Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fei Tong | -- | 3505 | 2023-06-14 10:05:18 | | | |
| 2 | Sirius Huang | Meta information modification | 3505 | 2023-06-16 03:05:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tong, F.; Sun, Z. Leadless Ventricular Pacemaker. Encyclopedia. Available online: https://encyclopedia.pub/entry/45553 (accessed on 15 January 2026).
Tong F, Sun Z. Leadless Ventricular Pacemaker. Encyclopedia. Available at: https://encyclopedia.pub/entry/45553. Accessed January 15, 2026.
Tong, Fei, Zhijun Sun. "Leadless Ventricular Pacemaker" Encyclopedia, https://encyclopedia.pub/entry/45553 (accessed January 15, 2026).
Tong, F., & Sun, Z. (2023, June 14). Leadless Ventricular Pacemaker. In Encyclopedia. https://encyclopedia.pub/entry/45553
Tong, Fei and Zhijun Sun. "Leadless Ventricular Pacemaker." Encyclopedia. Web. 14 June, 2023.
Copy Citation
Conventional transvenous pacemakers (TPMs) have been the cornerstone of the treatment of bradyarrhythmias. With technological advances in device miniaturization, communication, and battery longevity, leadless pacemakers (LPMs) have emerged as an alternative to TPMs to eliminate the complications associated with leads and subcutaneous pockets.
leadless single-chamber pacemaker
complication
atrioventricular synchrony
leadless dual-chamber pacemaker
1. Brief History and Current State of Two Leadless Systems
Nanostim (St.Jude, Saint Paul, MN, USA), as the first commercially available LPM capable of the VVI(R) pacing mode, was launched in 2012; however, the use of Nanostim implantation was discontinued due to the detachment of the docking button and premature battery failures, which occurred at two years after implantation [1]. The Aveir VR (Abbott, Abbott Park, IL, USA) by Abbott, which is an improvement of the Nanostim LPM, received Food and Drug Administration (FDA) approval on April 2022, and it could provide an expandable platform to support dual-chamber pacing once approved by the FDA [2].
The Micra VR (Medtronic, Minneapolis, MN, USA), which is now widely used all over the world, was first implanted in 2013 and it obtained FDA approval in 2016. The main indications for the Micra VR implantation are atrial fibrillation (AF) with slow ventricular response, as well as non-AF with low anticipated ventricular pacing, such as transient atrioventricular (AV) block and sinus node dysfunction [3][4]. The Micra AV (Medtronic) is the only currently available LPM that is capable of delivering the VDD pacing mode [5][6]. With an identical mass, appearance, design, and implant procedure to the Micra VR, the novel algorithm of the Micra AV discerns the signal of atrial mechanical contraction through the intracardiac accelerometer from the device in the right ventricle (RV) and fulfills AV synchrony. Limited by the mechanism of accelerometer-based atrial sensing rather than electric atrial sensing, and the absence of a pacing device in the right atrial (RA), the Micra AV is not suitable for those indicated for conventional DDD-TPMs, such as those with sick sinus syndrome and poor atrial contraction.
Elderly or malnourished patients with high infectious risk are prone to choosing LPMs [7][8]. Patients on haemodialysis would benefit from LPM implantation because it spares the subclavian and superior cava veins for dialysis treatment. The obstruction of the venous route used for TPM and potential pocket issues (e.g., in the case of dementia) are the indications for LPM [7]. Apart from patients with clinical frailty, younger patients also choose LPMs out of esthetical or active lifestyle concerns [9]. However, the scant clinical data regarding the end-of-life strategy and the possibility of implanting two or more LPMs in the same patient limit the routine use of LPMs in patients with a life expectancy of >20 years [7].
2. Evaluation of Clinical Performance and Recommendation of Strategies
The success rate of LPM implantation was extraordinarily high for the Micra VR, with 99.2% (719/725) in the Micra Investigational Device Exemption (IDE) trial [10] and 99.1% (1801/1817) in a real-world setting [11]. The success rate was 98% (196/200) for the Aveir VR in the LEADLESS II phase 2 IDE study. The mean pacing threshold and R-wave amplitude of the Micra VR were 0.66 ± 0.55 V at 0.24 ms and 11.1 ± 5.2 mV at implantation, and the electrical parameters remained stable during 18 months of follow-up [11]. A total of 196 patients were successfully implanted with the Aveir VR. For 95.9% of these patients, the pacing thresholds were less than 2.0 V at 0.4 ms and the R waves were greater than 5.0 mV at the 6 week follow-up [2]. However, no clear consensus has been determined in terms of the complication rate of LPMs when compared to TPMs. The Micra VR was associated with 48% and 63% lower risks of major complications than those of a historical TPM cohort in the Micra IDE trial [10] and the Micra Post-Approval Registry (PAR) [11], respectively, during the 12 month follow-up period. The analyses were based on the comparison with a historical TPM cohort and a long-term follow-up period [10][11]. However, a meta-analysis on four studies showed no difference in the incidences of any complications between LPMs and TPMs [12]. Moreover, in a prospective analysis, no significant difference at an almost 2 year complication rate was observed between LPMs and TPMs [13], which could be because the contemporary complication rate of TPMs is significantly lower than the historical one as a result of standard implantation procedures and improved techniques. However, a contemporary prospective propensity-matched analysis also demonstrated that the rate of complication in a TPM cohort was 4.9% vs. 0.9% in a LPM cohort, during 800 days of follow-up and after excluding the pacemaker advisory-related complications [14]. Furthermore, in real-world practice, Micra implantation (n = 16,825) is associated with a lower complication rate of 8.6%, which is lower than the 11.2% of contemporary TPM implantation (n = 564,100) [15]. A continuous enrollment study and contemporaneous comparison of the Micra and TPMs in the Micra Coverage with Evidence Development (CED) study observed that the Micra implantation was associated with 23% fewer and 31% fewer complications compared with TPMs over 6 months [16] and 2 years [17], respectively, indicating that the fewer LPM complications were due to a time-dependent effect, which was also manifested by the improved LPM complication rates from the 6 month follow-up to the 2 year follow-up, and the similar 30 day adjusted complication rate of LPMs to that of TPMs [16]. A large and real-world analysis of the national database from the United States showed that, overall, the unadjusted in-hospital complication rate of 16% for LPMs was higher than that of the 6.4% for TPMs; however, it should be noted that the patients with LPMs in this analysis were older and had more sepsis, chronic kidney disease, heart failure and malnutrition [8]. Transvenous lead- and subcutaneous-pocket-related complications account for most of these TPM complications [18][19], and they take time to occur. In contrast, the more frequent incidences of cardiac perforation and pericardial effusion in LPMs than TPMs and the relatively high incidence of vascular complications in LPMs are short-term complications, both of which form a non-conclusive picture of the complications encountered with LPMs vs. TPMs. The discrepancies of the complication percentages between studies are partly because some studies only report complications requiring reinterventions [14], while some studies describe all complications [15].
