Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | lingyun Wang | -- | 3719 | 2023-06-14 09:58:29 | | | |
| 2 | Catherine Yang | Meta information modification | 3719 | 2023-06-14 10:27:57 | | | | |
| 3 | lingyun Wang | Meta information modification | 3719 | 2023-07-07 14:57:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, L.; Chen, M.; Ran, X.; Tang, H.; Cao, D. Sorafenib-Based Nanomedicine. Encyclopedia. Available online: https://encyclopedia.pub/entry/45552 (accessed on 07 February 2026).
Wang L, Chen M, Ran X, Tang H, Cao D. Sorafenib-Based Nanomedicine. Encyclopedia. Available at: https://encyclopedia.pub/entry/45552. Accessed February 07, 2026.
Wang, Lingyun, Meihuan Chen, Xueguang Ran, Hao Tang, Derong Cao. "Sorafenib-Based Nanomedicine" Encyclopedia, https://encyclopedia.pub/entry/45552 (accessed February 07, 2026).
Wang, L., Chen, M., Ran, X., Tang, H., & Cao, D. (2023, June 14). Sorafenib-Based Nanomedicine. In Encyclopedia. https://encyclopedia.pub/entry/45552
Wang, Lingyun, et al. "Sorafenib-Based Nanomedicine." Encyclopedia. Web. 14 June, 2023.
Copy Citation
Sorafenib (SF) is a US Food and Drug Administration (FDA)-approved molecular-targeted chemotherapeutic agent, which is used as a clinic standard drug for hepatocellular carcinoma (HCC). The overall patient survival is increased by delaying the pathologic progression. SF is also an oral multikinase inhibitor by inhibiting the proliferation of tumor cells, neoplastic angiopoiesis, angiogenesis and invasion of cancer cells. So, SF is regarded as an effective chemotherapeutic agent against various types of tumors
sorafenib
multi-kinase inhibitor
nanodelivery systems
1. Biomacromolecule as Nanocarrier
As a kind of potential candidates for encapsulating hydrophobic drugs, nanostructured lipid carriers (NLCs) possess suitable size, good oral absorption, high biocompatibility, low toxicity and immunogenicity [1][2][3][4]. For example, the commercially pegylated liposomal doxorubicin (Doxil/Caelyx) and liposomal daunorubicin (Daunoxome, Galen) have been FDA approved as antitumor formulations. NLCs can be classified into solid lipid nanoparticles (SLNs) and liquid lipid nanoparticles (LLNs). The major difference between SLNs and LLNs is their lipid components. SLNs are prepared from solid lipids, whereas NLCs are modified by adding liquid lipids [3]. Both SLNs and LLNs are considered to be safe carriers since they are produced from physiological and biodegradable lipids. They can increase the stabilities of active ingredients and drug. However, LLNs exhibit higher drug loading capacities than SLNs, and lipid crystallization is avoided during drug storage because of the presence of liquid lipids, thus preventing drug expulsion. Until now, liposomes have been widely used as suitable nanovectors for the SF delivery since SF has high hydrophobicity and good lipid affinity.
Bondìa and coworkers found that an NLC mixture (from solid and liquid lipids) would be a better nanocarrier than solid lipids alone, where higher drug loading capacity and longer-term stability were shown [5]. In vitro results indicated that more efficiently inhibited HCC cell growth than free SF (70% vs. 40% at 10 mM, and 85% vs. 55% at 20 mM) were obtained, leading to enhanced anti-tumor activity. When SF-loaded SLNs were prepared by combined high-speed shearing with ultrasonic treatment, high average encapsulation efficiency (EE) and drug loading (DL) of 89.87 and 5.39%, respectively, were shown [6]. Due to good liver-targeting capability, a 2.20-times higher average drug selectivity index value was found compared to that of the free SF-suspension. After oral administration, SF is metabolized in the mucosa of the small intestine and in the liver, where it is transformed into eight metabolites. When SF was encapsulated in SLNs matrix, SF was protected from metabolism or slowed down SF release from SLNs. Thus, SF remained in systemic circulation for a prolonged duration. Therefore, reducing the dosage of SF-SLNs could be considered to achieve the same pharmacological effects as the SF-suspension. As a result, a low side effect via oral administration was obtained by the protective lipid matrix and improved bioavailability.
The charged nucleolipids possess good stability, non-toxicity and self-assembly properties. Benizri et al. reported charged SF-loaded SLNs entrapped by nucleolipids with improved water solubility of SF (>120 μM) and parallelepiped microstructure with 200 nm size [7]. Due to improved SF solubility, enhanced anticancer activities on four different cell lines (liver and breast cancers) were shown compared to free SF. Meanwhile, Ahiwale et al. used nanocarriers compressing 1,2-dioleoyl-sn-glycero-3-phospho-L-serine sodium salt and cholesterol for oral administration of SF tosylate [8]. The entrapment efficiency, particle size and zeta potential of 85.55%, 470 nm and −44.5 mV, respectively, was shown. The 2.18-fold improvement in oral bioavailability and a pronounced effect on liver cancer was found. In one case, SF was incorporated into the hydrophobic lipidic bilayer with a encapsulation efficiency rate of 75.35% [9]. In vitro cytotoxicity effect revealed the IC50 values decreased by ~30% at 24 h (12.25 µM for SK-HEP-1 and 11.61 µM for HepG2).
As a type of natural polymer, chitosan has good biodegradability, non-toxicity, and good affinity for adsorbing drugs. For instance, chitosan/heparin-immobilized Pluronic NPs were formulated to deliver SF, leading to enhanced antitumor efficacy of SF in gastric cancers [10]. Varshosaz et al. developed PEGylated trimethyl chitosan (TMC) emulsomes (EMs) conjugated with octreotide (TMC-octreotide) for targeted delivery of SF by the emulsion evaporation method (Figure 1) [11]. The optimized SF-loaded EMs had the particle size of 127 nm, zeta potential of −5.41 mV, loading efficiency of 95% and drug release efficiency of 62% within 52 h. Compared to free SF and non-targeted EMs, SF-loaded targeted EMs exhibited more cytotoxicity and more accumulation in HepG2 cells. Further study indicated chemically active free amine groups in TMC had a positive effect on enhancing the stability of EMs, cell adhesion capability and retaining the targeting at the tumor area. On the other hand, due to the acidic pH character of the tumor microenvironment, pH-sensitive carboxymethyl chitosan-modified liposomes for SF and siRNA co-delivery were reported [12]. When 4.5 mg/kg of nanoformulation was administrated intravenously, the controlled SF delivery led to a reduced tumor volume.
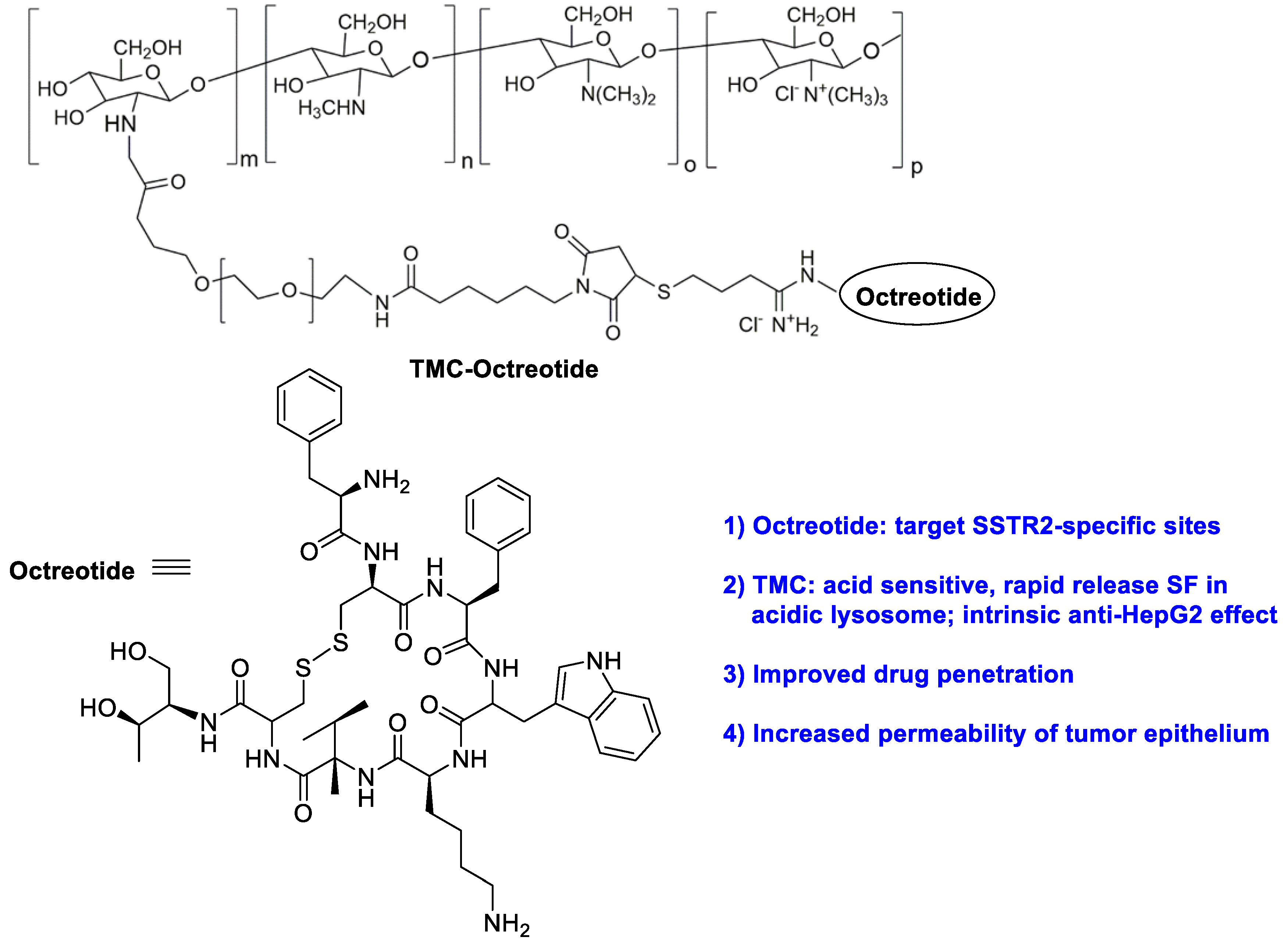
Figure 1. The chemical structure of trimethyl chitosan (TMC) -octreotide.
