Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ekaterina Sergeeva | -- | 1127 | 2023-06-14 11:26:03 | | | |
| 2 | Catherine Yang | Meta information modification | 1127 | 2023-06-15 03:59:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sergeeva, E.; Ruksha, T.; Fefelova, Y. Calorie Restriction under Malignant Tumors. Encyclopedia. Available online: https://encyclopedia.pub/entry/45565 (accessed on 07 February 2026).
Sergeeva E, Ruksha T, Fefelova Y. Calorie Restriction under Malignant Tumors. Encyclopedia. Available at: https://encyclopedia.pub/entry/45565. Accessed February 07, 2026.
Sergeeva, Ekaterina, Tatiana Ruksha, Yulia Fefelova. "Calorie Restriction under Malignant Tumors" Encyclopedia, https://encyclopedia.pub/entry/45565 (accessed February 07, 2026).
Sergeeva, E., Ruksha, T., & Fefelova, Y. (2023, June 14). Calorie Restriction under Malignant Tumors. In Encyclopedia. https://encyclopedia.pub/entry/45565
Sergeeva, Ekaterina, et al. "Calorie Restriction under Malignant Tumors." Encyclopedia. Web. 14 June, 2023.
Copy Citation
Excessive body weight leads to increased levels of estrogens, chronic inflammation and hypoxia, which can play an important role in the development of malignancies. Calorie restriction can improve the state of patients with various diseases. Decreased calorie uptake influences lipid, carbohydrate and protein metabolism, hormone levels and cell processes.
calorie restriction
obesity
cancer
1. Effects of Calorie Restriction in Laboratory Models with Malignant Tumors
Recently, a lot of investigations have been devoted to the effects of calorie restriction on cancer development in vitro and in vivo. The combined action of calorie restriction and a glutamine antagonist, 6-diazo-5-oxo-L-norleucine (DON), in a glioblastoma mouse model influenced glutaminolysis and decreased the glucose levels, resulting in the initiation of cancer cell death and an improvement in overall mouse survival. It is important to stress, however, that the mice used for these studies were not treated with surgery, radiation or standard chemotherapy, so it is unclear if a similar CR effect would be revealed in patients with glioblastoma [1]. A ketogenic diet in combination with calorie restriction led to the suppression of neuroblastoma cell growth and a significant decrease in tumor blood vessel density, which were accompanied by the up-regulation of AMP-activated protein kinase and decreased serum levels of essential amino acids in CD-nu mice with neuroblastoma xenografts. The influence of calorie restriction on energy metabolism promoted the anti-tumor and anti-angiogenic effects of chemotherapy [2]. It is interesting that calorie restriction in AKR/J female mice with a relatively short lifespan, because of leukemia development around 6 months of age, led to an increased survival rate of offspring [3]. Calorie restriction decreased the levels of glucose, cholesterol, triglycerides, low-density lipoproteins and non-esterified fatty acids and prevented the development of radiation-induced intestinal cancer in C3B6F1 ApcMin/+ mice [4]. A mouse model of triple-negative breast cancer was used for an evaluation of the influence of obesity and calorie restriction on breast cancer development. Obese C57BL/6 mice were orthotopically injected with E0771 cells. Calorie restriction decreased the expression of genes associated with the epithelial-to-mesenchymal transition. Furthermore, the expression of several genes in the normal mammary tissue that regulate hypoxia and reactive oxygen species production, and p53 signaling was also altered. The tumor weight, systemic cytokines and the incidence of lung metastases were decreased in calorie-restricted mice [5]. Non-small-cell lung cancer cells with the KRAS mutation and LKB1 loss demonstrated alterations to the activity of the enzymes involved in the regulation of glycolysis, glutaminolysis, tryptophan catabolism and phospholipid metabolism. Calorie restriction resulted in a decrease in cancer cell proliferation and an enhanced sensitivity to chemotherapy [6]. Glucose restriction combined with a curcumin treatment led to the inhibition of Na(+)-H(+) exchanger-1 (NHE1), vATPase, monocarboxylate transporter (MCT)-1, the MCT4 level and proton-extruding enzymes in hepatoma cells, with an intracellular pH reduction. It was revealed that the production of ATP and lactate decreased according to the pH change. Furthermore, this combined treatment initiated structural changes in the proteins of the mTOR signaling pathway. The consequences of this influence were the inhibition of cancer cell migration and proliferation [7]. Three types of preclinical models of colorectal cancer have been used to investigate the effects of calorie restriction on cancer development: carcinogenic-induced models with chemicals, the models with transplantation of CRC cell lines and genetically modified mouse models of CRC. It was revealed that all models are useful tools to investigate cellular and molecular response to calorie restriction. Nevertheless, it is necessary to stress that there are nutritional recommendations for cancer care, and weight-loss or reduction of protein may enhance risk of malnutrition or sarcopenia in cancer patients [8].
