Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ting qiu | -- | 1166 | 2023-06-14 12:48:37 | | | |
| 2 | Fanny Huang | Meta information modification | 1166 | 2023-06-19 07:48:01 | | | | |
| 3 | Fanny Huang | Meta information modification | 1166 | 2023-07-07 10:08:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kong, Y.; Jiang, C.; Wei, G.; Sun, K.; Wang, R.; Qiu, T. Fusion Genes in Diseases and Their Detection. Encyclopedia. Available online: https://encyclopedia.pub/entry/45577 (accessed on 08 February 2026).
Kong Y, Jiang C, Wei G, Sun K, Wang R, Qiu T. Fusion Genes in Diseases and Their Detection. Encyclopedia. Available at: https://encyclopedia.pub/entry/45577. Accessed February 08, 2026.
Kong, Yichao, Caihong Jiang, Guifeng Wei, Kai Sun, Ruijie Wang, Ting Qiu. "Fusion Genes in Diseases and Their Detection" Encyclopedia, https://encyclopedia.pub/entry/45577 (accessed February 08, 2026).
Kong, Y., Jiang, C., Wei, G., Sun, K., Wang, R., & Qiu, T. (2023, June 14). Fusion Genes in Diseases and Their Detection. In Encyclopedia. https://encyclopedia.pub/entry/45577
Kong, Yichao, et al. "Fusion Genes in Diseases and Their Detection." Encyclopedia. Web. 14 June, 2023.
Copy Citation
Oncogenic fusion proteins, arising from chromosomal rearrangements, have emerged as prominent drivers of tumorigenesis and crucial therapeutic targets in cancer research. The detection of gene fusions is essential for the identification of cancer and the development of targeted therapeutic approaches.
fusion proteins
inhibitor
therapeutic
cancer
1. Introduction
Genomic instability is a prominent factor in driving tumorigenesis [1]. Specifically, gene fusions represent significant genomic events in the progression of cancer [2]. Gene fusions result from the physical juxtaposition of two or more previously separate genes, generating a novel chimeric gene that comprises the head of one gene and the tail of another. These chimeric genes can be generated through several possible processes, including chromosomal inversions, tandem duplications, interstitial deletions, or translocation events [3]. Gene fusions often encode fusion proteins that possess unique functional properties distinct from the original proteins, thereby leading to alterations in cellular functions and promoting tumorigenesis. Gene fusions are frequently identified as primary tumor predisposing events in leukemias, lymphomas, solid malignancies, and benign tumors [4]. Various fusion proteins have emerged as critical biomarkers for clinical diagnosis and therapeutic targets [5]. Therefore, gene fusions have garnered substantial attention within the realm of cancer research.
The discovery of the BCR–ABL fusion protein in patients with chronic myeloid leukemia (CML) marked the inception of a new era in cancer research [6]. Subsequently, numerous gene fusions have been identified in various cancer types [7], with advancements in detection methods. The application of RNA sequencing technology has revealed a multitude of unique fusion events, surpassing a count of 2200, with 120 of them exhibiting high frequency. Among these, a substantial proportion encodes protein kinases, which are known to play crucial roles in multiple signaling pathways, with relatively high expression levels and kinase activity [8]. Fusion proteins have been recognized as pivotal drivers of cancer initiation and progression in a variety of malignancies. For instance, the BCR–ABL fusion protein has been shown to induce the transformation of benign cells into malignant ones [9]. In fibrolamellar hepatocellular carcinoma (FL-HCC), the DNAJB1–PRKACA fusion drives tumorigenesis by hyperactivating the Wnt signaling pathway [10]. RET/PTC have been observed in papillary carcinomas and shown to activate the RAS-MAPK pathway in a ligand-independent way [11]. Furthermore, MAN2A1–FER has been identified in various tumor types and has been demonstrated to enhance the FER kinase activation [12]. An increasing number of studies have illustrated the pivotal role of kinase fusion proteins in the pathogenesis of cancer by aberrantly activating downstream signaling pathways. Notably, a mounting body of research has also highlighted the significance of non-kinase fusion proteins, particularly transcription factor-related non-kinase fusion proteins, in cancer development. For instance, PML–RARα and FUS–DDIT3 have been considered as the key drivers of acute myeloid leukemia (AML) and mucinous liposarcoma (MLS) [13][14]. Therefore, comprehending the mechanistic and evolutionary processes underlying fusion proteins is crucial for understanding their role in driving carcinogenesis.