As a biodegradable, non-toxic and non-immunogenic protein, human serum albumin (HSA) is a promising carrier with multiple cellular receptors. Lactobionic acid (LA), the ligands for asialoglycoprotein (ASGP) receptors in HepG2 cells, was conjugated to HSA by amide bond formation between LA and HSA (Figure 2) [13]. The resulting LA-HSA conjugates were used as a targeted delivery system taking SF to HepG2 cells, which showed more cytotoxicity and cellular uptake than the untargeted ones with an IC50 value of 1.83 and 19.07 μM, respectively. The results indicated that LA as a targeting agent had a positive effect on tumor cell killing. Similarly, Gopakumar and coworkers showed core–shell protein nanoparticle as an albumin nanocarrier for SF showed a nearly two-fold enhancement in the oral bioavailability and enhanced therapeutic efficacy [14].
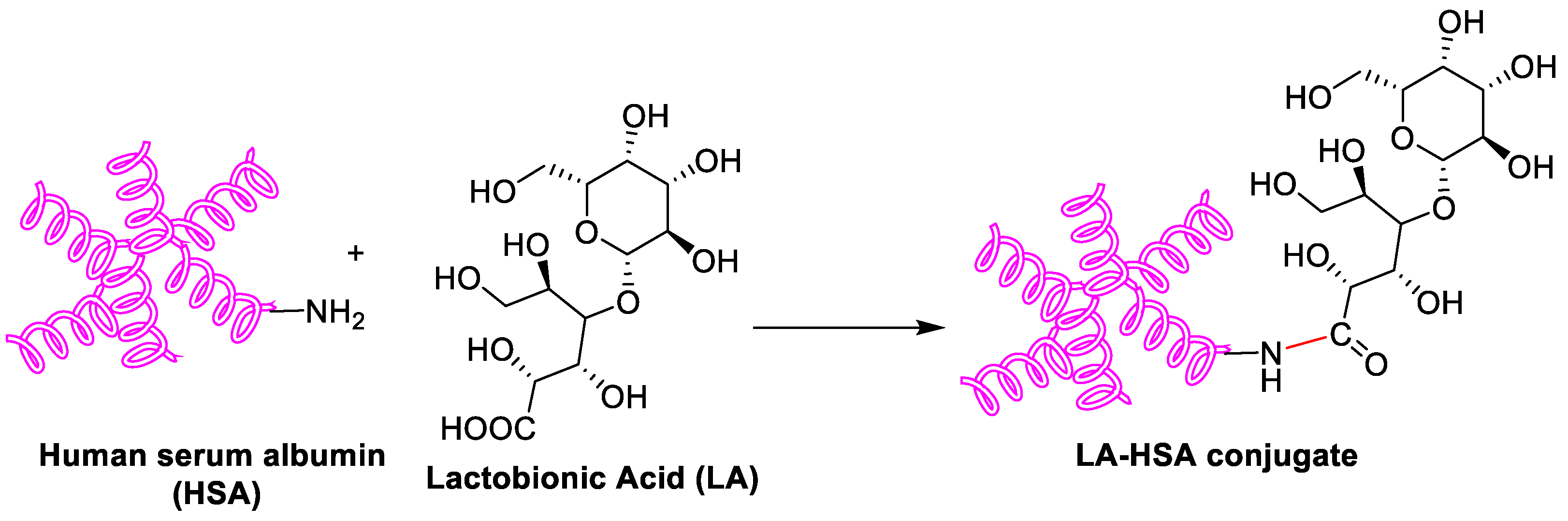
Figure 2. Schematic synthesis of LA-HSA conjugate.
Nonionic α-cyclodextrins (α-CD) are ideal carriers to deliver drugs [15]. Mazzaglia et al. reported supramolecular nanoassemblies based on α-CD and SF in aqueous solution for ablation of human HCC cells (Figure 3) [16]. The host–guest cooperation interaction between α-CD and SF produced highly water-dispersible colloidal nanoassemblies. The size of ~200 nm, negative zeta-potential (ζ = −11 mV), maximum loading capacity (~17%) and entrapment efficiency (~100%) were found. There was a slower release of SF from nanoassemblies with respect to the free SF. In addition, nanoassemblies showed low hemolytic activity and a high efficiency to inhibit the growth of three different HCC cell lines.
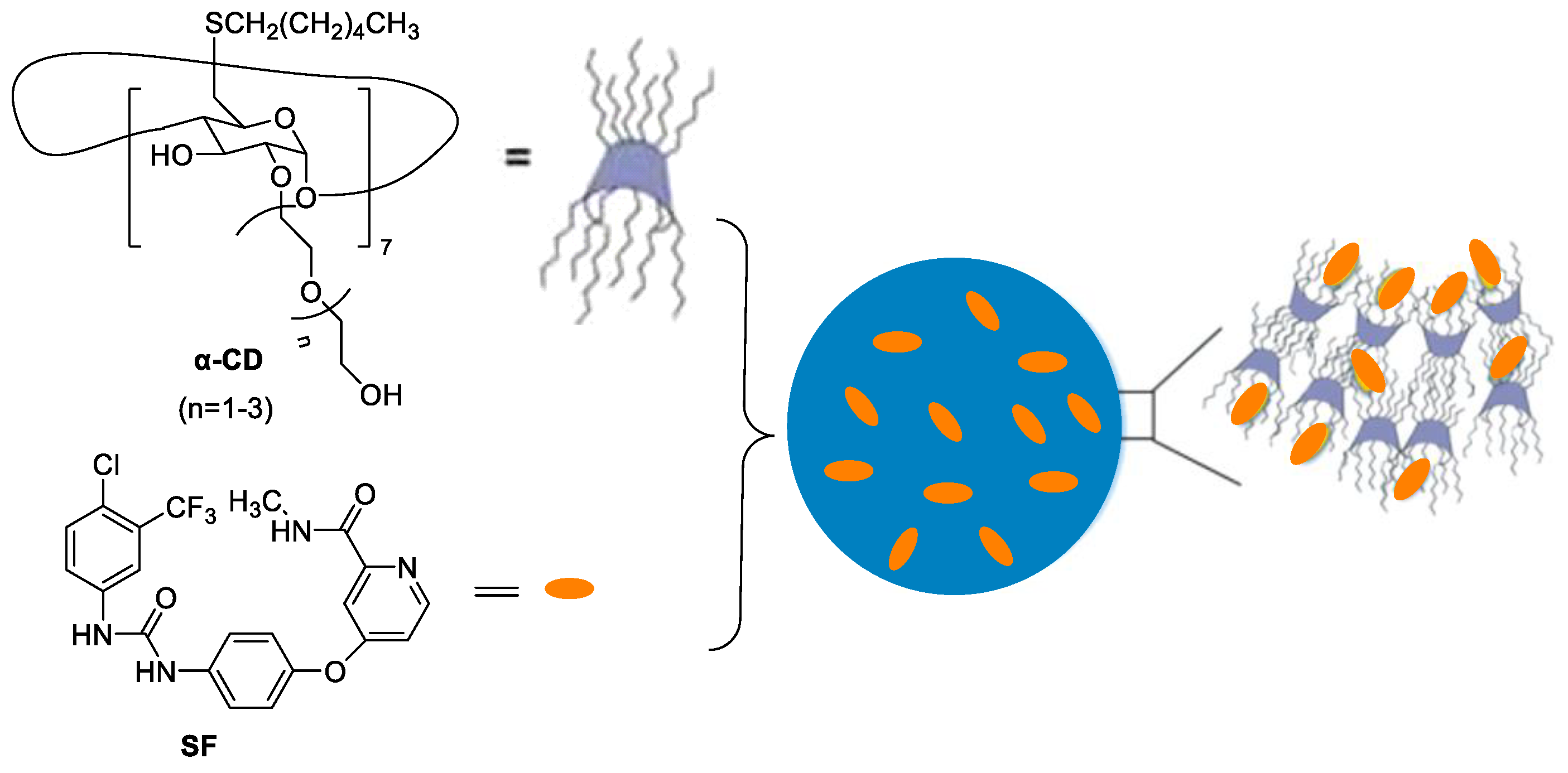
Figure 3. The supramolecular nanoassemblies based on α-CD and SF in aqueous solution.
As a natural anionic polysaccharide, hyaluronic acid (HA) directly binds to CD44, and RHAMM receptors overexpressed on various tumor cell surfaces. Mo et al. reported PEGylated HA-coated liposome for enhanced SF delivery and anti-tumor outcome via active tumor cell targeting and prolonged systemic exposure [17]. For MDA-MB-231 cells over expressing CD44, greater cellular uptake and cytotoxicity were found. However, there were no significant differences in MCF-7 cells with low CD44 expression. Compared with SF solution, PEG-HA-SF-Lip increased the systemic exposure and plasma half-life in rats by 3-fold and 2-fold, respectively. The effective tumor growth inhibition through CD44 targeting in the MDA-MB-231 tumor xenograft mouse model was demonstrated.
