
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Malgorzata Kloc | -- | 2948 | 2023-06-14 16:32:20 | | | |
| 2 | Peter Tang | Meta information modification | 2948 | 2023-06-15 04:47:30 | | |
Video Upload Options
Seahorses, together with sea dragons and pipefishes, belong to the Syngnathidae family of teleost fishes. Seahorses and other Syngnathidae species have a very peculiar feature: male pregnancy. Among different species, there is a gradation of paternal involvement in carrying for the offspring, from a simple attachment of the eggs to the skin surface, through various degrees of egg coverage by skin flaps, to the internal pregnancy within a brood pouch, which resembles mammalian uterus with the placenta. Because of the gradation of parental involvement and similarities to mammalian pregnancy, seahorses are a great model to study the evolution of pregnancy and the immunologic, metabolic, cellular, and molecular processes of pregnancy and embryo development. Seahorses are also very useful for studying the effects of pollutants and environmental changes on pregnancy, embryo development, and offspring fitness.
1. What Is a Seahorse, and Why Is It Used as a Model System?
2. Male Pregnancy
2.1. Brood Pouch Development
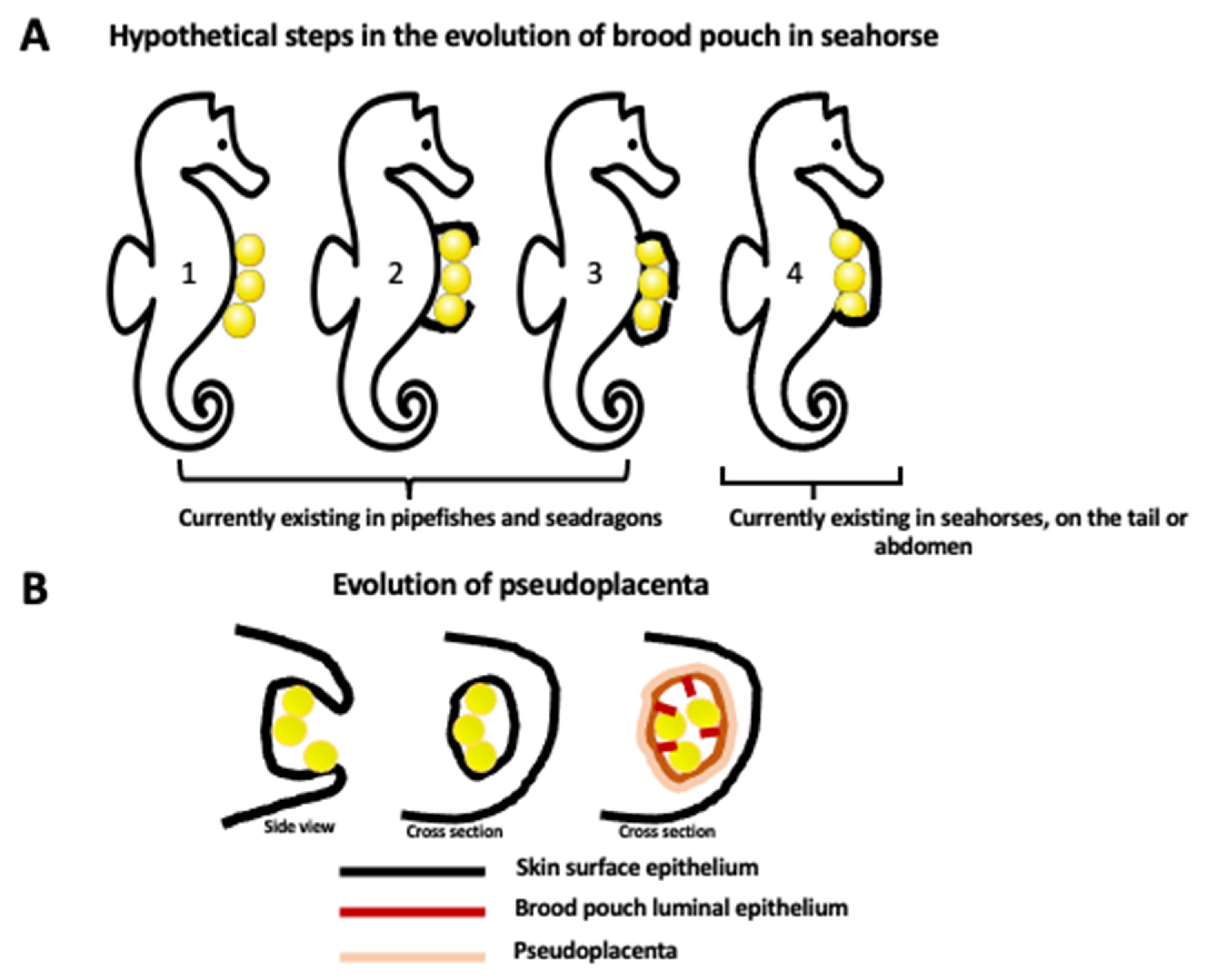
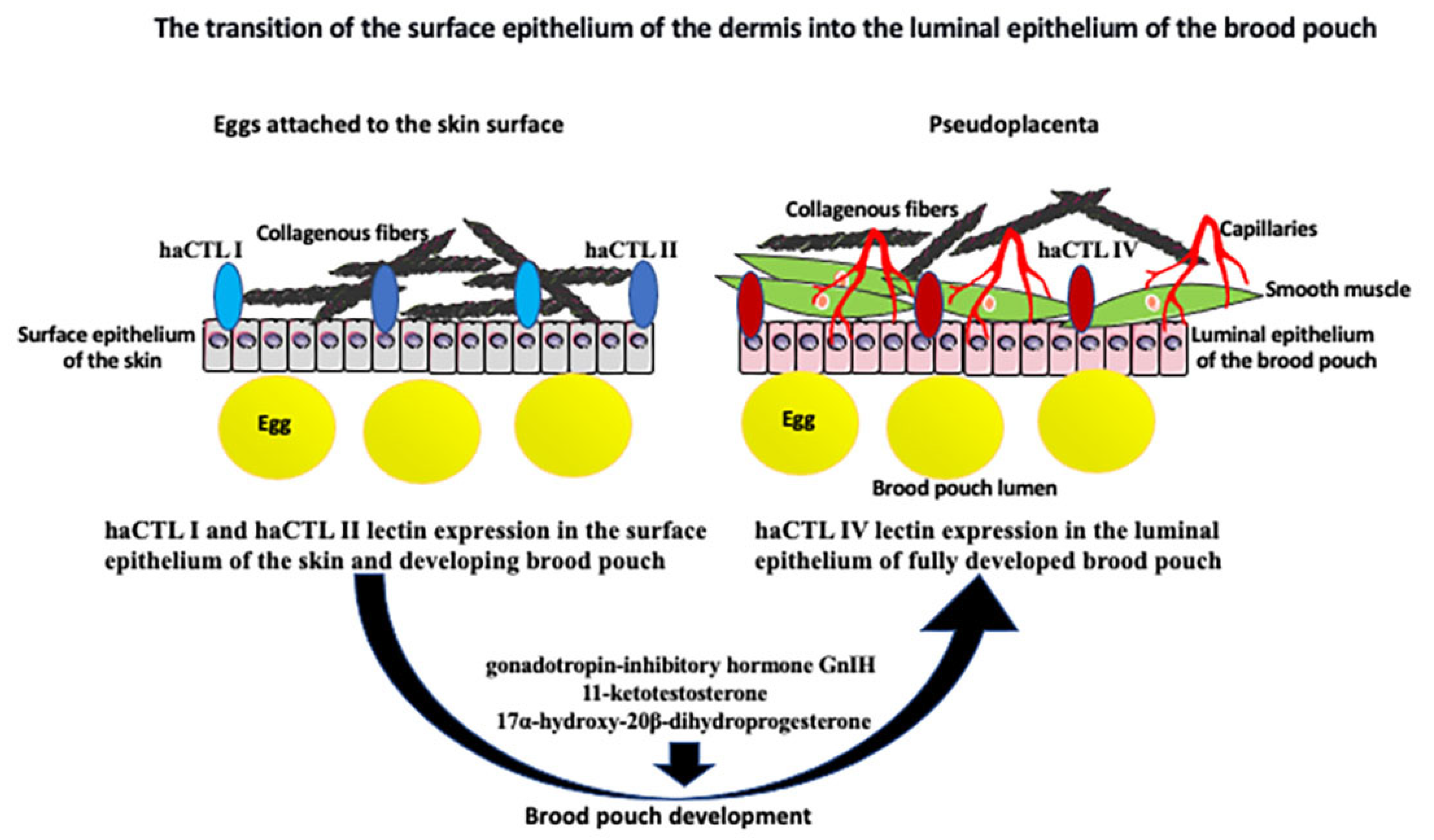
2.2. Hormonal Regulation of Seahorse Male Pregnancy
2.3. Retinoic Acid
3. Adaptations of the Immune System to Male Pregnancy
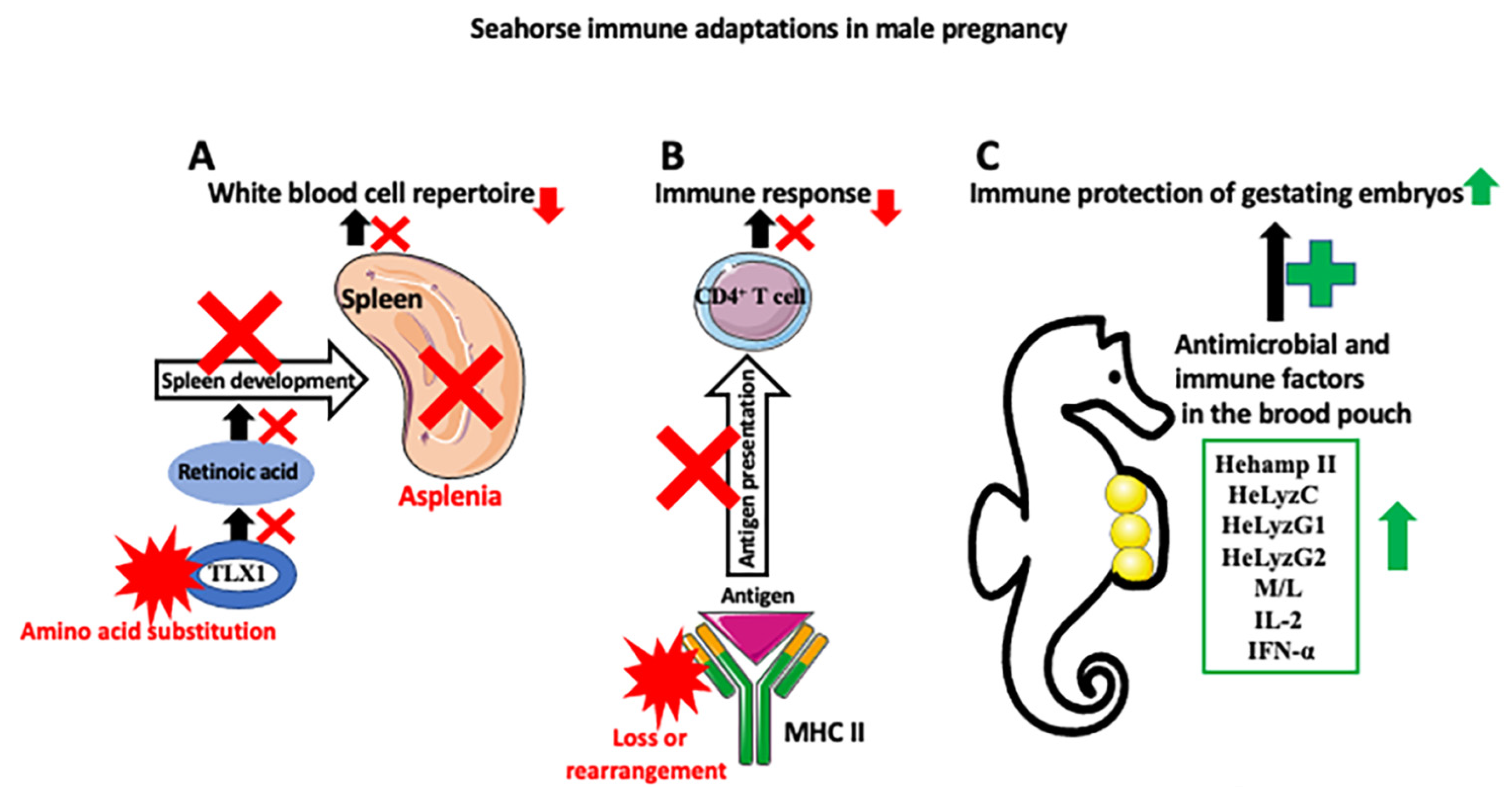
4. Effects of Male Pregnancy on the Microbiome
5. Effects of Environmental Changes and Pollutants on Seahorses
References
- Wilson, A.B.; Orr, J.W. The evolutionary origins of Syngnathidae: Pipefishes and seahorses. J. Fish. Biol. 2011, 78, 1603–1623.
