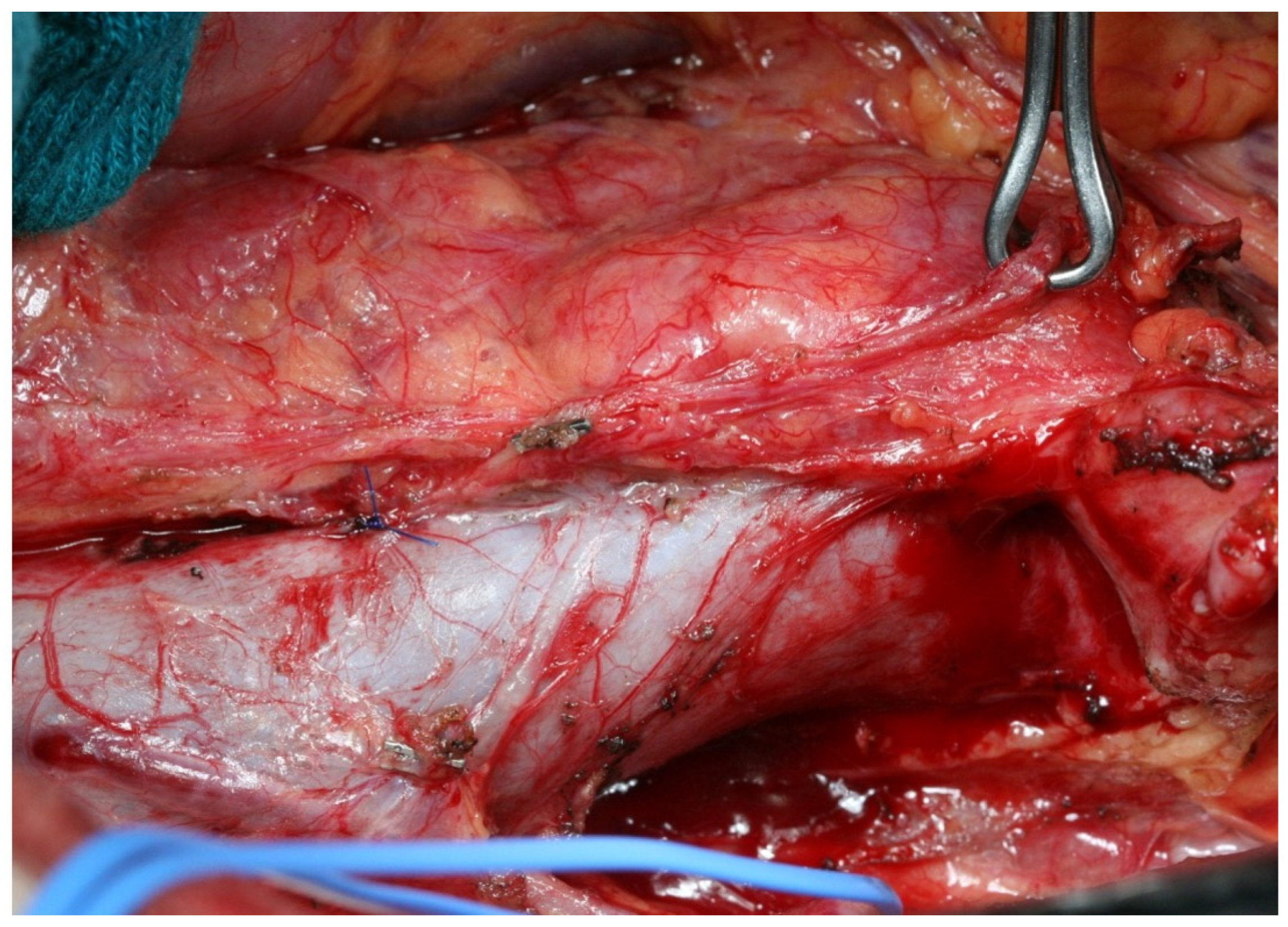
References
- Deng, J.-Y. Clinical Significance of Lymph Node Metastasis in Gastric Cancer. World J. Gastroenterol. 2014, 20, 3967.
- Biondi, A. R0 Resection in the Treatment of Gastric Cancer: Room for Improvement. World J. Gastroenterol. 2010, 16, 3358.