The remarkable specificity exhibited by fusion genes towards cancer cells has propelled their emergence as diagnostic markers and promising targets for cancer therapies. By targeting fusion proteins, the potential arises to mitigate the off-target effects commonly associated with traditional cancer treatments, such as chemotherapy and radiation therapy, which frequently lead to many side effects and adversely impact the patients’ quality of life. Consequently, the development of selective inhibitors for fusion proteins has become a critical goal in the realm of related-cancers therapies. Targeted therapies, particularly tyrosine kinase inhibitors (TKIs), have demonstrated remarkably efficacy in treating specific cancer types, including the first-, second- and third-generation TKIs. For example, Imatinib, a drug designed to target BCR–ABL, has exhibited effectiveness in the treatment of CML [15]. Recently approved drugs such as Larotrectinib and Entrectinib are capable of targeting fusion proteins harboring neurotrophic RTK (NRTK) genes [16]. Crizotinib has demonstrated efficient inhibition of ALK fusions [17]. Additionally, all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO) can be employed to treat or alleviate the blockage of granulocyte differentiation caused by the RARA fusion protein [18]. Despite the success achieved by inhibitors in the treatment of fusion-driven tumors, resistance often emerges as a result of secondary mutations [19]. Remarkably, the therapeutic strategy for cancers has undergone a paradigm shift with the advent of molecular-targeted therapy for oncogenes-driven cancers.
2. Fusion Genes in Diseases and the Detection of Fusion Genes
At the genomic level, the process of chromosome breakage and subsequent re-splicing gives rise to gene fusions, which are novel genes formed through this mechanism [20]. Unlike other recurrent oncogenic mutations, gene fusions are often regarded as pathognomonic factors for malignancy, and many of them are strongly associated with specific cancer types, such as prostate cancer [21][22], lung cancer [23][24], breast cancer, and so on.
Thus, the detection of gene fusions is essential for the identification of cancer and the development of targeted therapeutic approaches. These gene fusions generate novel chimeric proteins that can drive oncogenic transformation. The identification of gene fusions in cancer patients can provide valuable insight for personalized treatment strategies that target the aberrant fusion proteins or downstream signaling pathways, ultimately leading to improved therapeutic outcomes. Currently, a variety of techniques are wildly employed in clinical practice, include cell morphology, exon array analysis (EAA), immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), reverse transcription polymerase chain reaction (RT-PCR), droplet digital PCR (DD PCR), and next-generation sequencing (NGS) (Figure 1).