2. Effects of Calorie Restriction in Patients with Malignant Tumors
The influence of a calorie-restricted diet on patients with various types of cancer was also investigated. Short-term fasting in glioma patients led to significant metabolic changes, including decreased glucose and insulin levels, which can be treated as candidate markers for a better prognosis [9]. Short-term calorie restriction during chemotherapy in patients with diffuse large B-cell lymphoma led to better results and initiated a significant decrease in cancer cell proliferation [10].
One of the causes of chemotherapy resistance is diminished blood circulation and the formation of a hypoxic cancer microenvironment. This process is connected to anaerobic energy metabolism. It was revealed that calorie restriction reduced glycolysis in metastatic breast cancer patients and improved the effectiveness of treatment [11]. Calorie restriction prevents cardiotoxicity after anthracycline chemotherapy in patients with malignant tumors. This effect is associated with the modulation of apoptosis, inflammation, oxidative stress and endothelium-dependent vasodilation by diet [12]. The effects of calorie restriction on cancer development and progression are based on alterations to metabolism associated with various signaling pathways that are up- or down-regulated. Calorie restriction reduced glycemia, IGF-1 and the IGF-1/IGFBP3 ratio, and improved insulin sensitivity in patients with Barrett’s esophagus (BE). These alterations were associated with the down-regulation of the insulin/IGF-1 and ERK signaling pathways, which, in turn, decreased the risk of esophageal adenocarcinoma development [13]. Protein and calorie restriction altered the pharmacokinetics of irinotecan and improved the therapeutic window in patients with liver metastases of solid tumors treated with irinotecan [14].
Calorie restriction can influence melanoma development. Le Noci et al. showed that the use of caloric-restriction mimetics in mouse models of melanoma B16 reduced metastasis in the lungs. This effect was connected to the modulation of the immune microenvironment, including a significant increase in alveolar macrophages and CD103+ dendritic cells; an increase in M1 and a decrease in M2 markers in myeloid cells; the activation of CD3 T lymphocytes and NK cells; and an increased cytotoxic activity of effector cells in the lung [15]. The combined action of ropivacaine-loaded liposomes and calorie restriction effectively repressed melanoma B16 development and relieved cancer pain. These effects were associated with autophagy suppression and a reduction in the VEGF-A levels [16].
Thus, both obesity and calorie restriction regulate various signaling pathways associated with cancerogenesis, which can promote or suppress malignant tumor development (Figure 1).