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2018 (5th Edition). Gastric Cancer 2021, 24, 1–21.
- de Manzoni, G.; Marrelli, D.; Baiocchi, G.L.; Morgagni, P.; Saragoni, L.; Degiuli, M.; Donini, A.; Fumagalli, U.; Mazzei, M.A.; Pacelli, F.; et al. The Italian Research Group for Gastric Cancer (GIRCG) Guidelines for Gastric Cancer Staging and Treatment: 2015. Gastric Cancer 2017, 20, 20–30.
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2010 (Ver. 3). Gastric Cancer 2011, 14, 113–123.
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2014 (Ver. 4). Gastric Cancer 2017, 20, 1–19.
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192.
- Wang, F.-H.; Shen, L.; Li, J.; Zhou, Z.-W.; Liang, H.; Zhang, X.-T.; Tang, L.; Xin, Y.; Jin, J.; Zhang, Y.-J.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical Guidelines for the Diagnosis and Treatment of Gastric Cancer. Cancer Commun. 2019, 39, 10.
- Marrelli, D.; de Franco, L.; Iudici, L.; Polom, K.; Roviello, F. Lymphadenectomy: State of the Art. Transl. Gastroenterol. Hepatol. 2017, 2, 3.
- Bouvier, A.-M.; Haas, O.; Piard, F.; Roignot, P.; Bonithon-Kopp, C.; Faivre, J. How Many Nodes Must Be Examined to Accurately Stage Gastric Carcinomas? Cancer 2002, 94, 2862–2866.
- Smith, D.D.; Schwarz, R.R.; Schwarz, R.E. Impact of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: Data from a Large US-Population Database. J. Clin. Oncol. 2005, 23, 7114–7124.
- Mogal, H.; Fields, R.; Maithel, S.K.; Votanopoulos, K. In Patients with Localized and Resectable Gastric Cancer, What Is the Optimal Extent of Lymph Node Dissection—D1 Versus D2 Versus D3? Ann. Surg. Oncol. 2019, 26, 2912–2932.
- Cozzaglio, L.; Doci, R.; Celotti, S.; Roncalli, M.; Gennari, L. Gastric Cancer: Extent of Lymph Node Dissection and Requirements for a Correct Staging. Tumori 2004, 90, 467–472.
- Shen, J.Y.; Kim, S.; Cheong, J.-H.; Kim, Y.I.; Hyung, W.J.; Choi, W.H.; Choi, S.H.; Wang, L.B.; Noh, S.H. The Impact of Total Retrieved Lymph Nodes on Staging and Survival of Patients with PT3 Gastric Cancer. Cancer 2007, 110, 745–751.
- Liu, C.; Lu, Y.; Jun, Z.; Zhang, R.; Yao, F.; Lu, P.; Jin, F.; Li, H.; Xu, H.; Wang, S.; et al. Impact of Total Retrieved Lymph Nodes on Staging and Survival of Patients with Gastric Cancer Invading the Subserosa. Surg. Oncol. 2009, 18, 379–384.
- Son, T.; Hyung, W.J.; Lee, J.H.; Kim, Y.M.; Kim, H.-I.; An, J.Y.; Cheong, J.-H.; Noh, S.H. Clinical Implication of an Insufficient Number of Examined Lymph Nodes after Curative Resection for Gastric Cancer. Cancer 2012, 118, 4687–4693.
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 3rd English Edition. Gastric Cancer 2011, 14, 101–112.
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474.
- Coburn, N.; Seevaratnam, R.; Paszat, L.; Helyer, L.; Law, C.; Swallow, C.; Cardosa, R.; Mahar, A.; Lourenco, L.G.; Dixon, M.; et al. Optimal Management of Gastric Cancer. Ann. Surg. 2014, 259, 102–108.
- Waddell, T.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric Cancer: ESMO–ESSO–ESTRO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2013, 24, vi57–vi63.
- Lee, J.H.; Kim, J.G.; Jung, H.-K.; Kim, J.H.; Jeong, W.K.; Jeon, T.J.; Kim, J.M.; Kim, Y.I.; Ryu, K.W.; Kong, S.-H.; et al. Clinical Practice Guidelines for Gastric Cancer in Korea: An Evidence-Based Approach. J. Gastric Cancer 2014, 14, 87.