- Teske, P.R.; Beheregaray, L.B. Evolution of seahorses’ upright posture was linked to Oligocene expansion of seagrass habitats. Biol. Lett. 2009, 5, 521–523.
- Froese, R.; Pauly, D. (Eds.) Fish Base; World Wide Web Electronic Publication: 2013. Available online: www.fishbase.org (accessed on 25 May 2023).
- Roth, O.; Solbakken, M.H.; Tørresen, O.K.; Bayer, T.; Matschiner, M.; Baalsrud, H.T.; Hoff, S.N.K.; Brieuc, M.S.O.; Haase, D.; Hanel, R.; et al. Evolution of male pregnancy associated with remodeling of canonical vertebrate immunity in seahorses and pipefishes. Proc. Natl. Acad. Sci. USA 2020, 117, 9431–9439.
- Long, X.; Charlesworth, D.; Qi, J.; Wu, R.; Chen, M.; Wang, Z.; Xu, L.; Fu, H.; Zhang, X.; Chen, X.; et al. Independent Evolution of Sex Chromosomes and Male Pregnancy-Related Genes in Two Seahorse Species. Mol. Biol. Evol. 2023, 40, msac279.
- Dudley, J.S.; Paul, J.W.; Teh, V.; Mackenzie, T.E.; Butler, T.A.; Tolosa, J.M.; Smith, R.; Foley, M.; Dowland, S.; Thompson, M.B.; et al. Seahorse brood pouch morphology and control of male parturition in Hippocampus abdominalis. Placenta 2022, 127, 88–94.
- Harada, A.; Shiota, R.; Okubo, R.; Yorifuji, M.; Sogabe, A.; Motomura, H.; Hiroi, J.; Yasumasu, S.; Kawaguchi, M. Brood pouch evolution in pipefish and seahorse based on histological observation. Placenta 2022, 120, 88–96.
- Herald, E.S. From pipefish to seahorse—A study of phylogenetic relationships. Proc. Calif. Acad. Sci. 1959, 29, 465–473.
- Kawaguchi, M.; Okubo, R.; Harada, A.; Miyasaka, K.; Takada, K.; Hiroi, J.; Yasumasu, S. Morphology of brood pouch formation in the pot-bellied seahorse Hippocampus abdominalis. Zool. Lett. 2017, 3, 19.
- Vincent, A.; Ahnesjö, I.; Berglund, A.; Rosenqvist, G. Pipefishes and seahorses: Are they all sex role reversed? Trends Ecol. Evol. 1992, 7, 237–241.
- Wilson, A.B.; Vincent, A.; Ahnesjö, I.; Meyer, A. Male pregnancy in seahorses and pipefishes (family Syngnathidae): Rapid diversification of paternal brood pouch morphology inferred from a molecular phylogeny. J. Hered. 2001, 92, 159–166.
- Marques, P.; Skorupskaite, K.; Rozario, K.S.; Anderson, R.A.; George, J.T. Physiology of GnRH and Gonadotropin Secretion. In Endotext ; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000.
- Zhang, H.; Chen, L.; Zhang, B.; Lin, Q. Molecular identification of GnIH and its potential role in reproductive physiology and male pregnancy of the lined seahorse (Hippocampus erectus). Gen. Comp. Endocrinol. 2019, 279, 196–202.
