Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nykia D Walker | -- | 3609 | 2023-06-14 05:40:11 | | | |
| 2 | Nykia D Walker | + 6 word(s) | 3615 | 2023-06-14 05:41:39 | | | | |
| 3 | Jessie Wu | -3 word(s) | 3612 | 2023-06-14 07:08:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bhatia, R.; Chang, J.; Munoz, J.L.; Walker, N.D. Tumor-Derived Exosomes in Preparing the Pre-metastatic Niche. Encyclopedia. Available online: https://encyclopedia.pub/entry/45548 (accessed on 12 January 2026).
Bhatia R, Chang J, Munoz JL, Walker ND. Tumor-Derived Exosomes in Preparing the Pre-metastatic Niche. Encyclopedia. Available at: https://encyclopedia.pub/entry/45548. Accessed January 12, 2026.
Bhatia, Ranvir, Joanna Chang, Jessian L. Munoz, Nykia D Walker. "Tumor-Derived Exosomes in Preparing the Pre-metastatic Niche" Encyclopedia, https://encyclopedia.pub/entry/45548 (accessed January 12, 2026).
Bhatia, R., Chang, J., Munoz, J.L., & Walker, N.D. (2023, June 14). Tumor-Derived Exosomes in Preparing the Pre-metastatic Niche. In Encyclopedia. https://encyclopedia.pub/entry/45548
Bhatia, Ranvir, et al. "Tumor-Derived Exosomes in Preparing the Pre-metastatic Niche." Encyclopedia. Web. 14 June, 2023.
Copy Citation
Tumor-derived exosomes play a multifaceted role in preparing the pre-metastatic niche, promoting cancer dissemination, and regulating cancer cell dormancy. Tumor-derived exosomes are small vesicles that are released by tumor cells and contain a variety of molecules, including proteins, lipids and nucleic acids. They play a key role in cancer progression and metastasis by modulating the tumor microenvironment, promoting cancer cell survival and growth, and communicating with nearby cells. As such, these exosomes can act as vehicles for delivering pro-tumorigenic information and signals, helping to propagate cancer in the body.
cancer metastases
tumor derived extracellular vesicles
nanocarriers
niche
1. Tumor-Derived Exosomes (TD-EVs) Overview
In the endosome compartment, exosome synthesis occurs when multivesicular bodies mature into intraluminal vesicles (Figure 1A). Fusing intraluminal vesicles with a plasma membrane releases exosomes into the extracellular space [1][2]. Exosomes can be retrieved by endocytosis or receptor-mediated uptake, suggesting a selective intercellular communication between the donor and recipient cells [3][4]. Exosomes released from donor cells contain nucleic acids or proteins, which appear to be strategically used to modify the recipient cell’s function in a way that benefits the donor cell [5]. Thus, isolating exosomes, identifying their cargo and their intended target cell are potential biomarkers, diagnostic tools or therapeutic targets for disease progression [5][6]. In healthy cells, exosomes help to maintain normal physiology and are a type of intercellular communication system used mainly by immune cells [5][7]. However, the number of exosomes secreted by cancer cells exceeds those of healthy cells, especially during oncogenesis and tumor suppression [8][9]. The type of exosome cargo isolated from tumor cells consists of mainly nucleic acids associated with RNA processing, including microRNA (miRs), long-noncoding RNAs, and circular RNAs, which are useful cancer biomarkers that are distinguishable from those released from noncancerous cells [10][11][12]. Recent studies, using organoids from colon cancer cells, identified two distinct populations of exosomes, and both were enriched with a unique set of proteins with specific functions that supported colon cancer progression [13], suggesting that exosomes are heterogeneous, which means that their composition and function will differ, as well as what cells are targeted and how they are altered. Developing targeted therapeutic approaches requires studying these interactions and understanding what contributes to exosome heterogeneity within tumors.