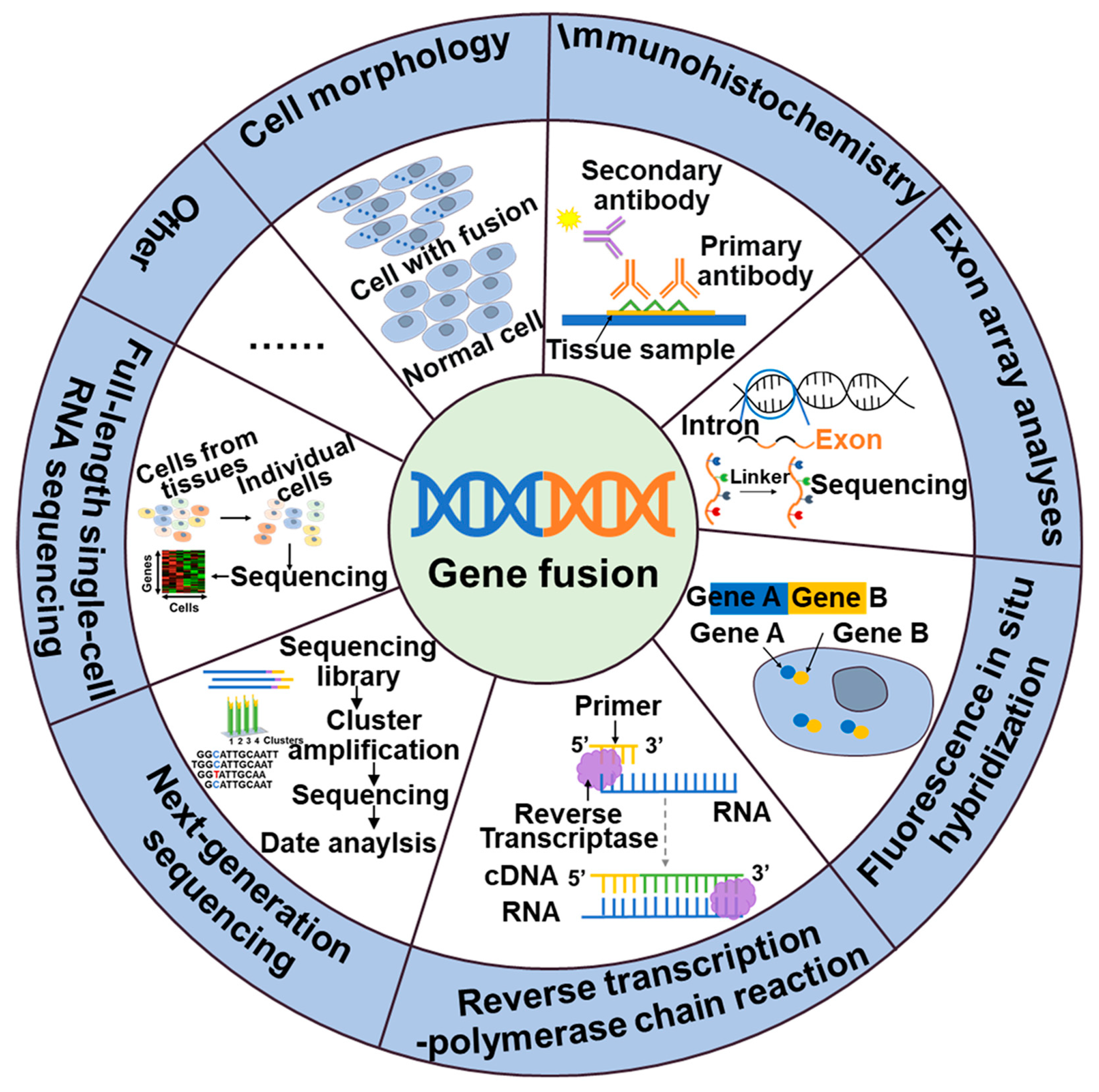
Figure 1. Methods of detection of gene fusions.
NTRK, ALK, NTRK1, BRAF, and RET fusions and many other gene fusions have previously been successfully detected by cytomorphological methods [25][26]. However, it may not always be sensitive enough to detect low-level fusion transcriptions or may not be able to differentiate between different fusion variants. The convenience and speed offered by IHC, the specificity provided by FISH, and the high detection sensitivity of RT-PCR have positioned them as widely utilized detection methods. These techniques have made great contributions to the identification of gene fusions, including ALK, NTRK, and RET. While IHC stands out for its cost-effectiveness and rapidity compared to other available assays, caution is warranted due to its tendency to yield false-positive results. Consequently, validation in combination with complementary techniques is often necessary to ensure reliable outcomes [27]. The emergence of NGS technology has facilitated targeted therapy for tumors by enabling the detection not only of fusions but also polygenes and fusion mutants [28]. In addition to the classical detection techniques, a large number of new assays are emerging, including targeted RNA sequencing (RNAseq) and full-length single-cell sequencing (scRNA-seq). Compared to FISH and RT-PCR methods, targeted RNAseq improves diagnostic yield by evaluating multiple genes simultaneously in a single assay, thereby increasing sequencing coverage and reducing diagnostic time. Additionally, it can also identify new gene fusions or complex structural rearrangements. However, the broader scope of detection in targeted RNAseq may lead to an increased likelihood of false-positive results [29]. The scRNA-seq [30], employing the detection algorithm scFusion, presents the opportunity to detect fusion genes at the single-cell level, thereby enhancing the detection power through co-analysis of relevant cells. However, the ability to detect rare fusions in highly heterogeneous tumor samples is limited. Furthermore, scRNAseq data often exhibit high levels of noise and contain various technical artifacts, which can also contribute to false-positive results [30].