2.1. Cardiac Perforation and Pericardial Effusion
The overall rate of cardiac perforation and pericardial effusion in the Micra IDE, Micra PAR, and Micra Continued Access study was 1.1% (32/2817) [20]. The risk of pericardial effusion after the implantation of Micra decreased from 1.8% to 0.8% over time [20]. The rate of pericardial effusion was 0.8% in the Micra CED study. The Manufacturer and User Facility Device Experience (MAUDE) report also estimated a 1% incidence of cardiac tamponade, and more than three times the cardiac tamponade of the Micra compared with the TPM ventricular lead [21]. In a real-world setting, 1.3% of LPM recipients suffered from cardiac effusion or perforation [8], and the figure was significantly higher than that of TPM recipients [15]. Similarly, as for Aveir VR implantation, the rate of cardiac tamponade was 1.5% (3/200) in the LEADLESS II-phase 2 IDE study [2]. A relatively high rate of cardiac perforation following LPM implantation could be worrying; hence, researchers recommend measures to be taken and strategies to limit those adverse events.
Patients’ characteristics: Apart from the patients’ characteristics, such as old age, female gender, and chronic obstructive pulmonary disease (COPD) that easily develops cardiac injury [10], a low body mass index (BMI) and congestive heart failure were also identified by the PAR investigators as predictors of perforation [11]. A novel risk score model, which included similar risk factors such as age of >85 years, BMI of <20, female gender, heart failure, prior myocardial infarction, COPD, haemodialysis, and the absence of previous cardiothoracic surgery, was developed and validated to predict pericardial effusion [20]. Additional attention should be paid to the presence of the abovementioned risk factors.
Procedure technique: The positioning of the Micra at the interventricular septum has been linked to a lower risk of cardiac perforation compared with the apex or free wall [10], and deployment in the mid-septum should be achieved [22], which leads to a narrower-paced QRS complex [23]. However, deploying a Micra near the anterior interventricular groove might increase the risk of perforation [24]. Contrast injection is recommended to ensure a mid-septal position in orthogonal fluoroscopic views prior to deployment [22], and RV trabeculation in front of and inferior to the tip of the delivery catheter suggests the septum location, as opposed to a lack of trabeculation, which suggests the apical location [22] (Figure 1). The combination of orthogonal fluoroscopic views and transthoracic echocardiography in the subxiphoid, parasternal, and apical views without contrast injection significantly reduces inadvertent non-septal implantation compared with ventriculography [25]. The pacing threshold will frequently improve over 2–3 min, and especially when the impedance suggests good myocardial apposition (>500 ohms). Therefore, it is reasonable to allow at least 3 min for the thresholds to improve before deciding on an alternate location [26]. As for the sensed R-wave, a 1.5-mV increase in R-wave amplitude after approximately 13 min since the first deployment is predictive of a reduction of pacing threshold below 1 V/0.24 ms at follow-up, which also suggests that waiting and evaluating a second electrical parameter may be capable to avoid unnecessary device repositioning in case of high pacing threshold recording at implant [27].
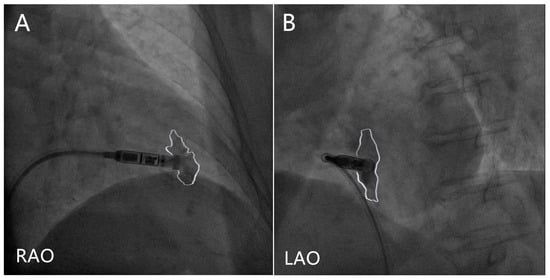
Figure 1. Contrast injection in right anterior oblique (RAO) and left anterior oblique (LAO) projections. The white lines delineate the contrast outlines. (A) The contrast seen both in front of and inferior to the tip of the delivery catheter in RAO projection suggests a non-apical location. (B) The contrast delineates the ventricular septum in LAO projection.