- American College of Surgeons. Cancer Programs Practice Profile Reports (CP3R). Available online: https://www.facs.org/~/media/files/quality%20programs/cancer/ncdb/measure%20specs%20gastric.ashx (accessed on 25 September 2022).
- Allum, W.H.; Blazeby, J.M.; Griffin, S.M.; Cunningham, D.; Jankowski, J.A.; Wong, R. Guidelines for the Management of Oesophageal and Gastric Cancer. Gut 2011, 60, 1449–1472.
- National Comprehensive Cancer Network. Practice Guidelines in Oncology (NCCN Guidelines), Gastric Cancer, Version 1. 2019. Available online: www.nccn.org (accessed on 25 September 2022).
- Marrelli, D.; Pedrazzani, C.; Morgagni, P.; de Manzoni, G.; Pacelli, F.; Coniglio, A.; Marchet, A.; Saragoni, L.; Giacopuzzi, S.; Roviello, F. Changing Clinical and Pathological Features of Gastric Cancer over Time. Br. J. Surg. 2011, 98, 1273–1283.
- Wohnrath, D.R.; Araujo, R.L.C. D2 Lymphadenectomy for Gastric Cancer as an Independent Prognostic Factor of 10-Year Overall Survival. Eur. J. Surg. Oncol. 2019, 45, 446–453.
- Naffouje, S.A.; Salti, G.I. Extensive Lymph Node Dissection Improves Survival among American Patients with Gastric Adenocarcinoma Treated Surgically: Analysis of the National Cancer Database. J. Gastric Cancer 2017, 17, 319.
- Shen, Z.; Ye, Y.; Xie, Q.; Liang, B.; Jiang, K.; Wang, S. Effect of the Number of Lymph Nodes Harvested on the Long-Term Survival of Gastric Cancer Patients According to Tumor Stage and Location: A 12-Year Study of 1,637 Cases. Am. J. Surg. 2015, 210, 431–440.e3.
- Macalindong, S.S.; Kim, K.H.; Nam, B.-H.; Ryu, K.W.; Kubo, N.; Kim, J.Y.; Eom, B.W.; Yoon, H.M.; Kook, M.-C.; Choi, I.J.; et al. Effect of Total Number of Harvested Lymph Nodes on Survival Outcomes after Curative Resection for Gastric Adenocarcinoma: Findings from an Eastern High-Volume Gastric Cancer Center. BMC Cancer 2018, 18, 73.
- Siewert, J.R.; Kestlmeier, R.; Busch, R.; Böttcher, K.; Roder, J.D.; Müller, J.; Fellbaum, C.; Höfler, H. Benefits of D2 Lymph Node Dissection for Patients with Gastric Cancer and PN0 and PN1 Lymph Node Metastases. Br. J. Surg. 2005, 83, 1144–1147.
- Baiocchi, G.L.; Tiberio, G.A.; Minicozzi, A.M.; Morgagni, P.; Marrelli, D.; Bruno, L.; Rosa, F.; Marchet, A.; Coniglio, A.; Saragoni, L.; et al. A Multicentric Western Analysis of Prognostic Factors in Advanced, Node-Negative Gastric Cancer Patients. Ann. Surg. 2010, 252, 70–73.
- di Leo, A.; Marrelli, D.; Roviello, F.; Bernini, M.; Minicozzi, A.; Giacopuzzi, S.; Pedrazzani, C.; Baiocchi, L.G.; de Manzoni, G. Lymph Node Involvement in Gastric Cancer for Different Tumor Sites and T Stage. J. Gastrointest. Surg. 2007, 11, 1146–1153.
- Zhang, Y.-X.; Yang, K. Significance of Nodal Dissection and Nodal Positivity in Gastric Cancer. Transl. Gastroenterol. Hepatol. 2020, 5, 17.
- Roviello, F.; Pedrazzani, C.; Marrelli, D.; di Leo, A.; Caruso, S.; Giacopuzzi, S.; Corso, G.; de Manzoni, G. Super-Extended (D3) Lymphadenectomy in Advanced Gastric Cancer. Eur. J. Surg. Oncol. EJSO 2010, 36, 439–446.