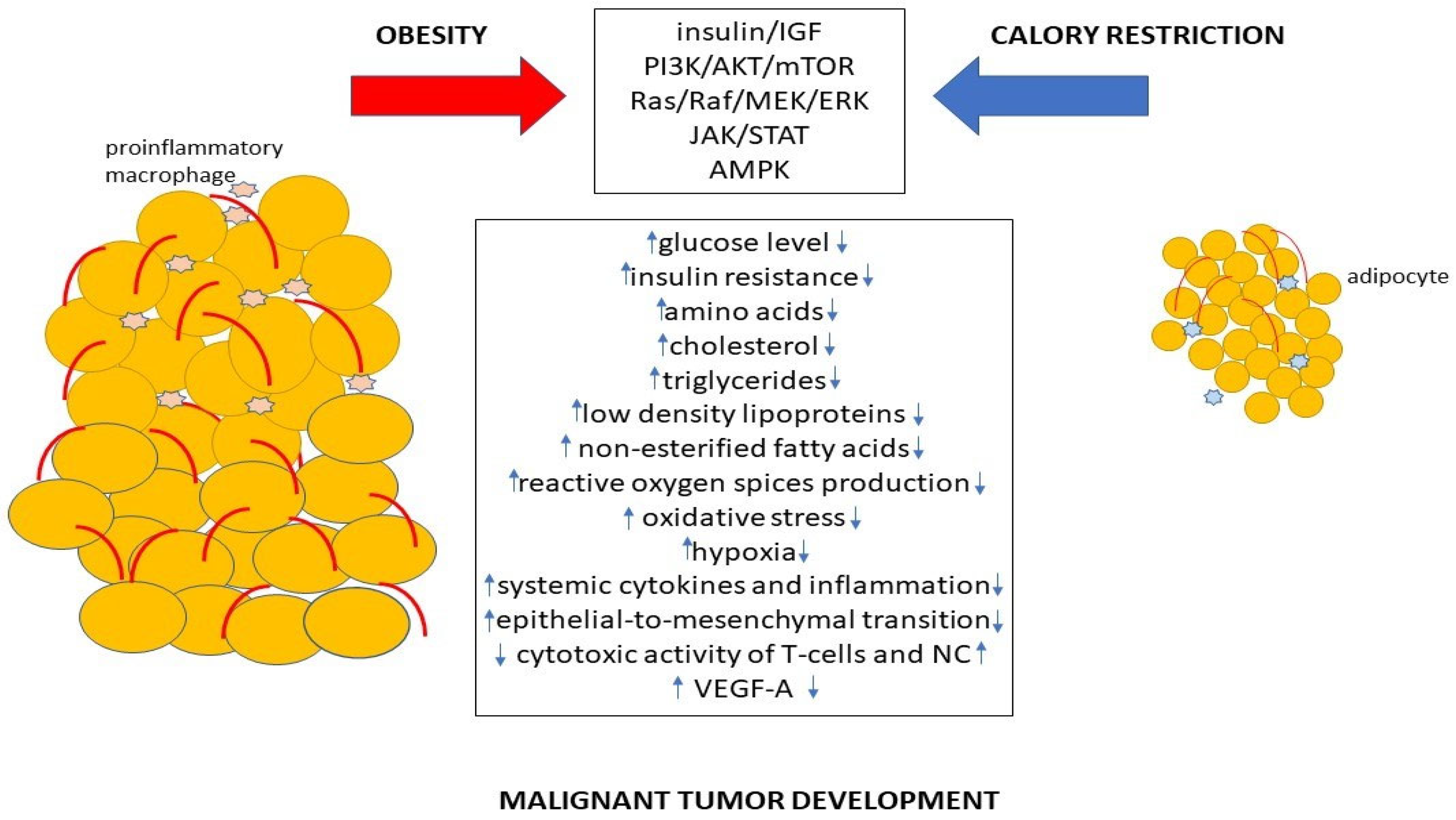
Figure 1. The mechanism of promotion or suppression of cancer development by obesity and calorie restriction.
The underlying mechanisms of calorie restriction influence on cancerogenesis is still unclear and can be associated with not only metabolic alterations. The CR effects can be connected with the regulation of splicing factors that leads to TORC pathway suppression [17]. Fasting reduces monocyte mobilization, which decreases the activity of inflammation via AMPK activation and suppression of CCL2 production [18]. CR can modulate enzyme activity. For example, decreased stearoyl-CoA desaturase (SCD) leads to imbalance between unsaturated and saturated fatty acids that can slow tumor growth [19]. The peculiarities of metabolism connected with race and age of patients can determine the CR effectivity [20]. On the other hand, starvation can increase mTORC1 signaling activity via deregulation of MiT/TFE-RagD-mTORC1-MiT/TFE feedback circuit [21]. Functional caloric restriction in T-cells triggered by elevated potassium ion levels lead to T cell disfunction and stemness [22].