Device design: The tine-based fixation mechanism of the Micra permits the measurement of the electrical parameters only after deployment and active fixation into the myocardium, which inevitably increases the need for repeated attempts at deployment once the parameters are unsatisfied and there is a risk of pericardial effusion [20][28]. Different from the Micra, the contact mapping capability of the Aveir VR helps to optimally position the device before fixation [2][29], which could prevent the need for multiple attempts. Of the successful implants, 83.2% (163/196) of the Aveir VR implantation did not require repositioning in the LEADLESS II phase 2, while one deployment sufficed in only 60.0% (1583/2638) of the Micra implantations [20]. In contrast to adequate forward pressure applied to form the “goose neck” appearance of the delivery system prior to the implantation of the tine-based Micra, firm pressure is not required nor recommended and a softer touch with tissue contact is sufficient for the Aveir VR to achieve stable fixation via the slow rotation of device [29].
Rescue strategies: Unlike transvenous lead perforations, Micra perforations are often large and life-threatening. The MAUDE reports a higher rate of mortality following perforations with the Micra compared with TPMs [21]; thus, intervention that includes pericardiocentesis and surgery is required to rescue patients. In the Micra PAR, 71% (10/14) of the cardiac effusion patients required pericardiocentesis and 14% (2/14) required surgical repair [11]. Data from a real-world setting showed that 36% (82/228) of pericardial effusion patients had the need for pericardiocentesis, and 11.5% (26/228) required a thoracotomy. A higher proportion (26% (146/563)) of Micra-related perforations requiring emergency surgery was also reported in the MAUDE [30].
2.2. Dislodgment
Micra dislodgment was rare and limited to case reports with no dislodgment in the Micra IDE trial [10]. A total of 0.06% of the implantations resulted in dislodgment in the Micra PAR [11], and 0.51% (40/7821) of the implantations resulted in dislodgment in the real-world setting, in comparison with the relatively high rate of lead dislodgment in TPMs, which ranged between 1% and 2.55% [31]. The dislodged device could migrate to a remote location, such as the pulmonary artery, causing acute respiratory failure [32], or could produce no symptoms if the LPM is wedged in a stable manner [33], or it could be stuck in the right ventricle, causing non-sustained ventricular tachycardia [34] and damage to the TV and papillary muscles. Some types of dislodgment only manifested the loss of capture without obvious dislocation [35].
Patients’ characteristics: Due to the limited amount of data, no risk factors concerning the underlying etiology for dislodgment were identified. Based on the currently available case reports, a complex heart anatomy [34][36] and myocardial fibrosis or scars in cardiac amyloidosis or ischemic cardiomyopathy might influence the engagement of the tines [34].
Procedure technique: The fixation of at least two tines is acceptable for the Micra according to the manufacturer’s training recommendation; however, several dislodgment cases met the criterion of two tines [32][37][38], which indicates that the movement of two tines by the pull-and-hold test is not a guarantee against dislodgment, and that the pull-and-hold test, per se, is not an objective evaluation of the tine movement. A stable and low pacing threshold (<1 V at 0.24 ms is ideal; however, ≤2 V at 0.24 ms is considered acceptable), high sensed R wave, high impedance, and the recorded current of the injury may indicate solid fixation. Unstable impedance by the repeated measurement could suggest an insecure connection between the device and myocardium [37]. Final implant thresholds above 2 V are not recommended [26]. In situations of multiple reposition failures, it is recommended to remove and re-flush the delivery system to clean off clots, and an effective strategy is to implant LPM in the apical position and to obtain the R-wave at approximately 10 mV and the pacing threshold far below 1V in a series of three to four interrogations [39]. This strategy can be attempted by experienced operators, and is not recommended for operators at the beginning of the learning curve in consideration of the risk of cardiac perforation [39].
Rescue strategies: A Micra introducer sheath with either a delivery catheter or steerable Agilis sheath is implemented to align with the Micra with an acute rise in capture threshold, but without obvious dislocation noted, and loop snares of different sizes (range 7–10 mm) and shape (single loop or multiple loop) with integrated protective sleeves are used to capture the proximal retrieval feature or the body of LPM if the proximal retrieval feature cannot be engaged [40]. In complex situations, such as the migration of the device to the pulmonary artery or its free movement in the RV, the two-snare technique is applied as follows: one snare captures any tine that is not engaged in the endocardium to minimize the Micra movement, and a second snare captures the retrieval feature or the body of the Micra capsule [34][35][37][41]. Additionally, a gooseneck snare from the femoral venous approach can also be used to retrieve LPM, embolizing the pulmonary artery [42].
2.3. Vascular Complications
The Micra VR and Aveir VR are both delivered through 27F (outer diameter) introducer sheaths. Large-bore venous access for LPM implantation could induce vascular complications, such as arteriovenous fistulas (AVFs), pseudoaneurysms, bleeding and hematomas. Incidences of 0.6–1.4% for vascular complications were reported in the Micra PAR study [11] and Micra CED study [16].
Medicine preparation: The Micra VR and Aveir VR are approved for patients with AF complicated by bradycardia. The results from the Micra PAR indicated whether the intermittent interruption of anticoagulation in the perioperative setting could not significantly influence vascular-related events [43], which meant that the Micra without the interruption of anticoagulation could be performed. Although no suggestions from the guidelines are provided on the perioperative anticoagulation management in LPM procedures, continued warfarin if the international normalized ratio is <3 and the temporary interruption of new oral anticoagulants 24 h before the LPM procedure may be safe and have already been applied in clinical practice [43][44][45].