- Hartgrink, H.H.; van de Velde, C.J.H.; Putter, H.; Bonenkamp, J.J.; Klein Kranenbarg, E.; Songun, I.; Welvaart, K.; van Krieken, J.H.J.M.; Meijer, S.; Plukker, J.T.M.; et al. Extended Lymph Node Dissection for Gastric Cancer: Who May Benefit? Final Results of the Randomized Dutch Gastric Cancer Group Trial. J. Clin. Oncol. 2004, 22, 2069–2077.
- Songun, I.; Putter, H.; Kranenbarg, E.M.-K.; Sasako, M.; van de Velde, C.J. Surgical Treatment of Gastric Cancer: 15-Year Follow-up Results of the Randomised Nationwide Dutch D1D2 Trial. Lancet Oncol. 2010, 11, 439–449.
- Degiuli, M.; Sasako, M.; Ponti, A.; Vendrame, A.; Tomatis, M.; Mazza, C.; Borasi, A.; Capussotti, L.; Fronda, G.; Morino, M.; et al. Randomized Clinical Trial Comparing Survival after D1 or D2 Gastrectomy for Gastric Cancer. Br. J. Surg. 2013, 101, 23–31.
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648.
- Degiuli, M.; Sasako, M.; Ponti, A. Morbidity and Mortality in the Italian Gastric Cancer Study Group Randomized Clinical Trial of D1 versus D2 Resection for Gastric Cancer. Br. J. Surg. 2010, 97, 643–649.
- Degiuli, M.; Reddavid, R.; Tomatis, M.; Ponti, A.; Morino, M.; Sasako, M.; Rebecchi, F.; Garino, M.; Vigano, L.; Scaglione, D.; et al. D2 Dissection Improves Disease-Specific Survival in Advanced Gastric Cancer Patients: 15-Year Follow-up Results of the Italian Gastric Cancer Study Group D1 versus D2 Randomised Controlled Trial. Eur. J. Cancer 2021, 150, 10–22.
- Wu, C.-W.; Hsiung, C.A.; Lo, S.-S.; Hsieh, M.-C.; Chen, J.-H.; Li, A.F.-Y.; Lui, W.-Y.; Whang-Peng, J. Nodal Dissection for Patients with Gastric Cancer: A Randomised Controlled Trial. Lancet Oncol. 2006, 7, 309–315.
- de Manzoni, G.; Roviello, F. Gastric Cancer: The 25-Year R-Evolution; Springer: Berlin/Heidelberg, Germany, 2022; pp. 111–118.
- Asoglu, O.; Matlim, T.; Kurt, A.; Onder, S.Y.; Kunduz, E.; Karanlik, H.; Sam, B.; Kapran, Y.; Bugra, D. Guidelines for Extended Lymphadenectomy in Gastric Cancer: A Prospective Comparative Study. Ann. Surg. Oncol. 2013, 20, 218–225.
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer 1998, 1, 10–24.
- Yu, P.; Du, Y.; Xu, Z.; Huang, L.; Cheng, X. Comparison of D2 and D2 plus Radical Surgery for Advanced Distal Gastric Cancer: A Randomized Controlled Study. World J. Surg. Oncol. 2019, 17, 28.
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247.
- Marrelli, D.; Mazzei, M.A.; Roviello, F. Gastric Cancer with Para-Aortic Lymph Node Metastases: Do Not Miss a Chance of Cure! Cancer Chemother. Pharm. 2014, 74, 433–434.
- Tsuburaya, A.; Mizusawa, J.; Tanaka, Y.; Fukushima, N.; Nashimoto, A.; Sasako, M. Neoadjuvant Chemotherapy with S-1 and Cisplatin Followed by D2 Gastrectomy with Para-Aortic Lymph Node Dissection for Gastric Cancer with Extensive Lymph Node Metastasis. Br. J. Surg. 2014, 101, 653–660.
- Yoshikawa, T.; Sasako, M.; Yamamoto, S.; Sano, T.; Imamura, H.; Fujitani, K.; Oshita, H.; Ito, S.; Kawashima, Y.; Fukushima, N. Phase II Study of Neoadjuvant Chemotherapy and Extended Surgery for Locally Advanced Gastric Cancer. Br. J. Surg. 2009, 96, 1015–1022.