SF and cisplatin (cis-Pt) are two chemotherapeutic drugs with different antitumor mechanisms. Zhang et al. developed pH-sensitive hyaluronic acid NPs encapsulating with SF and cis-Pt, where interactions between cisplatin and HA was involved [18]. The pH-sensitive property guaranteed the effective release of loaded drugs (cis-Pt and SF) in tumor sites. The HA@cis-Pt@SF showed prolonged circulation time and high accumulation rate, leading to exerted synergistic tumor-killing effects.
2. Synthetic Polymer as Nanocarriers
Poly(lactic-co-glycolic acid) (PLGA) is approved by the FDA as a drug carrier due to its hydrophobic properties and biocompatibility. Kim et al. prepared hydrophilic dextran-conjugated PLGA (PLGA-Dex, Figure 4a) [19]. In this case, dextran and PLGA acted as a hydrophilic and biodegradable hydrophobic domain, respectively. The SF-incorporated PLGA-Dex NPs were fabricated via the nanoprecipitation–dialysis method. The loading efficiency was higher than 35%. An initial burst of release and a sustained release of SF was observed. A dose-dependent antiproliferative effect against HuCC-T1 tumor cells was shown. In 2016, to regulate size polydispersity and the stability of NPs in the blood circulation, a mixture of amphiphilic copolymer of poly(ethylene glycol)-b-PLAG and PLGA (5/5) was co-formulated to entrapped SF [20]. The blood circulation of the cargo was significantly prolonged, resulting in increased uptake by liver fibrosis in CCl4-induced fibrosis models. In particular, vessel normalization in the fibrotic livers was observed.
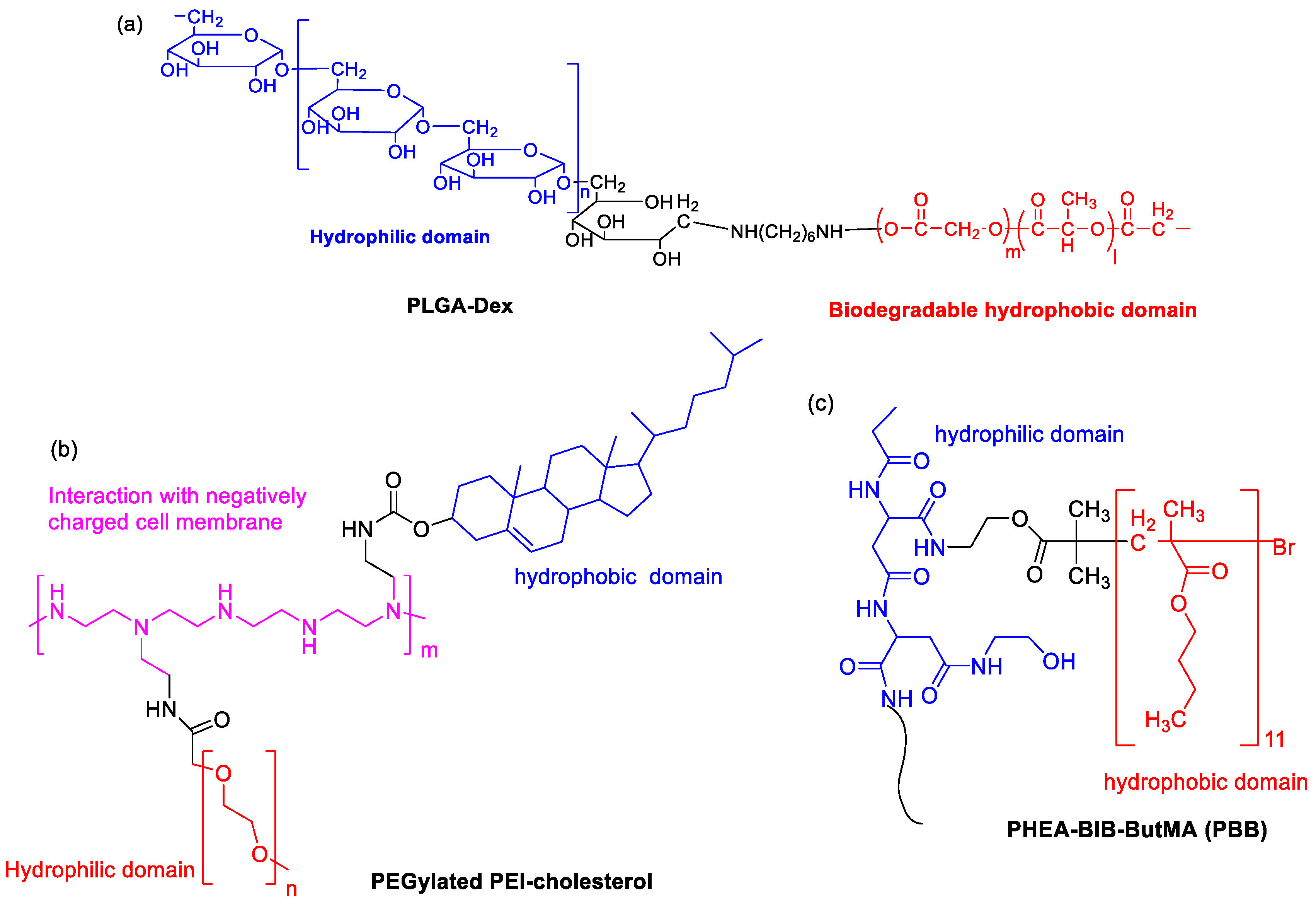
Figure 4. The chemical structures of (a) PLGA-Dex, (b) PEGylated PEI-cholesterol and (c) PBB.
Due to the presence of primary, secondary and tertiary amines in the polymer structure, cationic polyethyleneimine (PEI) exhibits “proton sponge” effect by interaction with negatively charged cell membrane. PEGylated PEI-cholesterol lipopolymers (Figure 4b) were synthesized, where cholesterol functionalization was helpful for endocytosis, enhancement of drug loading capacity and reduction in the drug burst release [21]. When they were used as nanocarriers, SF-loaded NPs exhibited maximum drug loading (13.1% ± 2.65) and concentration-dependent toxicity by pharmacological action through the charge shielding mechanism.
An amphiphilic brush copolymer PHEA-BIB-ButMA (PBB, Figure 4c) was synthesized by Atom Transfer Radical Polymerization (ATRP) [22]. SF was entrapped into its inner hydrophobic core in an aqueous environment as a drug reservoir. The spherical morphology (200 nm size), negative ζ potential and sustained SF release capability was found. Compared to free drug, SF-loaded NPs showed a higher inhibition efficacy of tumor growth and more accumulation in the solid tumor mass in in vivo xenograft models.
Liquid crystalline nanoparticles (LCN) have high stability, sustained drug release, and high drug payload to the target site [23]. Thapa et al. prepared a pH-responsive polymer-coated LCN with poly-L-lysine (PLL) and poly(ethylene glycol)-b-poly(aspartic acid) (PEG-b-PAsp) for SF delivery [24]. Due to selective depolymerization of polymer layers by acidic tumor microenvironment, the pH-responsive SF-loaded nanomedicine showed enhanced drug release. As a result, the intracellular uptake of SF was significantly improved, generating better antitumor efficacy against HepG2 cells.
The amphiphilic polymer based on polyacryic acid (PAA) with D-α-tocopherol succinate (VES) via a disulfide bond linker was synthesized [25]. SF was entrapped in the polymer micelles, where the release of SF was dependent on the concentration of glutathione (GSH) because of GSH sensitivity of disulfide linkage. A 2.8-fold bioavailability of SF was achieved by injecting the animal compared with free SF by this redox-responsive SF delivery system.
To control particle morphology, size distribution and reproducibility from batch-to-batch, a microfluidic process with glass-based microcapillary devices was developed for preparation of micellar-like PEG-b-PCL NPs loaded with SF free base [26]. Under optimized nanoprecipitation process, spherical NPs with loading degree (16%), encapsulation efficiency (54%), particle size less than 80 nm were obtained. More importantly, 20,000-fold increase in solubility was achieved as compared to free SF in PBS. They found process temperature, flow rate, nanoprecipitation temperature, and organic solvent removal method had the significant effects on particle size distribution, morphology distribution, zeta-potential, drug loading, encapsulation efficiency and process yield. The in vitro drug release study indicated that SF was released from the NPs in a sustained manner, where 13.6% and 25.3% release rate in 8 and 24 h at pH 7.4 was shown.
Colorectal cancer has overexpressed matrix metalloproteinase (MMPs), especially MMP-2. The MMP-2 responsive nanocarrier assembled by amphiphilic polyethylene glycol (PEG)-peptide copolymer for simultaneously loading camptothecin and SF for combination therapy (Figure 5) [27]. Two drugs were released upon exposure to tumor microenvironment because the peptide segments could be digested by MMP-2, leading to higher synergistic efficacy than that of single antitumor agents. The photoacoustic (PA) imaging signals kept constant for the whole week during detection the angiogenesis of in vivo HT-29 bearing mice.