Procedure technique: A puncture site of the femoral vein just higher or at the level of insertion of the great saphenous vein is recommended to reduce the risk of AVF [22]. A puncture site below the common femoral artery or vein is a risk factor for pseudoaneurysm. In complex cases in which multiple arteries are inadvertently punctured, vascular ultrasound guidance or micropuncture techniques could be considered to avoid vascular complications [43]. More than half of the cases of haemostasis following LPM implantation were achieved using figure-of-eight sutures [46], and the application of pressure alone should be avoided for the 27F introducer sheath [22]. Surgical isolation of the common femoral vein for the purpose of sheath insertion in patients with severe obesity and vascular haemostasis and suture performed by vascular surgeon were effective and safe in LPM implantation [47].
Rescue strategies: Studies on AVF indicated that 38% of the cases of iatrogenic femoral AVF self-resolved at 1 year [48] and that iatrogenic AVFs should be repaired using a covered stent to seal the shunting of the AVF only when the shunting has hemodynamic consequences [49]. However, these AVFs were vascular access complications of percutaneous coronary interventions, in which sheath sizes of 7F or 8F were used. As for LPM implantation, in which a sheath of 27F is used, intervention or surgical repair may be a necessary choice. A stable pseudoaneurysm diameter of <2 cm can be conservatively managed with observation, and a pseudoaneurysm diameter of >2 cm can be managed with ultrasound-guided thrombin injection, surgical repair, or covered stent placement [50].
2.4. Infection
By virtue of the elimination of surgical pockets and transvenous leads, less surface area and endovascular encapsulation, LPMs have a low incidence of infection, as no infections were identified among the 3726 patients with LPMs during the 6 month follow-up in the Micra CED study [16]. A total of 33 infections out of 726 cases was recorded in the Micra IDE trial [10]; however, none of these events were associated with device- or procedure-related infections. Procedure-related infections, including abdominal wall infections, infected groin hematomas, and sepsis, occurred at 0.17% in the Micra PAR [11]; however, none of these infection cases required device removal. As for the Aveir VR, no device-related infections have been reported so far [2]. LPMs have been shown to have some unique characteristics that make them suitable for patients with high infectious risk, and in situations in which infected transvenous leads or pockets have already occurred. Suggestions according to the different scenarios are listed as follows:
Medicine preparation: Almost all the patients in an Italian clinical practice were given a prophylactic dose of antibiotics before the LPM implantation procedure without additional adverse events [29][46]; however, the specific prophylactic antibiotic usage for LPMs in the perioperative setting needs further exploration.
Application strategies: No evidence of recurrent infection was found following the LPM implantation at or after the infected TPM removal [28][51][52][53]. In the case of lead- or pocket-related infections, the LPM implantation could even be performed before the extraction of an infected TPM for pacemaker-dependent patients, without the occurrence of reinfection [53][54]. In cases of bacteremia or endocarditis, it is recommended that the LPM not be implanted until the blood cultures turn negative, and for pacemaker-dependent patients, temporary pacing through jugular access is the interim solution after the removal of an infected TPM and prior to LPM implantation.
2.5. Tricuspid Valve Regurgitation
The development of tricuspid valve regurgitation (TR) after TPM implantation is primarily caused by the mechanical interference of the ventricular leads with the TV and its sub-valvular apparatus [55]. Despite the absence of leads crossing the TV, LPMs with lengths of 42 mm for the Nanostim, and with lengths of 25.9 mm for the Micra, still have the potential to interact with the valvular apparatus. The aggravation of TR was observed in 12% of patients with LPMs during the 48 month follow-up when compared with 9% of patients with TPMs [56]. However, an age- and sex-matched analysis showed a higher increase in the TR severity in patients with TPMs than in those with LPMs [57], and the LPMs had the advantage of reducing the TR effective regurgitant orifice area, compared with conventional leads 1 month after the device implantation [58]. The TR increased 12 months after the LPM implantation; however, it was comparable to that of TPMs [59]. The conclusion concerning TR following LPM implantation is controversial, which is possibly because of the differences in the definition of TR, the follow-up period, and the proportion of the Nanostim, with a greater length than the Micra. To minimize the potential influence of LPMs on the TV, suggestions are provided for consideration.
Procedure technique: A septal implantation of the Micra is recommended to reduce the risk of cardiac perforation, which may not be a risk factor for the worsening of TR. Apical septal implantation is considered desirable to avoid the entrapment of the docking button or proximal retrieval feature within the tricuspid valve apparatus. However, a basal implantation site close to the TV annulus should be avoided to minimize mechanical interference with the valvular apparatus [59]. Despite being designed 10% shorter than its predecessor the Nanostim, the Aveir VR, which is 38.0 mm in length, is still longer than the Micra [29], and therefore the Aveir VR implantation site should maintain more of a distance from the TV and anterior interventricular groove if possible. Physicians should balance the long-term benefit of the TR severity with the short-term risk of pericardial effusion.