- Xu, W.; Liu, W.; Wang, L.; He, C.; Lu, S.; Ni, Z.; Hua, Z.; Zhu, Z.; Sah, B.K.; Yang, Z.; et al. Is D2 Lymphadenectomy Alone Suitable for Gastric Cancer with Bulky N2 and/or Para-Aortic Lymph Node Metastases After Preoperative Chemotherapy? Front. Oncol. 2021, 11, 709617.
- Sasako, M.; Sano, T.; Yamamoto, S.; Nashimoto, A.; Kurita, A.; Furukawa, H.; Tsujinaka, T.; Kinoshita, T.; Arai, K. Randomized Phase III Trial of Standard D2 versus D2 + Para-Aortic Lymph Node (PAN) Dissection (D) for Clinically M0 Advanced Gastric Cancer: JCOG9501. J. Clin. Oncol. 2006, 24, LBA4015.
- Sasako, M.; Sano, T.; Yamamoto, S.; Kurokawa, Y.; Nashimoto, A.; Kurita, A.; Hiratsuka, M.; Tsujinaka, T.; Kinoshita, T.; Arai, K.; et al. D2 Lymphadenectomy Alone or with Para-Aortic Nodal Dissection for Gastric Cancer. N. Engl. J. Med. 2008, 359, 453–462.
- Endo, S.; Ikenaga, M.; Yamada, T.; Tamura, S.; Sasaki, Y.O. Prognostic Factors for Para-aortic Lymph Node Dissection After Neoadjuvant Chemotherapy for Gastric Cancer. Anticancer Res. 2020, 40, 2351–2357.
- Eom, B.W.; Joo, J.; Kim, Y.-W.; Park, B.; Park, J.Y.; Yoon, H.M.; Lee, J.H.; Ryu, K.W. Is There Any Role of Additional Retropancreatic Lymph Node Dissection on D2 Gastrectomy for Advanced Gastric Cancer? Ann. Surg. Oncol. 2013, 20, 2669–2675.
- Eom, B.W.; Joo, J.; Kim, Y.-W.; Reim, D.; Park, J.Y.; Yoon, H.M.; Ryu, K.W.; Lee, J.Y.; Kook, M.-C. Improved Survival after Adding Dissection of the Superior Mesenteric Vein Lymph Node (14v) to Standard D2 Gastrectomy for Advanced Distal Gastric Cancer. Surgery 2014, 155, 408–416.
- Marrelli, D.; Ferrara, F.; Giacopuzzi, S.; Morgagni, P.; di Leo, A.; de Franco, L.; Pedrazzani, C.; Saragoni, L.; de Manzoni, G.; Roviello, F. Incidence and Prognostic Value of Metastases to “Posterior” and Para-Aortic Lymph Nodes in Resectable Gastric Cancer. Ann. Surg. Oncol. 2017, 24, 2273–2280.
- Bencivenga, M.; Verlato, G.; Mengardo, V.; Scorsone, L.; Sacco, M.; Torroni, L.; Giacopuzzi, S.; de Manzoni, G. Is There Any Role for Super-Extended Limphadenectomy in Advanced Gastric Cancer? Results of an Observational Study from a Western High Volume Center. J. Clin. Med. 2019, 8, 1799.
- de Manzoni, G.; Verlato, G.; Bencivenga, M.; Marrelli, D.; di Leo, A.; Giacopuzzi, S.; Cipollari, C.; Roviello, F. Impact of Super-Extended Lymphadenectomy on Relapse in Advanced Gastric Cancer. Eur. J. Surg. Oncol. EJSO 2015, 41, 534–540.
- Bencivenga, M.; Torroni, L.; Verlato, G.; Mengardo, V.; Sacco, M.; Allum, W.H.; de Manzoni, G. Lymphadenectomy for Gastric Cancer at European Specialist Centres. Eur. J. Surg. Oncol. 2021, 47, 1048–1054.
- Marano, L.; Carbone, L.; Poto, G.E.; Calomino, N.; Neri, A.; Piagnerelli, R.; Fontani, A.; Verre, L.; Savelli, V.; Roviello, F.; et al. Antimicrobial Prophylaxis Reduces the Rate of Surgical Site Infection in Upper Gastrointestinal Surgery: A Systematic Review. Antibiotics 2022, 11, 230.