References
- Mukherjee, P.; Augur, Z.M.; Li, M.; Hill, C.; Greenwood, B.; Domin, M.A.; Kondakci, G.; Narain, N.R.; Kiebish, M.A.; Bronson, R.T.; et al. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun. Biol. 2019, 2, 200.
- Aminzadeh-Gohari, S.; Feichtinger, R.G.; Vidali, S.; Locker, F.; Rutherford, T.; O’donnel, M.; Stöger-Kleiber, A.; Mayr, J.A.; Sperl, W.; Kofler, B. A ketogenic diet supplemented with medium-chain triglycerides enhances the anti-tumor and anti-angiogenic efficacy of chemotherapy on neuroblastoma xenografts in a CD1-nu mouse model. Oncotarget 2017, 8, 64728–64744.
- Palliyaguru, D.L.; Rudderow, A.L.; Sossong, A.M.; Lewis, K.N.; Younts, C.; Pearson, K.J.; Bernier, M.; de Cabo, R. Perinatal diet influences health and survival in a mouse model of leukemia. Geroscience 2020, 42, 1147–1155.
- Morioka, T.; Yamazaki, S.; Yanagihara, H.; Sunaoshi, M.; Kaminishi, M.; Kakinuma, S. Calorie Restriction Suppresses the Progression of Radiation-Induced Intestinal Tumours in C3B6F1 ApcMin/+ Mice. Anticancer Res. 2021, 41, 1365–1375.
- Bowers, L.W.; Doerstling, S.S.; Shamsunder, M.G.; Lineberger, C.G.; Rossi, E.L.; Montgomery, S.A.; Coleman, M.F.; Gong, W.; Parker, J.S.; Howell, A.; et al. Reversing the Genomic, Epigenetic, and Triple-Negative Breast Cancer–Enhancing Effects of Obesity. Cancer Prev. Res. 2022, 15, 581–594.
- Caiola, E.; Falcetta, F.; Giordano, S.; Marabese, M.; Garassino, M.C.; Broggini, M.; Pastorelli, R.; Brunelli, L. Co-occurring KRAS mutation/LKB1 loss in non-small cell lung cancer cells results in enhanced metabolic activity susceptible to caloric restriction: An in vitro integrated multilevel approach. J. Exp. Clin. Cancer Res. 2018, 37, 302.
- Kim, S.W.; Cha, M.-J.; Lee, S.-K.; Song, B.-W.; Jin, X.; Lee, J.M.; Park, J.H.; Lee, J.D. Curcumin Treatment in Combination with Glucose Restriction Inhibits Intracellular Alkalinization and Tumor Growth in Hepatoma Cells. Int. J. Mol. Sci. 2019, 20, 2375.
- Castejón, M.; Plaza, A.; Martinez-Romero, J.; Fernandez-Marcos, P.J.; Cabo, R.; Diaz-Ruiz, A. Energy Restriction and Colorectal Cancer: A Call for Additional Research. Nutrients 2020, 12, 114.
- Voss, M.; Wenger, K.J.; von Mettenheim, N.; Bojunga, J.; Vetter, M.; Diehl, B.; Franz, K.; Gerlach, R.; Ronellenfitsch, M.W.; Harter, P.; et al. Short-term fasting in glioma patients: Analysis of diet diaries and metabolic parameters of the ERGO2 trial. Eur. J. Nutr. 2022, 61, 477–487.
- Tang, C.-C.; Huang, T.-C.; Tien, F.-M.; Lin, J.-M.; Yeh, Y.-C.; Lee, C.-Y. Safety, Feasibility, and Effects of Short-Term Calorie Reduction during Induction Chemotherapy in Patients with Diffuse Large B-Cell Lymphoma: A Pilot Study. Nutrients 2021, 13, 3268.