3. Gaps in Experience
Worldwide experience demonstrated that the early retrieval of Micra (median 46 days, range 1–95 days) was feasible and can be accomplished with low risk of serious complications, such as cardiac perforation, and device embolization [40]. However, the long-term experience with Micra retrieval is limited. The Micra VR was designed with 12 years of battery life, and the battery longevity was confirmed in the real-world setting [60]. As for the Micra AV, the original design of an 11.8 year battery life (1 V, 0.24 ms) with 100% pacing was reduced to 10.5 years in the real-world setting [61]. The anticipated encapsulation of LPMs during the whole battery life could further impede the extractability of LPMs. There is no recommended treatment for LPMs after battery depletion. Physicians can either retrieve the nonfunctioning LPMs and subsequently implant a new device, or abandon the nonfunctioning LPMs and implant a new adjacent one [62]. Even though the RV can host up to three Micra devices [63], this could still lead to geometric alterations of the cardiac anatomy and have a negative impact on the ventricular volume.
References
- Lakkireddy, D.; Knops, R.; Atwater, B.; Neuzil, P.; Ip, J.; Gonzalez, E.; Friedman, P.; Defaye, P.; Exner, D.; Aonuma, K.; et al. A worldwide experience of the management of battery failures and chronic device retrieval of the nanostim leadless pacemaker. Hear Rhythm 2017, 14, 1756–1763.
- Reddy, V.Y.; Exner, D.V.; Doshi, R.; Tomassoni, G.; Bunch, T.J.; Estes, N.A.M.; Neužil, P.; Paulin, F.L.; Garcia Guerrero, J.J.; Cantillon, D.J.; et al. Primary results on safety and efficacy from the LEADLESS II-phase 2 worldwide clinical trial. JACC Clin. Electrophysiol. 2022, 8, 115–117.
- Piccini, J.P.; Stromberg, K.; Jackson, K.P.; Kowal, R.C.; Duray, G.Z.; El-Chami, M.F.; Crossley, G.H.; Hummel, J.D.; Narasimhan, C.; Omar, R.; et al. Patient selection, pacing indications, and subsequent outcomes with de novo leadless single-chamber VVI pacing. Europace 2019, 21, 1686–1693.
- Steinwender, C.; Lercher, P.; Schukro, C.; Blessberger, H.; Prenner, G.; Andreas, M.; Kraus, J.; Ammer, M.; Stühlinger, M. State of the art: Leadless ventricular pacing:a national expert consensus of the Austrian Society of Cardiology. J. Interv. Card. Electrophysiol. 2020, 57, 27–37.
- Chinitz, L.; Ritter, P.; Khelae, S.K.; Iacopino, S.; Garweg, C.; Grazia-Bongiorni, M.; Neuzil, P.; Johansen, J.B.; Mont, L.; Gonzalez, E.; et al. Accelerometer-based atrioventricular synchronous pacing with a ventricular leadless pacemaker: Results from the Micra atrioventricular feasibility studies. Heart Rhythm 2018, 15, 1363–1371.
- Steinwender, C.; Khelae, S.K.; Garweg, C.; Chan, J.Y.S.; Ritter, P.; Johansen, J.B.; Sagi, V.; Epstein, L.M.; Piccini, J.P.; Pascual, M.; et al. Atrioventricular synchronous pacing using a leadless ventricular pacemaker: Results from the MARVEL 2 study. JACC Clin. Electrophysiol. 2020, 6, 94–106.
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520.
- Tonegawa-Kuji, R.; Kanaoka, K.; Mori, M.; Nakai, M.; Iwanaga, Y. Mortality and 30-day readmission rates after inpatient leadless pacemaker implantation: Insights from a nationwide readmissions database. Can. J. Cardiol. 2022, 38, 1697–1705.
- Gulletta, S.; Schiavone, M.; Gasperetti, A.; Breitenstein, A.; Palmisano, P.; Mitacchione, G.; Chierchia, G.B.; Montemerlo, E.; Statuto, G.; Russo, G.; et al. Peri-procedural and mid-term follow-up age-related differences in leadless pacemaker implantation: Insights from a multicenter European registry. Int. J. Cardiol. 2023, 371, 197–203.
- Reynolds, D.; Duray, G.Z.; Omar, R.; Soejima, K.; Neuzil, P.; Zhang, S.; Narasimhan, C.; Steinwender, C.; Brugada, J.; Lloyd, M.; et al. A leadless intracardiac transcatheter pacing system. N. Engl. J. Med. 2016, 374, 533–541.
- El-Chami, M.F.; Al-Samadi, F.; Clementy, N.; Garweg, C.; Martinez-Sande, J.L.; Piccini, J.P.; Iacopino, S.; Lloyd, M.; Viñolas Prat, X.; Jacobsen, M.D.; et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control. Heart Rhythm 2018, 15, 1800–1807.
- Darlington, D.; Brown, P.; Carvalho, V.; Bourne, H.; Mayer, J.; Jones, N.; Walker, V.; Siddiqui, S.; Patwala, A.; Kwok, C.S. Efficacy and safety of leadless pacemaker: A systematic review, pooled analysis and meta-analysis. Indian Pacing Electrophysiol. 2022, 22, 77–86.
- Bertelli, M.; Toniolo, S.; Ziacchi, M.; Gasperetti, A.; Schiavone, M.; Arosio, R.; Capobianco, C.; Mitacchione, G.; Statuto, G.; Angeletti, A.; et al. Is less always more? A prospective two-centre study addressing clinical outcomes in leadless versus transvenous single-chamber pacemaker recipients. J. Clin. Med. 2022, 11, 6071.