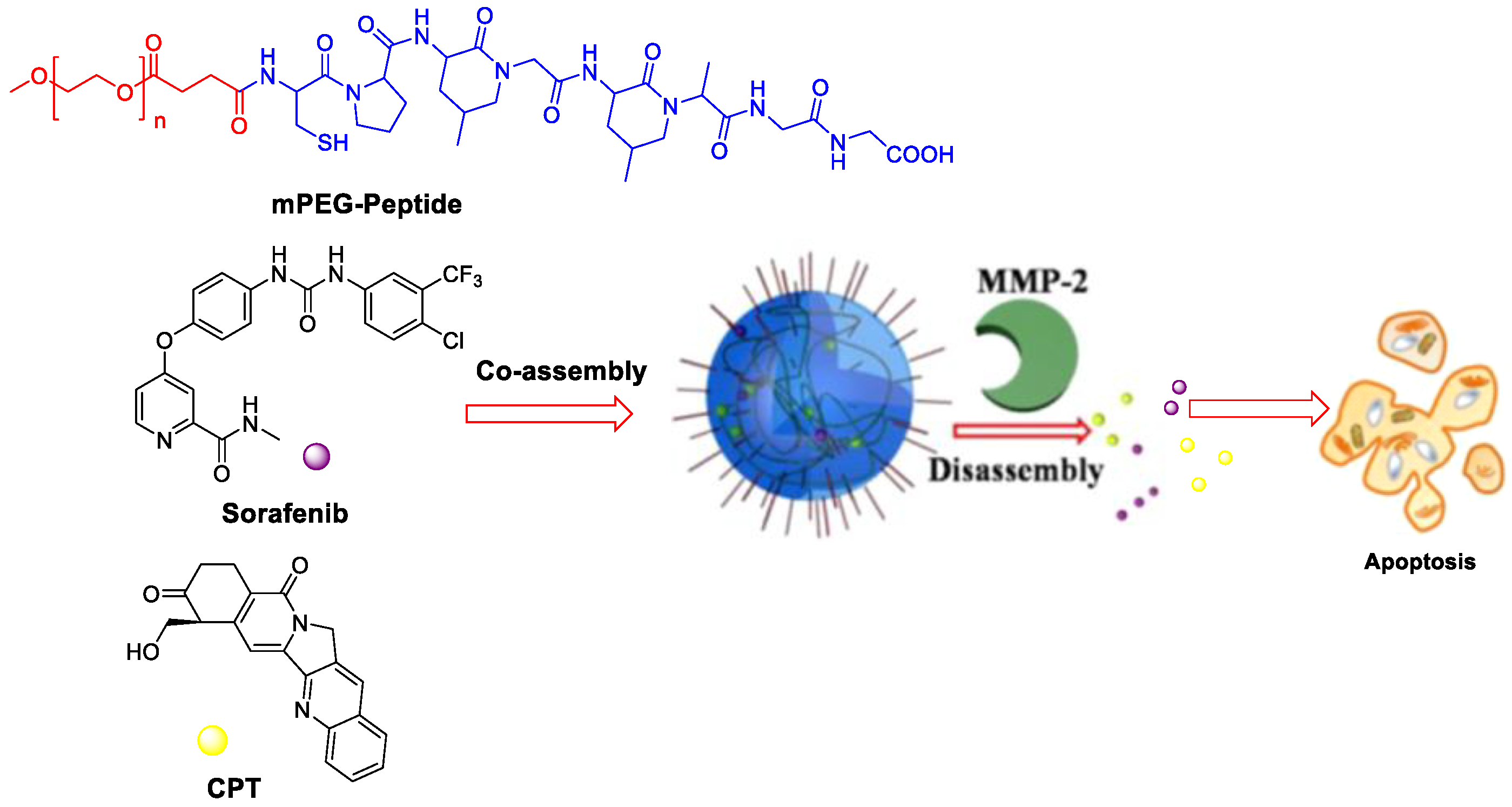
Figure 5. MMP-2-responsive nanoparticles for synergistic antitumor effect of anti-angiogenesis and chemotherapy. Adapted with permission from [27]. Copyright 2016, American Chemical Society.
3. Inorganic and Metal Nanocarriers
Hollow mesoporous silica nanoparticles (MSNs) show uniform particle size distribution, excellent water dispersion, large specific surface area and good biocompatibility [28], which are ideal drug delivery nanocarriers [29]. Shao’s group reported a series of MSNs-based SF NPs [30][31]. For example, MSNs were used as nanocarriers for the co-delivery of SF and siRNA, leading to enhanced cytotoxicity and improved the tumor target of SF in asialoglycoprotein receptor (ASGPR)-overexpressing Huh7 cells [30]. Since lactobionic acid (LA) could target human HCC effectively, an ASGPR-targeting SF delivery system based on MSNs co-delivery of vascular endothelial growth factor (VEGF)-targeted siRNA (siVEGF) and LA was synthesized as follows [30]. NPs based on MSN-NH2 and SF were fabricated by non-covalent interactions. Then, LA was chemically linked to the surface of the NPs. Finally, siVEGF were loaded into the nanocarrier by electrostatic interaction. The improvement in the targeting property resulted in enhanced anti-cancer efficacy of SF. Recently, SF was loaded into manganese-doped MSNs by physical adsorption [32]. In this case, high-concentration GSH could break manganese-oxidation bonds leading to the degradation of MSNs. This dual GSH-exhausting (consumption or synthesis inhibition of GSH) nanomedicine showed a significantly enhanced in vitro inhibitory effect.
To decrease the risk of post-surgical HCC relapse, a wound-targeted nanodrug containing immune checkpoint inhibitor (anti-PD-L1) and SF as an angiogenesis inhibitor was developed, where a mesoporous silica with a surface-coated platelet membrane as surgical site-targeting nanocarriers was used [33]. The resulting nano-formulation efficiently inhibited post-surgical HCC relapse without obvious side effects.
Gold NPs with high biocompatibility and negligible toxicity, small size and precise targeting ability are suitable candidates for a drug delivery system for the treatment of various diseases. FA-b-PEG-modified gold NPs loaded with SF tosylate were fabricated, providing an effective treatment for retinal neovascularization in patients of diabetic retinopathy [34]. MiR-221 plays a role in promoting tumorigenesis in HCC by inhibiting the expression of p27. Cai et al. investigated the synergistic anti-tumor effects of SF and gold NPs-loaded anti-miR221, leading to a strong synergistic effect on inhibiting proliferation of HCC cells [35]. Recently, alginate/CaCO3 hybrid loaded with SF tosylate and gold hexagons was developed for the dual (chemo-radio) treatment of HepG2 cells [36].
As a versatile inorganic compound, sodium selenite (Na2Se) NPs exert anticancer effect by inducing apoptosis. A co-delivery system for SF and Na2Se was prepared when tripolyphosphate was used as a crosslinker via the solvent evaporation technique [37]. The resulting NPs had a zeta potential of −37.5 mV and size (diameter) in the range of 208 nm to 0.2 µm. The sustained release of the drug to elicit synergetic action was developed. Selenium NPs have distinct characteristics of high biocompatibility, low-toxicity and degradability in vivo, and radiosensitization effects with X-ray. An injectable thermosensitive nanosystem based on SF, Se NPs and PLGA-PEG-PLGA was prepared [38]. When it was injected in HepG2 tumor-bearing nude mice, the resulting hydrogel for long-acting therapy with the degradation of PLGA-PEG-PLGA was obtained. The raised expression of cleaved caspase-3 in tumor tissue was observed based on the synergistic local treatment and chemoradiotherapy.
ZnO-NPs possess excellent physicochemical properties of safety, biodegradability, fast delivery rate and cytotoxicity against tumor cells [39]. ZnO-NPs might be a safe candidate in combination with SF to generate enhanced treatment effect [40].
4. Co-Delivery of SF and Other Drugs
The SF-based monotherapy is often hampered by its modest efficacy, serve systemic toxicity and high occurrence of drug resistance. In order to enhance the antitumor effect of SF and reduce its side effects, different drugs are co-loaded in nanomedicine. Doxorubicin (DOX) is an effective chemotherapy agent, but it suffers from some drawbacks such as low response rate, severe side effects and cytotoxicity to normal tissues. Although DOX and SF have completely different chemical characteristics, Babos et al. reported the entrapment of DOX and SF by PLGA/PEG-PLGA using the double emulsion solvent evaporation method [41]. The DOX-SF NPs showed a suitable size (~142 and 177 nm), high drug encapsulation efficiency (69% for DOX and 88% for SF) and high drug loading. The DOX was released continuously within 6 days, while the SF was released quickly during 24 h under biorelevant conditions. Duan et al. formulated a specific sugar-modified pH sensitive lipid system for the co-delivery of DOX and SF for combination HCC chemotherapy [42]. In this case, DOX prodrug (NAcGal-DOX, Figure 6) contained pH-sensitive hydrazine moiety and N-acetylgalactosamine, allowing a rapid DOX release at the tumor site and targeting to highly expressed asialoglycoprotein receptors in HCC. DOX and SF were entrapped by single-step nanoprecipitation in the presence of soya lecithin and polysorbate 80. The targeted ability of sugar to HCC and strong synergy between DOX and SF generated a higher anti-tumor efficiency of drugs.