- Cole, K.S.; Stacey, N.E. Prostaglandin induction of spawning behavior in Cichlasoma bimaculatum (Pisces cichlidae). Horm. Behav. 1984, 18, 235–248.
- Omony, J.B.; Biran, J.; Kahwa, D.; Aizen, J.; Golan, M.; Nyatia, E.; Levavi-Sivan, B.; Rutaisire, J. Cloning of gonadotropin Gph-alpha, FSH-beta and LH-beta subunits and seasonal profiles of steroid hormones in wild-caught Nile perch, Lates niloticus. Gen. Comp. Endocrinol. 2022, 323–324, 114035.
- Scobell, S.K.; Mackenzie, D.S. Reproductive endocrinology of Syngnathidae. J. Fish. Biol. 2011, 78, 1662–1680.
- Yazawa, T.; Uesaka, M.; Inaoka, Y.; Mizutani, T.; Sekiguchi, T.; Kajitani, T.; Kitano, T.; Umezawa, A.; Miyamoto, K. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: Conservation and evolution of the androgen metabolic pathway. Endocrinology 2008, 149, 1786–1792.
- Barannikova, I.A.; Dyubin, V.P.; Bayunova, L.V.; Semenkova, T.B. Steroids in the control of reproductive function in fish. Neurosci. Behav. Physiol. 2002, 32, 141–148.
- Lovat, P.E.; Annicchiarico-Petruzzelli, M.; Corazzari, M.; Dobson, M.G.; Malcolm, A.J.; Pearson, A.D.; Melino, G.; Redfern, C.P. Differential effects of retinoic acid isomers on the expression of nuclear receptor co-regulators in neuroblastoma. FEBS Lett. 1999, 445, 415–419.
- Brown, G. Targeting the Retinoic Acid Pathway to Eradicate Cancer Stem Cells. Int. J. Mol. Sci. 2023, 24, 2373.
- Heeg, M.; Goldrath, A.W. License to kill: Retinoic acid programs T cells for tissue residency. J. Exp. Med. 2023, 220, e20230161.
- Koop, D.; Holland, N.D.; Sémon, M.; Alvarez, S.; de Lera, A.R.; Laudet, V.; Holland, L.Z.; Schubert, M. Retinoic acid signaling targets Hox genes during the amphioxus gastrula stage: Insights into early anterior-posterior patterning of the chordate body plan. Dev. Biol. 2010, 338, 98–106.
- Bozzo, M.; Bellitto, D.; Amaroli, A.; Ferrando, S.; Schubert, M.; Candiani, S. Retinoic Acid and POU Genes in Developing Amphioxus: A Focus on Neural Development. Cells 2023, 12, 614.
- Yeung, K.W.Y.; Lai, R.W.S.; Zhou, G.J.; Leung, K.M.Y. Concentration-response of six marine species to all-trans-retinoic acid and its ecological risk to the marine environment. Ecotoxicol. Environ. Saf. 2022, 235, 113455.
- Li, C.; Li, Y.; Qin, G.; Chen, Z.; Qu, M.; Zhang, B.; Han, X.; Wang, X.; Qian, P.Y.; Lin, Q. Regulatory Role of Retinoic Acid in Male Pregnancy of the Seahorse. Innovation 2020, 1, 100052.
- Powell, J.R.; Kim, D.H.; Ausubel, F.M. The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proc. Natl. Acad. Sci. USA 2009, 106, 2782–2787.
- Zhang, J.; Wang, X.; Jiang, H.; Yang, F.; Du, Y.; Wang, L.; Hong, B. MicroRNA-185 modulates CYP7A1 mediated cholesterol-bile acid metabolism through post-transcriptional and post-translational regulation of FoxO1. Atherosclerosis 2022, 348, 56–67.
- Monteiro, N. Mom and dad are not that different after all: Immune modulation as a prerequisite for the evolution of pregnancy. Mol. Ecol. 2023, 32, 753–755.
- Chavan, A.R.; Griffith, O.W.; Wagner, G.P. The inflammation paradox in the evolution of mammalian pregnancy: Turning a foe into a friend. Curr. Opin. Genet. Dev. 2017, 47, 24–32.