- Tjong, F.V.Y.; Knops, R.E.; Udo, E.O.; Brouwer, T.F.; Dukkipati, S.R.; Koruth, J.S.; Petru, J.; Sediva, L.; van Hemel, N.M.; Neuzil, P.; et al. Leadless pacemaker versus transvenous single-chamber pacemaker therapy: A propensity matched analysis. Heart Rhythm 2018, 15, 1387–1393.
- Vincent, L.; Grant, J.; Peñalver, J.; Ebner, B.; Maning, J.; Olorunfemi, O.; Goldberger, J.J.; Mitrani, R.D. Early trends in leadless pacemaker implantation: Evaluating nationwide in-hospital outcomes. Heart Rhythm 2022, 19, 1334–1342.
- Piccini, J.P.; El-Chami, M.; Wherry, K.; Crossley, G.H.; Kowal, R.C.; Stromberg, K.; Longacre, C.; Hinnenthal, J.; Bockstedt, L. Contemporaneous comparison of outcomes among patients implanted with a leadless vs. transvenous single chamber ventricular pacemaker. JAMA Cardiol. 2021, 6, 1187–1195.
- El-Chami, M.F.; Bockstedt, L.; Longacre, C.; Higuera, L.; Stromberg, K.; Crossley, G.; Kowal, R.C.; Piccini, J.P. Leadless vs. transvenous single-chamber ventricular pacing in the Micra CED study: 2-year follow-up. Eur. Heart J. 2022, 43, 1207–1215.
- Udo, E.O.; Zuithoff, N.P.; van Hemel, N.M.; de Cock, C.C.; Hendriks, T.; Doevendans, P.A.; Moons, K.G. Incidence and predictors of short- and long-term complications in pacemaker therapy: The FOLLOWPACE study. Heart Rhythm 2012, 9, 728–735.
- Kirkfeldt, R.E.; Johansen, J.B.; Nohr, E.A.; Jorgensen, O.D.; Nielsen, J.C. Complications after cardiac implantable electronic device implantations: An analysis of a complete, nationwide cohort in Denmark. Eur. Heart J. 2014, 35, 1186–1194.
- Piccini, J.P.; Cunnane, R.; Steffel, J.; El-Chami, M.F.; Reynolds, D.; Roberts, P.R.; Soejima, K.; Steinwender, C.; Garweg, C.; Chinitz, L.; et al. Development and validation of a risk score for predicting pericardial effusion in patients undergoing leadless pacemaker implantation: Experience with the Micra transcatheter pacemaker. Europace 2022, 24, 1119–1126.
- Hauser, R.G.; Gornick, C.C.; Abdelhadi, R.H.; Tang, C.Y.; Casey, S.A.; Sengupta, J.D. Major adverse clinical events associated with implantation of a leadless intracardiac pacemaker. Heart Rhythm 2021, 18, 1132–1139.
- Okabe, T.; Afzal, M.R.; Houmsse, M.; Makary, M.S.; Elliot, E.D.; Daoud, E.G.; Augostini, R.S.; Hummel, J.D. Tine-based leadless pacemaker: Strategies for safe implantation in unconventional clinical scenarios. JACC Clin. Electrophysiol. 2020, 6, 1318–1331.
- Garweg, C.; Vandenberk, B.; Foulon, S.; Haemers, P.; Ector, J.; Willems, R. Leadless pacing with Micra TPS: A comparison between right ventricular outflow tract, mid-septal, and apical implant sites. J. Cardiovasc. Electrophysiol. 2019, 30, 2002–2011.
- Chen, X.; Huang, W. Strategies to overcome complicated situations in leadless pacemaker implantation. Pacing Clin. Electrophysiol. 2021, 44, 1959–1962.
- Bhardwaj, R.; Kewcharoen, J.; Contractor, T.; Nayak, S.; Ai, S.; Kim, U.; Mandapati, R.; Garg, J. Echocardiogram-guided leadless pacemaker implantation. JACC Clin. Electrophysiol. 2022, 8, 1581–1582.
- Lloyd, M.S.; El-Chami, M.F.; Nilsson, K.R., Jr.; Cantillon, D.J. Transcatheter/leadless pacing. Heart Rhythm 2018, 15, 624–628.
- Mitacchione, G.; Arabia, G.; Schiavone, M.; Cerini, M.; Gasperetti, A.; Salghetti, F.; Bontempi, L.; Viecca, M.; Curnis, A.; Forleo, G.B. Intraoperative sensing increase predicts long-term pacing threshold in leadless pacemakers. J. Interv. Card. Electrophysiol. 2022, 63, 679–686.
- Mitacchione, G.; Schiavone, M.; Gasperetti, A.; Arabia, G.; Breitenstein, A.; Cerini, M.; Palmisano, P.; Montemerlo, E.; Ziacchi, M.; Gulletta, S.; et al. Outcomes of Leadless Pacemaker implantation following transvenous lead extraction in high-volume referral centers: Real-world data from a large international registry. Heart Rhythm 2022, 20, 395–404.
- Laczay, B.; Aguilera, J.; Cantillon, D.J. Leadless cardiac ventricular pacing using helix fixation: Step-by-step guide to implantation. J. Cardiovasc. Electrophysiol. 2023, 34, 748–759.
- Hauser, R.G.; Gornick, C.C.; Abdelhadi, R.H.; Tang, C.Y.; Kapphahn-Bergs, M.; Casey, S.A.; Okeson, B.K.; Steele, E.A.; Sengupta, J.D. Leadless pacemaker perforations: Clinical consequences and related device and user problems. J. Cardiovasc. Electrophysiol. 2022, 33, 154–159.