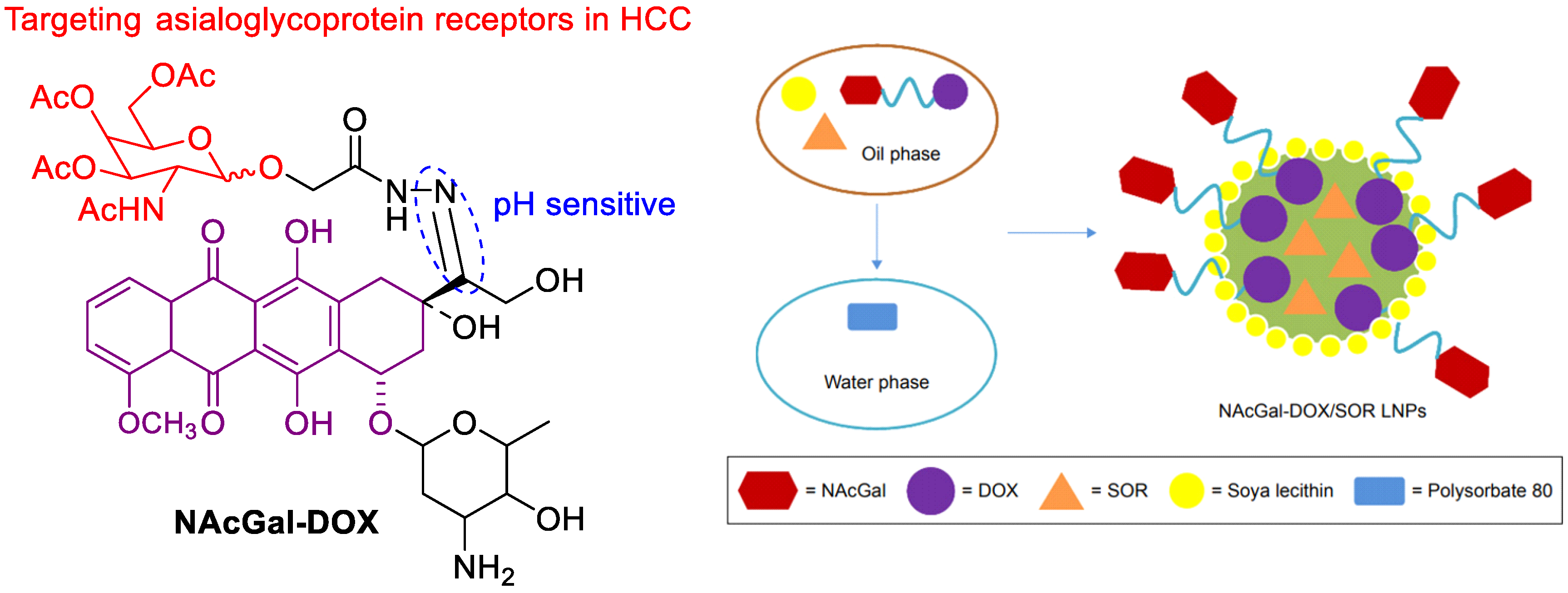
Figure 6. The chemical structure of NAcGal-DOX and co-loading in NAcGal-DOX/SF NPs.
In order to deliver SF in a targeted fashion into undifferentiated/anaplastic thyroid carcinoma cells, Mato et al. developed SF-loaded PLGA NPs covalently attached with cetuximab [43]. SF-loaded PLGA NPs was firstly prepared by the single emulsion evaporation method. Then, EDC/Sulfo-NHS cross linking chemistry was utilized to conjugated cetuximab to SF-loaded PLGA NPs, yielding 51% cetuximab incorporation. The resulting NPs showed suitable size (252 nm) and drug entrapment efficiency (58%). A higher specificity to the anaplastic cells line (CAL-62) that overexpress EGFR was shown, providing a promising targeting approach for the treatment of epithelial thyroid cancer.
5. Imaging-Guided SF Nanodrugs
Nanomedicines combining drug delivery and imaging functions have been employed as theranostic tools for imaging-guided cancer therapy. Superparamagnetic iron oxide nanoparticles (SPIONs) are suitable nanocarrier systems for cancer therapeutics [44][45][46]. SPIONs within the lipid matrix were used to construct magnetically responsive SLNs. Grillone et al. developed SF-loaded magnetic SLNs (SF-Mag-SLNs) to enhance SF delivery with the help of a remote magnetic field using cetyl palmitateas lipid matrix [47]. The resulting NPs had a 90% SF loading efficiency, a regular spherical shape and an average diameter of less than 300 nm. SF-Mag-SLNs were able to inhibit cancer cell proliferation through the SF cytotoxic action. Good targeting properties and attenuated side effects were achieved via a magnetically driven accumulation of SF. However, the preparation process is extremely complex and tedious.
The use of poly(vinyl alcohol)-coated SPIONs as a polymeric-magnetic delivery system for SF with significantly enhanced activity was developed via co-precipitation and physical entrapment methods [48]. An entrapment value of 76.37% and a release rate of SF up to 66.7% in 80 h were obtained. The MTT assay showed that the cytotoxicity of SF-loaded PVA/SPIONs was comparable or higher than that of free SF. The apoptosis study in HepG2 cells indicated that the combination of SF with PVA/SPIONs showed a higher percentage of early apoptotic cells than free SF (13.8% vs. 11.4%).
A reduction and pH dual-sensitive nano-formulation co-encapsulating SPIONs and SF was developed and further functionalized by anti-GPC3 antibody (AbGPC3) for magnetic resonance imaging (MRI)-monitorable HCC-targeted therapy [49]. The AbGPC3-mediated active targeting enhanced cancer cell uptake and tumor accumulation of nanodrug. Consequently, the nanodrug displayed prominent anticancer effects (the lowest tumor weight was only 12.3% of that for the saline group) in an animal study via responding to the cytoplasmic glutathione and lysosomal acidity. Moreover, tumor detection and monitoring of drug delivery process by MRI were obtained.
Faramarzi et al. developed functionalized magnetic nanoparticles with chitosan for adsorption of SF and thermosensitive N-isopropylacrylamide to control release (Figure 7) [50]. The radical copolymerization of allyl glycidyl ether (AGE), N-isopropylacrylamide (NIP) and silica-coated magnetic NPs was firstly involved. The following coupling reaction between chitosan and epoxy ring of the AGE afforded thermosensitive magnetic nanocarrier (TSMNC). Due to superparamagnetic properties, rapid adsorption and slow release at low and high temperatures, Fickian diffusion-controlled drug release was obtained, where about 88% of SF was released within 35 h at 45 °C. Recently, SF tosylate-loaded PCL-superparamagnetic nanoparticles were prepared by an emulsion solvent evaporation method and optimized using Box–Behnken design [51].
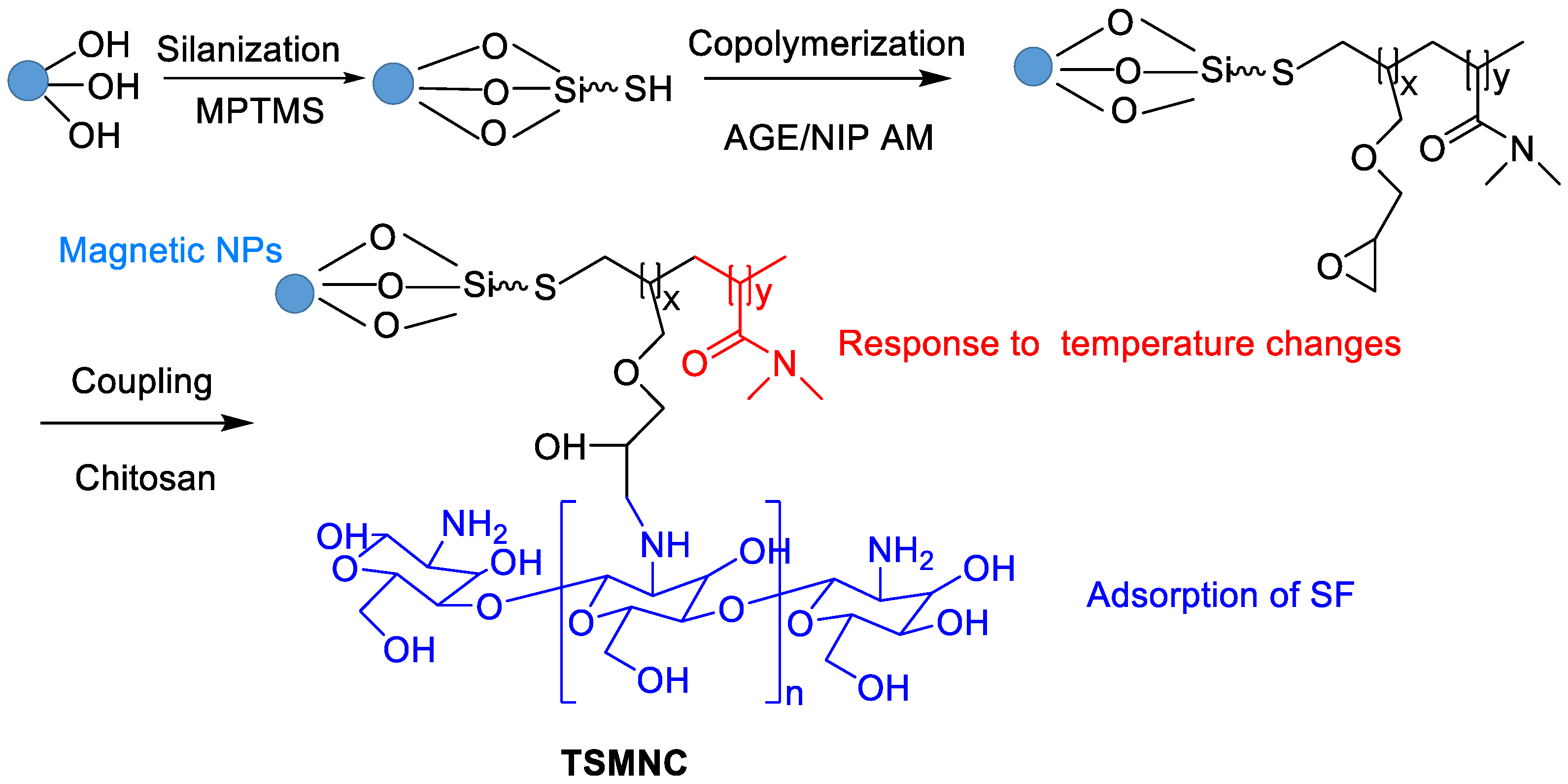
Figure 7. Synthetic route of TSMNC.
