Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Excessive body weight leads to increased levels of estrogens, chronic inflammation and hypoxia, which can play an important role in the development of malignancies. Calorie restriction can improve the state of patients with various diseases. Decreased calorie uptake influences lipid, carbohydrate and protein metabolism, hormone levels and cell processes.
- calorie restriction
- obesity
- cancer
1. Effects of Calorie Restriction in Laboratory Models with Malignant Tumors
Recently, a lot of investigations have been devoted to the effects of calorie restriction on cancer development in vitro and in vivo. The combined action of calorie restriction and a glutamine antagonist, 6-diazo-5-oxo-L-norleucine (DON), in a glioblastoma mouse model influenced glutaminolysis and decreased the glucose levels, resulting in the initiation of cancer cell death and an improvement in overall mouse survival. It is important to stress, however, that the mice used for these studies were not treated with surgery, radiation or standard chemotherapy, so it is unclear if a similar CR effect would be revealed in patients with glioblastoma [107]. A ketogenic diet in combination with calorie restriction led to the suppression of neuroblastoma cell growth and a significant decrease in tumor blood vessel density, which were accompanied by the up-regulation of AMP-activated protein kinase and decreased serum levels of essential amino acids in CD-nu mice with neuroblastoma xenografts. The influence of calorie restriction on energy metabolism promoted the anti-tumor and anti-angiogenic effects of chemotherapy [108]. It is interesting that calorie restriction in AKR/J female mice with a relatively short lifespan, because of leukemia development around 6 months of age, led to an increased survival rate of offspring [109]. Calorie restriction decreased the levels of glucose, cholesterol, triglycerides, low-density lipoproteins and non-esterified fatty acids and prevented the development of radiation-induced intestinal cancer in C3B6F1 ApcMin/+ mice [110]. A mouse model of triple-negative breast cancer was used for an evaluation of the influence of obesity and calorie restriction on breast cancer development. Obese C57BL/6 mice were orthotopically injected with E0771 cells. Calorie restriction decreased the expression of genes associated with the epithelial-to-mesenchymal transition. Furthermore, the expression of several genes in the normal mammary tissue that regulate hypoxia and reactive oxygen species production, and p53 signaling was also altered. The tumor weight, systemic cytokines and the incidence of lung metastases were decreased in calorie-restricted mice [111]. Non-small-cell lung cancer cells with the KRAS mutation and LKB1 loss demonstrated alterations to the activity of the enzymes involved in the regulation of glycolysis, glutaminolysis, tryptophan catabolism and phospholipid metabolism. Calorie restriction resulted in a decrease in cancer cell proliferation and an enhanced sensitivity to chemotherapy [112]. Glucose restriction combined with a curcumin treatment led to the inhibition of Na(+)-H(+) exchanger-1 (NHE1), vATPase, monocarboxylate transporter (MCT)-1, the MCT4 level and proton-extruding enzymes in hepatoma cells, with an intracellular pH reduction. It was revealed that the production of ATP and lactate decreased according to the pH change. Furthermore, this combined treatment initiated structural changes in the proteins of the mTOR signaling pathway. The consequences of this influence were the inhibition of cancer cell migration and proliferation [113]. Three types of preclinical models of colorectal cancer have been used to investigate the effects of calorie restriction on cancer development: carcinogenic-induced models with chemicals, the models with transplantation of CRC cell lines and genetically modified mouse models of CRC. It was revealed that all models are useful tools to investigate cellular and molecular response to calorie restriction. Nevertheless, it is necessary to stress that there are nutritional recommendations for cancer care, and weight-loss or reduction of protein may enhance risk of malnutrition or sarcopenia in cancer patients [114].
2. Effects of Calorie Restriction in Patients with Malignant Tumors
The influence of a calorie-restricted diet on patients with various types of cancer was also investigated. Short-term fasting in glioma patients led to significant metabolic changes, including decreased glucose and insulin levels, which can be treated as candidate markers for a better prognosis [115]. Short-term calorie restriction during chemotherapy in patients with diffuse large B-cell lymphoma led to better results and initiated a significant decrease in cancer cell proliferation [116].
One of the causes of chemotherapy resistance is diminished blood circulation and the formation of a hypoxic cancer microenvironment. This process is connected to anaerobic energy metabolism. It was revealed that calorie restriction reduced glycolysis in metastatic breast cancer patients and improved the effectiveness of treatment [117]. Calorie restriction prevents cardiotoxicity after anthracycline chemotherapy in patients with malignant tumors. This effect is associated with the modulation of apoptosis, inflammation, oxidative stress and endothelium-dependent vasodilation by diet [118]. The effects of calorie restriction on cancer development and progression are based on alterations to metabolism associated with various signaling pathways that are up- or down-regulated. Calorie restriction reduced glycemia, IGF-1 and the IGF-1/IGFBP3 ratio, and improved insulin sensitivity in patients with Barrett’s esophagus (BE). These alterations were associated with the down-regulation of the insulin/IGF-1 and ERK signaling pathways, which, in turn, decreased the risk of esophageal adenocarcinoma development [119]. Protein and calorie restriction altered the pharmacokinetics of irinotecan and improved the therapeutic window in patients with liver metastases of solid tumors treated with irinotecan [120].
Calorie restriction can influence melanoma development. Le Noci et al. showed that the use of caloric-restriction mimetics in mouse models of melanoma B16 reduced metastasis in the lungs. This effect was connected to the modulation of the immune microenvironment, including a significant increase in alveolar macrophages and CD103+ dendritic cells; an increase in M1 and a decrease in M2 markers in myeloid cells; the activation of CD3 T lymphocytes and NK cells; and an increased cytotoxic activity of effector cells in the lung [121]. The combined action of ropivacaine-loaded liposomes and calorie restriction effectively repressed melanoma B16 development and relieved cancer pain. These effects were associated with autophagy suppression and a reduction in the VEGF-A levels [122].
Thus, both obesity and calorie restriction regulate various signaling pathways associated with cancerogenesis, which can promote or suppress malignant tumor development (Figure 2).
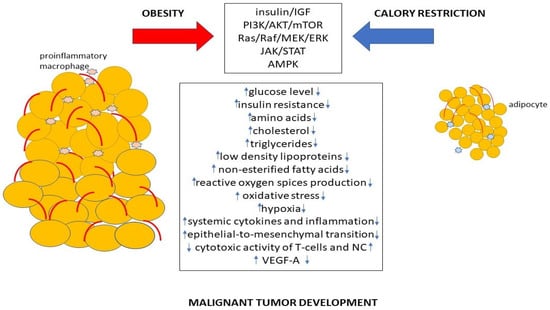
Figure 2. The mechanism of promotion or suppression of cancer development by obesity and calorie restriction.
The underlying mechanisms of calorie restriction influence on cancerogenesis is still unclear and can be associated with not only metabolic alterations. The CR effects can be connected with the regulation of splicing factors that leads to TORC pathway suppression [123]. Fasting reduces monocyte mobilization, which decreases the activity of inflammation via AMPK activation and suppression of CCL2 production [124]. CR can modulate enzyme activity. For example, decreased stearoyl-CoA desaturase (SCD) leads to imbalance between unsaturated and saturated fatty acids that can slow tumor growth [125]. The peculiarities of metabolism connected with race and age of patients can determine the CR effectivity [126]. On the other hand, starvation can increase mTORC1 signaling activity via deregulation of MiT/TFE-RagD-mTORC1-MiT/TFE feedback circuit [127]. Functional caloric restriction in T-cells triggered by elevated potassium ion levels lead to T cell disfunction and stemness [128].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24119601
This entry is offline, you can click here to edit this entry!