- Wang, Y.; Hou, W.; Zhou, C.; Yin, Y.; Lu, S.; Liu, G.; Duan, C.; Cao, M.; Li, M.; Toft, E.S.; et al. Meta-analysis of the incidence of lead dislodgement with conventional and leadless pacemaker systems. Pacing Clin. Electrophysiol. 2018, 41, 1365–1371.
- Terricabras, M.; Khaykin, Y. Successful leadless pacemaker retrieval from the left pulmonary artery: A case report. Heart Rhythm Case Rep. 2020, 6, 798–799.
- Sugiura, K.; Baba, Y.; Hirota, T.; Kubo, T.; Kitaoka, H. A drifting dislodged leadless pacemaker in the bilateral pulmonary arteries. JACC Case Rep. 2022, 4, 844–846.
- Fichtner, S.; Estner, H.L.; Näbauer, M.; Hausleiter, J. Percutaneous extraction of a leadless Micra pacemaker after dislocation: A case report. Eur. Heart J. Case Rep. 2019, 3, ytz113.
- Karim, S.; Abdelmessih, M.; Marieb, M.; Reiner, E.; Grubman, E. Extraction of a Micra transcatheter pacing system: First-in-human experience. Heart Rhythm Case Rep. 2015, 2, 60–62.
- Sterliński, M.; Demkow, M.; Plaskota, K.; Oręziak, A. Percutaneous extraction of a leadless Micra pacemaker from the pulmonary artery in a patient with complex congenital heart disease and complete heart block. EuroIntervention 2018, 14, 236–237.
- Hasegawa-Tamba, S.; Ikeda, Y.; Tsutsui, K.; Kato, R.; Muramatsu, T.; Matsumoto, K. Two-directional snare technique to rescue detaching leadless pacemaker. Heart Rhythm Case Rep. 2020, 6, 711–714.
- Roberts, P.R.; Clementy, N.; Al Samadi, F.; Garweg, C.; Martinez-Sande, J.L.; Iacopino, S.; Johansen, J.B.; Vinolas Prat, X.; Kowal, R.C.; Klug, D.; et al. A leadless pacemaker in the real-world setting: The Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm 2017, 14, 1375–1379.
- Sterliński, M.; Demkow, M.; Oręziak, A.; Szumowski, Ł. What is retrieved must dislocate first: Few consideration how to avoid leadless pacemaker escape. Pacing Clin. Electrophysiol. 2021, 44, 1137–1138.
- Afzal, M.R.; Daoud, E.G.; Cunnane, R.; Mulpuru, S.K.; Koay, A.; Hussain, A.; Omar, R.; Wei, K.K.; Amin, A.; Kidwell, G.; et al. Techniques for successful early retrieval of the Micra transcatheter pacing system: A worldwide experience. Heart Rhythm 2018, 15, 841–846.
- Romeo, E.; D’Alto, M.; Cappelli, M.; Nigro, G.; Correra, A.; Colonna, D.; Sarubbi, B.; Golino, P. Retrieval of a leadless transcatheter pacemaker from the right pulmonary artery: A case report. Pacing Clin. Electrophysiol. 2021, 44, 952–954.
- Sundaram, S.; Choe, W. The one that got away: A leadless pacemaker embolizes to the lungs. Heart Rhythm 2016, 13, 2316.
- El-Chami, M.F.; Garweg, C.; Iacopino, S.; Al-Samadi, F.; Martinez-Sande, J.L.; Tondo, C.; Johansen, J.B.; Prat, X.V.; Piccini, J.P.; Cha, Y.M.; et al. Leadless pacemaker implant, anticoagulation status, and outcomes: Results from the Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm 2022, 19, 228–234.
- Kiani, S.; Black, G.B.; Rao, B.; Thakkar, N.; Massad, C.; Patel, A.V.; Merchant, F.M.; Hoskins, M.H.; De Lurgio, D.B.; Patel, A.M.; et al. Outcomes of Micra leadless pacemaker implantation with uninterrupted anticoagulation. J. Cardiovasc. Electrophysiol. 2019, 30, 1313–1318.
- San Antonio, R.; Chipa-Ccasani, F.; Apolo, J.; Linhart, M.; Trotta, O.; Pujol-López, M.; Niebla, M.; Alarcón, F.; Trucco, E.; Arbelo, E.; et al. Management of anticoagulation in patients undergoing leadless pacemaker implantation. Heart Rhythm. 2019, 16, 1849–1854.
- Palmisano, P.; Iacopino, S.; De Vivo, S.; D’Agostino, C.; Tomasi, L.; Startari, U.; Ziacchi, M.; Pisanò, E.C.L.; Santobuono, V.E.; Caccavo, V.P.; et al. Leadless transcatheter pacemaker: Indications, implantation technique and peri-procedural patient management in the Italian clinical practice. Int. J. Cardiol. 2022, 365, 49–56.
- Malagù, M.; D’Aniello, E.; Vitali, F.; Balla, C.; Gasbarro, V.; Bertini, M. Leadless pacemaker implantation in superobese patient. Rev. Cardiovasc. Med. 2022, 23, 125.