- Kirkham, A.A.; King, K.; Joy, A.A.; Pelletier, A.B.; Mackey, J.R.; Young, K.; Zhu, X.; Meza-Junco, J.; Basi, S.K.; Hiller, J.P.; et al. Rationale and design of the Diet Restriction and Exercise-induced Adaptations in Metastatic breast cancer (DREAM) study: A 2-arm, parallel-group, phase II, randomized control trial of a short-term, calorie-restricted, and ketogenic diet plus exercise during intravenous chemotherapy versus usual care. BMC Cancer 2021, 21, 1093.
- Kirkham, A.A.; Paterson, D.I.; Prado, C.M.; Mackey, J.R.; Courneya, K.S.; Pituskin, E.; Thompson, R.B. Rationale and design of the Caloric Restriction and Exercise protection from Anthracycline Toxic Effects (CREATE) study: A 3-arm parallel group phase II randomized controlled trial in early breast cancer. BMC Cancer 2018, 18, 864.
- Arcidiacono, D.; Zaramella, A.; Fabris, F.; Sánchez-Rodríguez, R.; Nucci, D.; Fassan, M.; Nardi, M.; Benna, C.; Cristofori, C.; Morbin, T.; et al. Insulin/IGF-1 Signaling Is Downregulated in Barrett’s Esophagus Patients Undergoing a Moderate Calorie and Protein Restriction Program: A Randomized 2-Year Trial. Nutrients 2021, 13, 3638.
- De Man, F.M.; van Eerden, R.A.; van Doorn, G.M.; Hoop, E.; Koolen, S.L.W.; Olieman, J.F.; de Bruijn, P.; Veraart, J.N.; van Halteren, H.K.; Sandberg, Y.; et al. Effects of Protein and Calorie Restriction on the Metabolism and Toxicity Profile of Irinotecan in Cancer Patients. Clin. Pharmacol. Ther. 2021, 109, 1304–1313.
- Le Noci, V.; Sommariva, M.; Bianchi, F.; Triulzi, T.; Tagliabue, E.; Balsari, A.; Sfondrini, L. Local Administration of Caloric Restriction Mimetics to Promote the Immune Control of Lung Metastases. J. Immunol. Res. 2019, 2019, 2015892.
- Zhang, J.; Zhu, S.; Tan, Q.; Cheng, D.; Dai, Q.; Yang, Z.; Zhang, L.; Li, F.; Zuo, Y.; Dai, W.; et al. Combination therapy with ropivacaine-loaded liposomes and nutrient deprivation for simultaneous cancer therapy and cancer pain relief. Theranostics 2020, 10, 4885–4899.
- Heintz, C.; Doktor, T.K.; Lanjuin, A.; Escoubas, C.; Zhang, Y.; Weir, H.J.; Dutta, S.; Silva-García, C.G.; Bruun, G.H.; Morantte, I.; et al. Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature 2017, 541, 102–106.
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019, 178, 1102–1114.
- Lien, E.C.; Westermark, A.M.; Zhang, Y.; Yuan, C.; Li, Z.; Lau, A.N.; Sapp, K.M.; Wolpin, B.M.; Heiden, M.G.V. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 2021, 599, 302–307.
- Katzmarzyk, P.T.; Martin, C.K.; Newton, R.L.; Apolzan, J.W.; Arnold, C.L.; Davis, T.C.; Price-Haywood, E.G.; Denstel, K.D.; Mire, E.F.; Thethi, T.K.; et al. Weight Loss in Underserved Patients—A Cluster-Randomized Trial. N. Engl. J. Med. 2020, 383, 909–918.
- Di Malta, C.; Siciliano, D.; Calcagni, A.; Monfregola, J.; Punzi, S.; Pastore, N.; Eastes, A.N.; Davis, O.; De Cegli, R.; Zampelli, A.; et al. Transcriptional activation of RagD GTPase controls mTORC1 and promotes cancer growth. Science 2017, 356, 1188–1192.
- Vodnala, S.K.; Eil, R.; Kishton, R.J.; Sukumar, M.; Yamamoto, T.N.; Ha, N.-H.; Lee, P.-H.; Shin, M.; Patel, S.J.; Yu, Z.; et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 2019, 363, eaau0135.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
572
Revisions:
2 times
(View History)
Update Date:
15 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