6. Actively Targeted Nanosystems
Feng et al. designed a lipid-coated nanocarrier functionalized with peptides specific for glypican-3, which showed targeted drug delivery to human HCC xenograft tumors [52]. PLGA and 1,2-dioleoyl-snglycero-3-phosphocholine (DOPC) were utilized as a hydrophobic core to entrap SF and as a lipid shell to prevent drug leakage, respectively (Figure 8). The peptides are attached to the nanocarrier surface via a thioether-mediated conjugation. The entrapment efficiency (%EE) for SF was > 80% with concentrations up to 69.5 mg/L, which showed a 1900-fold improvement in aqueous solubility versus free SF. The half-life release ratio in vitro was 22.7 h. The greater tumor regression was shown due to the target peptide versus controls after 21 days of therapy.
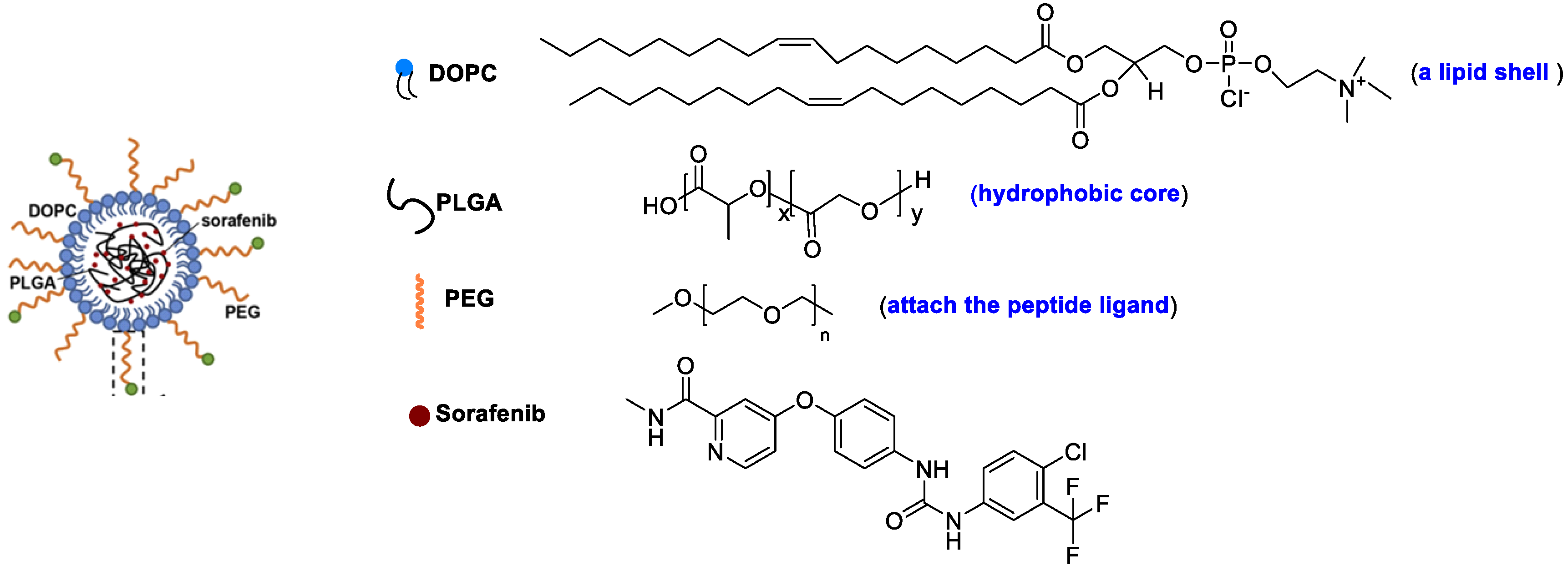
Figure 8. A lipid-coated nanocarrier.
SLN appended with PEGylated galactose (Figure 9) was developed to modulate the site-specific oral delivery of SF for HCC treatment because galactose is a C-type lectin receptor binding ligand [53]. The encapsulation of SF into SLN and use of PEGylated galactose as targeting ligand provided a liver-targeting, biocompatible drug delivery system and increased circulation half-life. The oral bioavailability of the SF can be improved by encapsulating it in SLN and grafting it with PEGylated galactose.
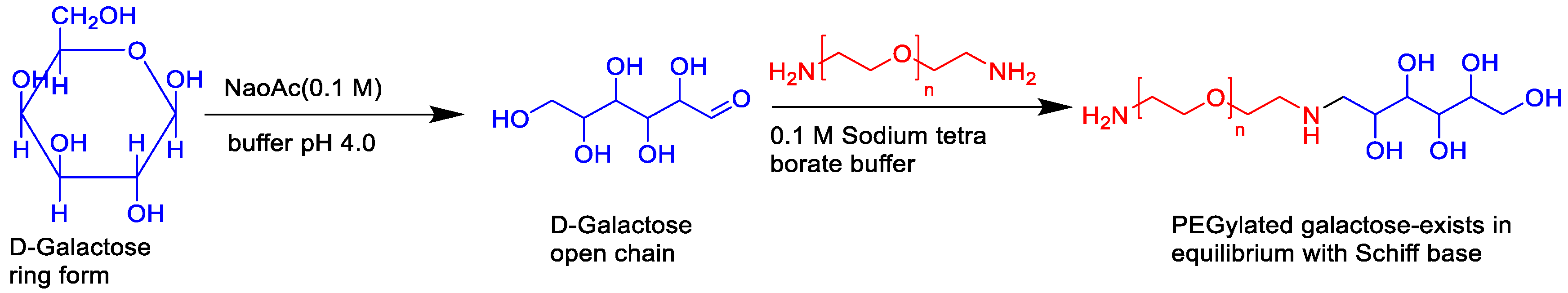
Figure 9. The synthetic route of PEGylated galactose.
Folate (FA) can enter cells through a receptor-mediated endocytosis pathway. It is highly expressed in tumor tissue at a level 100–300 times higher than that in normal tissues. The FA receptor is a good target for targeting delivery systems for many solid tumors. Good stability for more than 1 month at room temperature was found. The average particle size (158 nm), zeta potential (−16.27 mV), entrapment efficiency (77.25%), and drug loading (7.73%) were obtained. As compared with SF-BSA NPs and SF solution, SF-BSA-FA NPs showed the best intracellular uptake of hepatoma cells (SMMC-7721) and the strongest inhibitory effect. Moreover, excellent tumor targeting ability with higher Ctumor/Cblood value (0.66) than those of the former two (0.56 and 0.41) in a nude mice liver cancer model were found.
FA-modified PEG-nitroimidazole grafts exhibited a targeting SF delivery ability in the treatment of hypoxic HCC [54]. Since antibody hGC33 has important antitumor activity, Shen et al. described hGC33-modified PEG-b-PLGA for SF delivery to increase the targeting to HCC cells [55].
7. Combination of Ferroptosis and Others
Ferroptosis is an alternative to the traditional apoptotic pathways for cancer therapy, which is regarded as an iron-based Fenton-type reaction. Since SF can induce the occurrence of ferroptosis, some combinational therapy methods have been proposed [56]. Zhou et al. described a metal-polyphenol network-based SF nanodrug for combinational ferroptosis and PDT, showing a more excellent inhibitory effect on tumor cells [57].
The novel drug–mate strategy has been developed in the nanomedicine field. Namely, amphiphilic small molecule and hydrophobic drugs with poor solubility are selected as a mate and drug, respectively (Figure 10). Taking SF/D-α-tocopherol succinate nanoassemblies as an example, drug loading content (46%) was shown compared with the lipid-based SF nanodrugs (11%) and SF liposomes (4%) [58]. The highest SF water dispersion was due to stronger interaction between small molecular mate (SMA) and SF than that between SF itself. In vitro cytotoxicity assay indicated the IC50 of SF NPs based on the drug–mate strategy was close to that of free SF (3.44 vs. 4.44 μg/mL). However, the pharmacokinetics of the former were largely improved, where the plasma concentrations of SF was 2 and 20 times than that of the SF solution and SF suspension, respectively. In vivo antitumor efficacy study further revealed the increased bioavailability and enhanced therapeutic efficacy of SF.
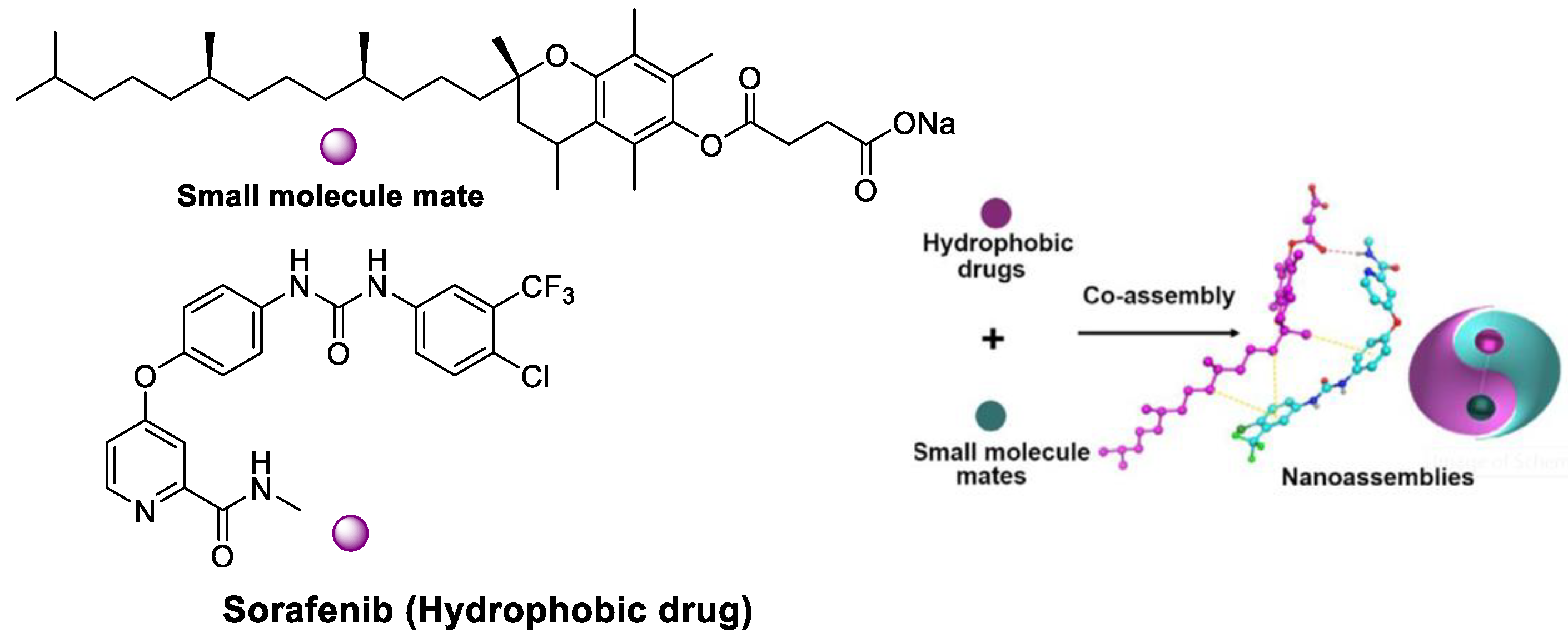
Figure 10. SF/D-α-tocopherol succinate nanoassemblies based on the drug–mate strategy.