References
- Li, Y.; Roberts, N.D.; Wala, J.A.; Shapira, O.; Schumacher, S.E.; Kumar, K.; Khurana, E.; Waszak, S.; Korbel, J.O.; Haber, J.E.; et al. Patterns of somatic structural variation in human cancer genomes. Nature 2020, 578, 112–121.
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421.
- Mitelman, F.; Johansson, B.; Mertens, F. The impact of translocations and gene fusions on cancer causation. Nat. Rev. Cancer 2007, 7, 233–245.
- Panagopoulos, I.; Heim, S. Interstitial deletions generating fusion genes. Cancer Genom. Proteom. 2021, 18, 167–196.
- Pederzoli, F.; Bandini, M.; Marandino, L.; Ali, S.M.; Madison, R.; Chung, J.; Ross, J.S.; Necchi, A. Targetable gene fusions and aberrations in genitourinary oncology. Nat. Rev. Urol. 2020, 17, 613–625.
- Rowley, J.D. A New Consistent Chromosomal Abnormality in Chronic Myelogenous Leukaemia identified by Quinacrine Fluorescence and Giemsa Staining. Nature 1973, 243, 290–293.
- Heydt, C.; Wölwer, C.B.; Velazquez Camacho, O.; Wagener-Ryczek, S.; Pappesch, R.; Siemanowski, J.; Rehker, J.; Haller, F.; Agaimy, A.; Worm, K.; et al. Detection of gene fusions using targeted next-generation sequencing: A comparative evaluation. BMC Med. Genom. 2021, 14, 62.
- Klijn, C.; Durinck, S.; Stawiski, E.W.; Haverty, P.M.; Jiang, Z.; Liu, H.; Degenhardt, J.; Mayba, O.; Gnad, F.; Liu, J.; et al. A comprehensive transcriptional portrait of human cancer cell lines. Nat. Biotechnol. 2015, 33, 306–312.
- Cilloni, D.; Saglio, G. Molecular pathways: BCR-ABL. Clin. Cancer Res. 2012, 18, 930–937.
- Kastenhuber, E.R.; Lalazar, G.; Houlihan, S.L.; Tschaharganeh, D.F.; Baslan, T.; Chen, C.C.; Requena, D.; Tian, S.; Bosbach, B.; Wilkinson, J.E.; et al. DNAJB1-PRKACA fusion kinase interacts with β-catenin and the liver regenerative response to drive fibrolamellar hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2017, 114, 13076–13084.
- Asai, N.; Murakami, H.; Iwashita, T.; Takahashi, M. A mutation at tyrosine 1062 in MEN2A-Ret and MEN2B-Ret impairs their transforming activity and association with shc adaptor proteins. J. Biol. Chem. 1996, 271, 17644–17649.
- Chen, Z.H.; Yu, Y.P.; Tao, J.; Liu, S.; Tseng, G.; Nalesnik, M.; Hamilton, R.; Bhargava, R.; Nelson, J.B.; Pennathur, A.; et al. MAN2A1-FER fusion gene is expressed by human liver and other tumor types and has oncogenic activity in mice. Gastroenterology 2017, 153, 1120–1132.
- Tan, Y.; Wang, X.; Song, H.; Zhang, Y.; Zhang, R.; Li, S.; Jin, W.; Chen, S.; Fang, H.; Chen, Z.; et al. A PML/RARα direct target atlas redefines transcriptional deregulation in acute promyelocytic leukemia. Blood 2021, 137, 1503–1516.