- Kelm, M.; Perings, S.M.; Jax, T.; Lauer, T.; Schoebel, F.C.; Heintzen, M.P.; Perings, C.; Strauer, B.E. Incidence and clinical outcome of iatrogenic femoral arteriovenous fistulas: Implications for risk stratification and treatment. J. Am. Coll. Cardiol. 2002, 40, 291–297.
- Zilinyi, R.S.; Sethi, S.S.; Parikh, M.A.; Parikh, S.A. Iatrogenic arteriovenous fistula following femoral access precipitating high-output heart failure. JACC Case Rep. 2021, 3, 421–424.
- Madia, C. Management trends for postcatheterization femoral artery pseudoaneurysms. JAAPA 2019, 32, 15–18.
- Chang, D.; Gabriels, J.K.; Soo Kim, B.; Ismail, H.; Willner, J.; Beldner, S.J.; John, R.M.; Epstein, L.M. Concomitant leadless pacemaker implantation and lead extraction during an active infection. J. Cardiovasc. Electrophysiol. 2020, 31, 860–867.
- Beurskens, N.E.G.; Tjong, F.V.Y.; Dasselaar, K.J.; Kuijt, W.J.; Wilde, A.A.M.; Knops, R.E. Leadless pacemaker implantation after explantation of infected conventional pacemaker systems: A viable solution? Heart Rhythm 2019, 16, 66–71.
- Breeman, K.T.N.; Beurskens, N.E.G.; Driessen, A.H.G.; Wilde, A.A.M.; Tjong, F.V.Y.; Knops, R.E. Timing and mid-term outcomes of using leadless pacemakers as replacement for infected cardiac implantable electronic devices. J. Interv. Card. Electrophysiol. 2022.
- Bicong, L.; Allen, J.C.; Arps, K.; Al-Khatib, S.M.; Bahnson, T.D.; Daubert, J.P.; Frazier-Mills, C.; Hegland, D.D.; Jackson, K.P.; Jackson, L.R.; et al. Leadless pacemaker implantation after lead extraction for cardiac implanted electronic device infection. J. Cardiovasc. Electrophysiol. 2022, 33, 464–470.
- Al-Mohaissen, M.A.; Chan, K.L. Prevalence and mechanism of tricuspid regurgitation following implantation of endocardial leads for pacemaker or cardioverter-defibrillator. J. Am. Soc. Echocardiogr. 2012, 25, 245–252.
- Sasaki, K.; Togashi, D.; Nakajima, I.; Suchi, T.; Nakayama, Y.; Harada, T.; Akashi, Y.J. Clinical outcomes of non-atrial fibrillation bradyarrhythmias treated with a ventricular demand leadless pacemaker compared with an atrioventricular synchronous transvenous pacemaker-a propensity score-matched analysis. Circ. J. 2022, 86, 1283–1291.
- Vaidya, V.R.; Dai, M.; Asirvatham, S.J.; Rea, R.F.; Thome, T.M.; Srivathsan, K.; Mulpuru, S.K.; Kusumoto, F.; Venkatachalam, K.L.; Ryan, J.D.; et al. Real-world experience with leadless cardiac pacing. Pacing Clin. Electrophysiol. 2019, 42, 366–373.
- Ohta, Y.; Goda, A.; Daimon, A.; Manabe, E.; Masai, K.; Kishima, H.; Mine, T.; Asakura, M.; Ishihara, M. The differences between conventional lead, thin lead, and leadless pacemakers regarding effects on tricuspid regurgitation in the early phase. J. Med. Ultrason. 2023, 50, 51–56.
- Beurskens, N.E.G.; Tjong, F.V.Y.; de Bruin-Bon, R.H.A.; Dasselaar, K.J.; Kuijt, W.J.; Wilde, A.A.M.; Knops, R.E. Impact of leadless pacemaker therapy on cardiac and atrioventricular valve function through 12 months of follow-up. Circ. Arrhythm. Electrophysiol. 2019, 12, e007124.
- Breeman, K.T.N.; Oosterwerff, E.F.J.; Dijkshoorn, L.A.; Salavati, A.; Beurskens, N.E.G.; Wilde, A.A.M.; Delnoy, P.H.M.; Tjong, F.V.Y.; Knops, R.E. Real-world long-term battery longevity of Micra leadless pacemakers. J. Interv. Card. Electrophysiol. 2022.
- Garweg, C.; Piccini, J.P.; Epstein, L.M.; Frazier-Mills, C.; Chinitz, L.A.; Steinwender, C.; Stromberg, K.; Sheldon, T.; Fagan, D.H.; El-Chami, M.F. Correlation between AV synchrony and device collected AM-VP sequence counter in atrioventricular synchronous leadless pacemakers: A real-world assessment. J. Cardiovasc. Electrophysiol. 2023, 34, 197–206.
- Grubman, E.; Ritter, P.; Ellis, C.R.; Giocondo, M.; Augostini, R.; Neuzil, P.; Ravindran, B.; Patel, A.M.; Omdahl, P.; Pieper, K.; et al. To retrieve, or not to retrieve: System revisions with the Micra transcatheter pacemaker. Heart Rhythm 2017, 14, 1801–1806.
- Omdahl, P.; Eggen, M.D.; Bonner, M.D.; Iaizzo, P.A.; Wika, K. Right ventricular anatomy can accommodate multiple Micra transcatheter pacemakers. Pacing Clin. Electrophysiol. 2016, 39, 393–397.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
578
Revisions:
2 times
(View History)
Update Date:
16 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