References
- Li, M.; Su, Y.; Zhang, F.G.; Chen, K.R.; Xu, X.T.; Xu, L.; Zhou, J.P.; Wang, W. A dual-targeting reconstituted high density lipoprotein leveraging the synergy of sorafenib and anti-miRNA21 for enhanced hepatocellular carcinoma therapy. Acta Biomater. 2018, 75, 413–426.
- Muller, R.H.; Runge, S.A.; Ravelli, V.; Thunemann, A.F.; Mehnert, W.; Souto, E.B. Cyclosporine-loaded solid lipid nanoparticles (SLN): Drug-lipid physicochemical interactions and characterization of drug incorporation. Eur. J. Pharm. Biopharm. 2008, 68, 535–544.
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of active targeting lipid nanoparticles: Challenges and perspectives. Mater. Today Adv. 2022, 16, 100299.
- Mahmoodi, N.O.; Alavi, S.M.; Yahyazadeh, A. Formulation and therapeutic efficacy of PEG-liposomes of sorafenib for the production of NL-PEG-SOR FUM and NL-PEG-SOR TOS. Res. Chem. Intermed. 2022, 48, 3915–3935.
- Bondì, M.L.; Botto, C.; Amore, E.; Emma, M.R.; Augello, G.; Craparo, E.F.; Cervello, C. Lipid nanocarriers containing sorafenib inhibit colonies formation inhuman hepatocarcinoma cells. Int. J. Pharm. 2015, 493, 75–85.
- Wang, H.; Wang, H.Y.; Yang, W.L.; Yu, M.J.; Sun, S.L.; Xie, B.G. Improved oral bioavailability and liver targeting of sorafenib solid lipid nanoparticles in rats. AAPS PharmSciTech. 2018, 19, 761–768.
- Sebastien, B.; Ferey, L.; Bruno, A.; Mebarek, N.; Gaelle, V.; Ananda, A.; Cathy, S.; Karen, G.; Philippe, B. Nucleoside-lipid-based nanocarriers for sorafenib delivery. Nanoscale Res. Lett. 2018, 13, 17–24.
- Ahiwale, R.J.; Chellampillai, B.; Pawar, A.P. Investigation of novel sorafenib tosylate loaded biomaterial-based nano-cochleates dispersion system for treatment of hepatocellular carcinoma. J. Dispers. Sci. Technol. 2022, 43, 1568–1586.
- Bartos, A.; Iancu, I.; Ciobanu, L.; Onaciu, A.; Moldovan, C.; Moldovan, A.; Moldovan, R.C.; Tigu, A.B.; Stiufiuc, G.F.; Toma, V.; et al. Hybrid lipid nanoformulationsfor hepatoma therapy: Sorafenib loaded nanoliposomes—A preliminary study. Nanomaterials 2022, 12, 2833.
- Yang, Y.C.; Cai, J.; Yin, J.; Zhang, J.; Wang, K.L.; Zhang, Z.T. Heparin-functionalized pluronic nanoparticles to enhance the antitumor efficacy of sorafenib in gastric cancers. Carbohydr. Polym. 2016, 136, 782–790.
- Jaleh, V.; Fatemeh, R.; Mahboubeh, R.; Ali, J. PEGylated trimethylchitosan emulsomes conjugated to octreotide for targeted delivery of sorafenib to hepatocellular carcinoma cells of HepG2. J. Liposome Res. 2019, 31, 64–78.
- Yao, Y.; Su, Z.; Liang, Y.; Zhang, N. pH-Sensitive carboxymethyl chitosan-modified cationic liposomes for sorafenib and siRNA co-delivery. Int. J. Nanomed. 2015, 10, 6185–6197.
- Dayani, L.; Dehghani, M.; Aghaei, M.; Taymouri, S.; Taheri, A. Preparation and evaluation of targeted albumin lipid nanoparticles with lactobionic acid for targeted drug delivery of sorafenib in hepatocellular carcinoma. J. Drug Deliv. Sci. Tech. 2022, 69, 103142.
- Gopakumar, L.; Sreeranganathan, M.; Chappan, S. Enhanced oral bioavailability and antitumor therapeutic efficacy of sorafenib administered in core–shell protein nanoparticle. Drug Deliv. Transl. Res. 2022, 12, 2824–2837.
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621.
- Bondì, M.L.; Scala, A.; Sortino, G.; Amore, E.; Botto, C.; Azzolina, A.; Balasus, D.; Cervello, M.; Mazzaglia, A. Nanoassemblies based on supramolecular complexes of nonionic amphiphilic cyclodextrin and sorafenib as effective weapons to kill human HCC cells. Biomacromolecules 2015, 16, 3784–3791.
- Mo, L.X.; Song, J.G.; Lee, H.K.; Zhao, M.J.; Kim, H.Y.; Lee, Y.J.; Ko, H.W.; Han, H.Y. PEGylated hyaluronic acid-coated liposome for enhanced in vivo efficacy of sorafenib via active tumor cell targeting and prolonged systemic exposure. Nanomedicine 2018, 14, 557–567.
- Zhang, W.; Cai, J.; Wu, B.; Shen, Z. pH-responsive hyaluronic acid nanoparticles coloaded with sorafenib and cisplatin for treatment of hepatocellular carcinoma. J. Biomater. Appl. 2019, 34, 219–228.
- Kim, D.H.; Kim, M.D.; Choi, C.W.; Chung, C.W.; Ha, S.H.; Kim, C.H.; Shim, Y.H.; Jeong, Y.; Kang, D.H. Antitumor activity of sorafenib-incorporated nanoparticles of dextran/poly(dl-lactide-co-glycolide) block copolymer. Nanoscale. Res Lett. 2012, 7, 91.
- Lin, T.T.; Gao, D.Y.; Liu, Y.C.; Sung, Y.C.; Wan, D.H.; Liu, J.Y.; Chiang, T.; Wang, L.Y.; Chen, Y.C. Development and characterization of sorafenib-loaded PLGA nanoparticles for the systemic treatment of liver fibrosis. J. Control. Release 2016, 221, 62–70.
- Monajati, M.; Tavakoli, S.; Abolmaali, S.S.; Yousefi, G.; Tamaddon, A. Effect of PEGylation on assembly morphology and cellular uptake of poly ethyleneimine-cholesterol conjugates for delivery of sorafenib tosylate in hepatocellular carcinoma. BioImpacts 2018, 8, 1–252.
- Melchiorre, C.; Giovanna, P.; Antonella, B.V.; Barbara, P.; Daniele, B.; Maria, R.E.; Antonina, A.; Roberto, P.; Guido, R.L.; Stefano, P.; et al. Nanoparticles of a polyaspartamide-based brush copolymer for modified release of sorafenib: In vitro and in vivo evaluation. J. Control. Release 2017, 266, 47–56.
- Yang, D.; Armitage, B.; Marder, S.R. Cubic Liquid-crystalline nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 4402–4409.
- Thapa, R.K.; Choi, J.Y.; Poudel, B.K.; Hiep, T.T.; Pathak, S.; Gupta, B. Multilayer-coated liquid crystalline nanoparticles for effective sorafenib delivery to hepatocellulr carcinoma. ACS Appl. Mater. Interfaces 2015, 7, 20360–20368.
- Liu, Y.; Yang, J.; Wang, X.; Liu, J.; Wang, Z.; Liu, H. In vitro and in vivo evaluation of redox-responsive sorafenib carrier nanomicelles synthesized from poly(acryic acid)-cystamine hydrochloride-D-alpha-tocopherol succinate. J. Biomater. Sci. Polym. Ed. 2016, 27, 1729–1747.
- Känkänen, V.; Fernandes, M.; Liu, Z.; Seitsonen, J.; Hirvonen, S.; Ruokolainen, J.; Pinto, J.F.; Hirvonen, J.; Balasubramanian, V.; Santos, H.A. Microfluidic preparation and optimization of sorafenib-loaded poly(ethylene glycol-block-caprolactone) nanoparticles for cancer therapy applications. J. Colloid Interface Sci. 2023, 633, 383–395.
- Shi, L.; Hu, Y.; Lin, A.; Ma, C.; Zhang, C.; Su, Y. Matrix metalloproteinase responsive nanoparticles for synergistic treatment of colorectal cancer via simultaneous anti-angiogenesis and chemotherapy. Bioconjug. Chem. 2016, 27, 2943–2953.