- Yu, J.S.E.; Colborne, S.; Hughes, C.S.; Morin, G.B.; Nielsen, T.O. The FUS-DDIT3 Interactome in Myxoid Liposarcoma. Neoplasia 2019, 21, 740–751.
- Capdeville, R.; Silberman, S. Imatinib: A targeted clinical drug development. Semin. Hematol. 2003, 40, 15–20.
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747.
- Cui, J.J.; Tran-Dubé, M.; Shen, H.; Nambu, M.; Kung, P.P.; Pairish, M.; Jia, L.; Meng, J.; Funk, L.; Botrous, I.; et al. Structure based drug design of Crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J. Med. Chem. 2011, 54, 6342–6363.
- Lallemand-Breitenbach, V.; Zhu, J.; Chen, Z.; de Thé, H. Curing APL through PML/RARA degradation by As2O3. Trends Mol. Med. 2012, 18, 36–42.
- Schram, A.M.; Chang, M.T.; Jonsson, P.; Drilon, A. Fusions in solid tumours: Diagnostic strategies, targeted therapy, and acquired resistance. Nat. Rev. Clin. Oncol. 2017, 14, 735–748.
- Hahn, Y.; Bera, T.K.; Gehlhaus, K.; Kirsch, I.R.; Pastan, I.H.; Lee, B. Finding fusion genes resulting from chromosome rearrangement by analyzing the expressed sequence databases. Proc. Natl. Acad. Sci. USA 2004, 101, 13257–13261.
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648.
- Tomlins, S.A.; Laxman, B.; Dhanasekaran, S.M.; Helgeson, B.E.; Cao, X.; Morris, D.S.; Menon, A.; Jing, X.; Cao, Q.; Han, B.; et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 2007, 448, 595–599.
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566.
- Koivunen, J.P.; Mermel, C.; Zejnullahu, K.; Murphy, C.; Lifshits, E.; Holmes, A.J.; Choi, H.G.; Kim, J.; Chiang, D.; Thomas, R.; et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin. Cancer Res. 2008, 14, 4275–4283.
- Amin, S.M.; Haugh, A.M.; Lee, C.Y.; Zhang, B.; Bubley, J.A.; Merkel, E.A.; Verzi, A.E.; Gerami, P. A comparison of morphologic and molecular features of BRAF, ALK, and NTRK1 fusion Spitzoid Neoplasms. Am. J. Pathol. 2017, 41, 491–498.
- De la Fouchardière, A.; Tee, M.K.; Peternel, S.; Valdebran, M.; Pissaloux, D.; Tirode, F.; Busam, K.J.; LeBoit, P.E.; McCalmont, T.H.; Bastian, B.C.; et al. Fusion partners of NTRK3 affect subcellular localization of the fusion kinase and cytomorphology of melanocytes. Mod. Pathol. 2021, 34, 735–747.
- Batra, U.; Nathany, S.; Sharma, M.; Pasricha, S.; Bansal, A.; Jain, P.; Mehta, A. IHC versus FISH versus NGS to detect ALK gene rearrangement in NSCLC: All questions answered? J. Clin. Pathol. 2022, 75, 405–409.
- Mangolini, A.; Rocca, C.; Bassi, C.; Ippolito, C.; Negrini, M.; Dell’Atti, L.; Lanza, G.; Gafà, R.; Bianchi, N.; Pinton, P.; et al. Detection of disease-causing mutations in prostate cancer by NGS sequencing. Cell Biol. Int. 2022, 46, 1047–1061.
- Wagener-Ryczek, S.; Pappesch, R. Targeted RNA-sequencing for the evaluation of gene fusions in lung tumors: Current status and future prospects. Expert Rev. Mol. Diagn. 2021, 21, 531–534.
- Jin, Z.; Huang, W.; Shen, N.; Li, J.; Wang, X.; Dong, J.; Park, P.J.; Xi, R. Single-cell gene fusion detection by scFusion. Nat. Commun. 2022, 13, 1084.
More
Information
Subjects:
Allergy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
801
Revisions:
3 times
(View History)
Update Date:
07 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