- Griffith, O.W.; Chavan, A.R.; Protopapas, S.; Maziarz, J.; Romero, R.; Wagner, G.P. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. USA 2017, 114, E6566–E6575.
- Murphy, S.P.; Choi, J.C.; Holtz, R. Regulation of major histocompatibility complex class II gene expression in trophoblast cells. Reprod. Biol. Endocrinol. 2004, 2, 52.
- Rock, K.L.; Reits, E.; Neefjes, J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737.
- Rutigliano, H.M.; Thomas, A.J.; Wilhelm, A.; Sessions, B.R.; Hicks, B.A.; Schlafer, D.H.; White, K.L.; Davies, C.J. Trophoblast Major Histocompatibility Complex Class I Expression Is Associated with Immune-Mediated Rejection of Bovine Fetuses Produced by Cloning. Biol. Reprod. 2016, 95, 39.
- Tsuda, S.; Nakashima, A.; Shima, T.; Saito, S. New Paradigm in the Role of Regulatory T Cells during Pregnancy. Front. Immunol. 2019, 10, 573.
- Parker, J.; Dubin, A.; Schneider, R.; Wagner, K.S.; Jentoft, S.; Böhne, A.; Bayer, T.; Roth, O. Immunological tolerance in the evolution of male pregnancy. Mol. Ecol. 2023, 32, 819–840.
- Parker, J.; Dubin, A.; Roth, O. Genome rearrangements, male pregnancy and immunological tolerance—The curious case of the syngnathid immune system. Front. Mar. Sci. 2023, 10, 1099231.
- Beemelmanns, A.; Poirier, M.; Bayer, T.; Kuenzel, S.; Roth, O. Microbial embryonal colonization during pipefish male pregnancy. Sci. Rep. 2019, 9, 3.
- Vincent, A.C.; Foster, S.J.; Koldewey, H.J. Conservation and management of seahorses and other Syngnathidae. J. Fish. Biol. 2011, 78, 1681–1724.
- Delunardo, F.A.; de Carvalho, L.R.; da Silva, B.F.; Galão, M.; Val, A.L.; Chippari-Gomes, A.R. Seahorse (Hippocampus reidi) as a bioindicator of crude oil exposure. Ecotoxicol. Environ. Saf. 2015, 117, 28–33.
- Faleiro, F.; Baptista, M.; Santos, C.; Aurélio, M.L.; Pimentel, M.; Pegado, M.R.; Paula, J.R.; Calado, R.; Repolho, T.; Rosa, R. Seahorses under a changing ocean: The impact of warming and acidification on the behaviour and physiology of a poor-swimming bony-armoured fish. Conserv. Physiol. 2015, 3, cov009.
- Aurélio, M.; Faleiro, F.; Lopes, V.M.; Pires, V.; Lopes, A.R.; Pimentel, M.S.; Repolho, T.; Baptista, M.; Narciso, L.; Rosa, R. Physiological and behavioral responses of temperate seahorses (Hippocampus guttulatus) to environmental warming. Mar. Biol. 2013, 160, 2663–2670.
- Liu, Y.; Shang, D.; Yang, Y.; Cui, P.; Sun, J. Bioaccumulation of contaminants in wild seahorses collected from coastal China. Front. Mar. Sci. 2022, 9, 1021170.
- Liu, Y.; Shang, D.; Yang, Y.; Cui, P.; Sun, J. Transcriptomic Analysis Provides Insights into Microplastic and Heavy Metal Challenges in the Line Seahorse (Hippocampus erectus). Fishes 2022, 7, 338.
- Nenciu, M.-I.; Coatu, V.; Oros, A.; Rosiorua, D.; Tiganus, D.; Rosoiu, N. Pollutant bioaccumulation in the long-snouted seahorse at the Romanian coast. J. Environ. Prot. Ecol. 2014, 15, 1650–1659.
- Zhang, W.; Zhang, Y.; Zhang, L.; Lin, Q. Bioaccumulation of Metals in Tissues of Seahorses Collected from Coastal China. Bull. Environ. Contam. Toxicol. 2016, 96, 281–288.
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The Physical Impacts of Microplastics on Marine Organisms: A Review. Environ. Pollut. 2013, 178, 483–492.