- Fan, W.; Shen, B.; Bu, W.; Chen, F.; He, Q.; Zhao, K.; Zhang, S.; Zhou, L.; Xiao, Q.; Ni, D.; et al. A smart upconversion-based mesoporous silica nanotheranostic system for synergetic chemo-/radio-/photodynamic therapy and simultaneous MR/UCL imaging. Biomaterials 2014, 35, 8992–9002.
- Barkat, A.; Beg, S.; Panda, S.K.; Alharbi, S.K.; Rahman, M.; Ahmed, F.J. Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Semin Cancer Biol. 2021, 69, 365–375.
- Zheng, G.; Zhao, R.; Xu, A.; Shen, Z.; Chen, X.; Shao, J. Co-delivery of sorafenib and siVEGF based on mesoporous silica nanoparticles for ASGPR mediated targeted HCC therapy. Eur. J. Pharmac. Sci. 2018, 111, 492–502.
- Zhang, B.C.; Luo, B.Y.; Zou, J.J.; Wu, P.Y.; Jiang, J.L.; Le, J.L.; Zhao, R.R.; Chen, L.; Shao, J.W. Co-delivery of sorafenib and CRISPR/Cas9 based on targeted core–Shell hollow mesoporous organosilica nanoparticles for synergistic HCC therapy. ACS Appl. Mater. Interfaces. 2020, 12, 57362–57372.
- Tang, H.; Chen, D.; Li, C.; Zheng, C.; Wu, X.; Zhang, Y. Dual GSH-exhausting sorafenib loaded manganese-silica nanodrugs for inducing the ferroptosis of hepatocellular carcinoma cells. Int. J. Pharm. 2019, 572, 118782.
- Li, B.; Zhang, X.; Wu, Z.; Chu, T.; Yang, Z.; Xu, S.; Wu, S.; Qie, Y.; Lu, Z.; Qi, F.; et al. Reducing postoperative recurrence of early-stage hepatocellular carcinoma by a wound-targeted nanodrug. Adv. Sci. 2022, 9, 2200477.
- Dave, V.; Sharma, R.; Gupta, C.; Sur, C. Folic acid modified gold nanoparticle for targeted delivery of Sorafenib tosylate towards the treatment of diabetic retinopathy. Colloids Surf. B Biointerfaces 2020, 194, 111151.
- Cai, H.; Yang, Y.; Peng, Y.; Liu, Y.; Fu, X.; Ji, B. Gold nanoparticles-loaded anti-mir221 enhances antitumor effect of sorafenib in hepatocellular carcinoma cells. Int. J. Med. Sci. 2019, 16, 1541–1548.
- Sukkar, F.; Shafaa, M.; Nagdy, M.; Darwish, W.; Korraa, S. Alginate/CaCO3 hybrid loaded with sorafenib tosylate and gold hexagons: A model for efficient dual (Chemo-Radio) treatment of HepG2 cells. Egypt. J. Chem. 2021, 16, 8309–8321.
- Moni, S.S.; Alam, M.F.; Safhi, M.M.; Sultan, M.H.; Makeen, H.A.; Elmobark, M.E. Development of formulation methods and physical characterization of injectable sodium selenite nanoparticles for the delivery of sorafenib tosylate. Curr. Pharm. Biotechnol. 2020, 21, 659–666.
- Zheng, L.; Li, C.; Huang, X.; Lin, X.; Lin, W.; Yang, F.; Chen, T. Thermosensitive hydrogels for sustained release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials 2019, 216, 119220.
- Jin, S.E.; Jin, H.E. Synthesis, characterization, and three-dimensional structure generation of zinc oxide-based nanomedicine for biomedical applications. Pharmaceutics 2019, 11, 575.
- Nabil, A. Zinc oxide nanoparticle synergizes sorafenib anticancer efficacy with minimizing its cytotoxicity. Oxid. Med. Cell. Longev. 2020, 2020, 1362104.
- György, B.; Biró, E.; Mónika, M.; Tivadar, F. Dual drug delivery of sorafenib and doxorubicin from PLGA and PEG-PLGA polymeric nanoparticles. Polymers 2018, 10, 895.
- Duan, D.W.; Liu, Y. Targeted and synergistic therapy for hepatocellular carcinoma: Monosaccharide modified lipid nanoparticles for the co-delivery of doxorubicin and sorafenib. Drug Des. Devel. Ther. 2018, 12, 2149–2161.
- Mato, E.; Puras, G.; Bell, O.; Agirre, M.; Hernández, R.M.; Igartua, M.; Moreno, R.; Gonzalez, G.; Leiva, A.; Pedraz, J.L. Selective antitumoral effect of sorafenib loaded PLGA nanoparticles conjugated with cetuximab on undifferentiated/anaplastic thyroid carcinoma cells. J. Nanomed. Nanotechnol. 2015, 6, 1000281.
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: Opportunities and challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470.
- Rao, Y.F.; Chen, W.; Liang, X.G.; Huang, Y.Z.; Miao, J.; Liu, Y.; Lou, Y.; Zhang, X.G.; Wang, B.; Tang, R.K. Epirubicin-loaded superparamagnetic iron-oxide nanoparticles for transdermal delivery: Cancer therapy by circumventing the skin barrier. Small 2015, 11, 239–247.
- Taratula, O.; Garbuzenko, O.; Savla, R.; Wang, Y.A.; Minko, H.T. Multifunctional nanomedicine platform for cancer-specific delivery of siRNA by superparamagnetic iron oxide nanoparticles-dendrimer complexes. Curr. Drug Deliv. 2011, 8, 59–69.
- Grillone, A.; Riva, E.R.; Mondini, A.; Forte, C.; Calucci, L.; Innocenti, C.; de Julian Fernandez, C.; Cappello, V.; Gemmi, M.; Moscato, S.; et al. Active targeting of sorafenib: Preparation, characterization, and in vitro testing of drug-loaded magnetic solid lipid nanoparticles. Adv. Healthc. Mater. 2015, 4, 1681–1690.
- Greeshma, T.; Sheena, P.; Rimal, I.; Praseetha, P.K.; Jiji, S.G.; Asha, V.V. Preparation of an efficient and safe polymeric-magnetic nanoparticle delivery system for sorafenib in hepatocellular carcinoma. Life Sci. 2018, 206, 10–21.
- Cai, M.; Li, B.; Lin, L.; Huang, J.; An, Y.; Huang, W.; Zhou, Z.; Wang, Y.; Shuai, X.; Zhu, K. A reduction and pH dual-sensitive nanodrug for targeted theranostics in hepatocellular carcinoma. Biomater. Sci. 2020, 8, 3485–3499.
- Amir, H.; Homayon, A.P.; Mehdi, F.; Fatemeh, F. Synthesis of thermosensitive magnetic nanocarrier for controlled sorafenib delivery. Mater. Sci. Eng. C. 2016, 67, 42–50.
- Dahiya, M.; Awasthi, R.; Dua, K.; Dureja, H. Sorafenib tosylate loaded superparamagnetic nanoparticles: Development, optimization and cytotoxicity analysis on HepG2 human hepatocellular carcinoma cell line. J. Drug Deliv. Sci. Tech. 2023, 79, 104044.
- Feng, S.; Zhou, J.; Li, Z.; Appelman, H.D.; Zhao, L.L.; Zhu, J.Y.; Thomas, D. Sorafenib encapsulated in nanocarrier functionalized with glypican-3 specific peptide for targeted therapy of hepatocellular carcinoma. Colloids Surf. B. 2019, 184, 10.
- Lakshmi, T.K.; Hitesh, K.; Lakshma, N.V.; Madhusudana, M.; Suresh, B.; Deep, P.; Ramakrishna, S. Modulating the site-specific oral delivery of sorafenib using sugar-grafted nanoparticles for hepatocellular carcinoma treatment. Eur. J. Pharm. Sci. 2019, 137, 104978.
- Meng, T.; Li, Y.; Tian, Y.; Ma, M.; Shi, K.; Shang, X.; Yuan, H.; Hu, F. A Hypoxia-sensitive drug delivery system constructed by nitroimidazole and its application in the treatment of hepatocellular carcinoma. AAPS PharmSciTech. 2022, 23, 167.
- Shen, J.; Cai, W.; Ma, Y.; Xu, R.; Huo, Z.; Song, L. hGC33-Modified and sorafenib-loaded nanoparticles have a synergistic anti-hepatoma effect by inhibiting Wnt signaling pathway. Nanoscale Res. Lett. 2020, 15, 220.
- Lin, J.; Zhang, J.; Wang, K.; Guo, S.; Yang, W. Zwitterionic polymer coated sorafenib-loaded Fe3O4 composite nanoparticles induced ferroptosis for cancer therapy. J. Mater. Chem. B 2022, 10, 5784–5795.
- Zhou, K.; Xu, W.; Zhang, X.; Wang, S.; Li, Y.; Yang, L.; Cai, R.; Xu, Q.; Li, G.; Guo, X. Complex of nanocarriers based on the metal polyphenol network: Multi-modal synergistic inhibition of tumor cell proliferation by inducing ferroptosis and photodynamic effect. New J. Chem. 2022, 46, 21962–21967.
- Han, L.; Liang, S.; Mu, W.; Zhang, Z.; Wang, L.; Ouyang, S.; Yao, B.; Liu, Y.; Zhang, N. Amphiphilic small molecular mates match hydrophobic drugs to form nanoassemblies based on drug-mate strategy. Asian J. Pharm. Sci. 2022, 17, 129–138.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
680
Revisions:
3 times
(View History)
Update Date:
07 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