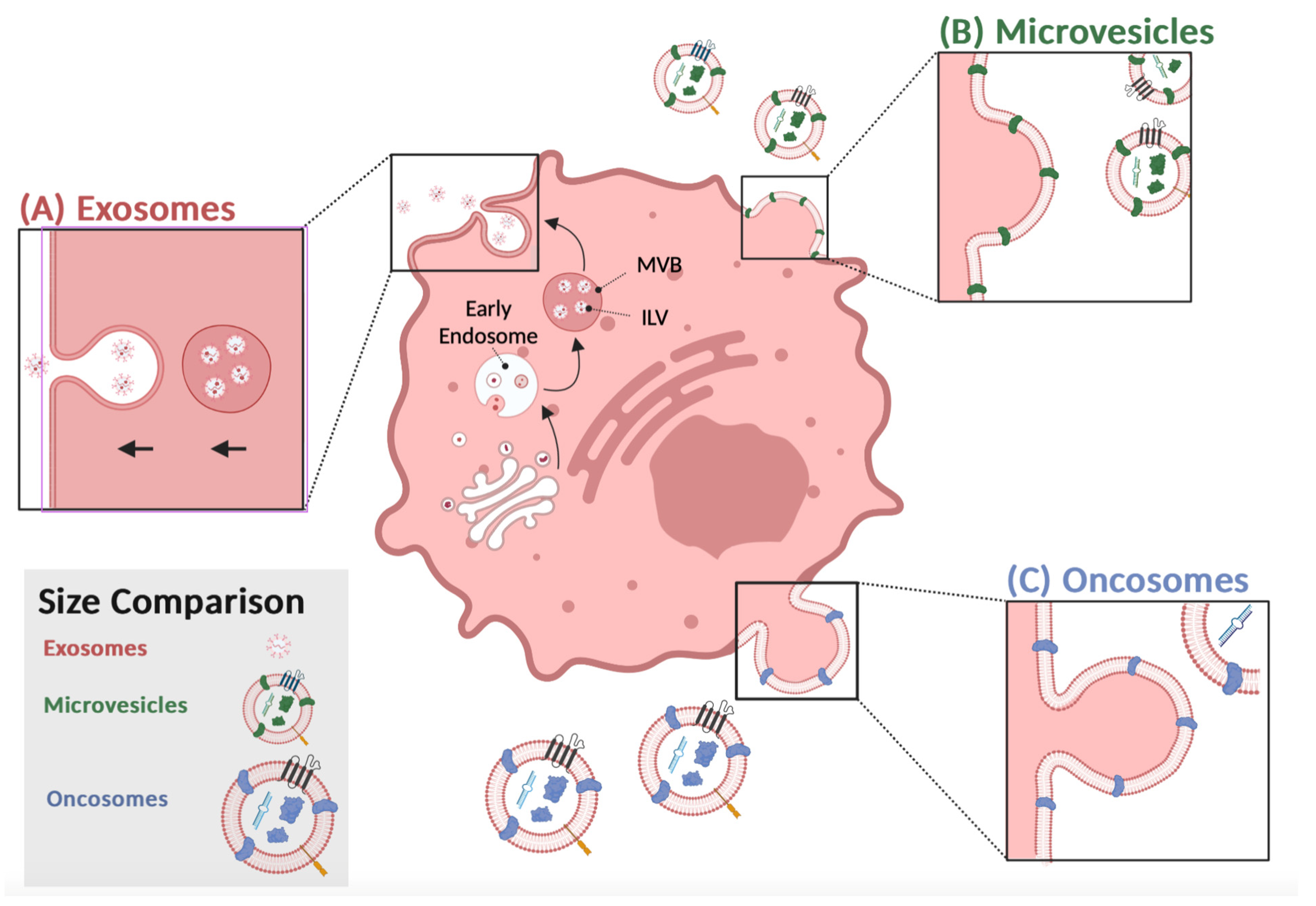
Figure 1. Tumor-derived extracellular vesicles release: (A) Proteins from the membrane, cytosol, or the endomembrane system invaginate in an early endosome. Late endosomes, or multivesicular bodies (MVB), sort proteins in intraluminal vesicles (ILV). Once MVBs fuse with the plasma membrane and release exosomes into the extracellular space. (B) Microvesicles (MV) bleb directly from the membrane, adapting a portion of the parent cell. Microvesicles are released through outward budding. (C) Oncosomes are released through pinched blebbing. The surface proteins and nucleic acids of different colors depict the unique cargo of MV and oncosomes. Proteins of the same color depict overlaps between the vesicles. This figure was created using BioRender.com.
By secreting exosomes into the circulation, tumor cells can communicate with noncancerous cells at distant sites in preparation for tumor dissemination in an autocrine-, paracrine-, or endocrine-like manner [14]. In the bone marrow, exosomes can induce cell-to-cell interactions via gap juxtracrine communication to facilitate cellular dormancy [15][16]. Since exosomes regularly migrate through the circulatory system as extracellular messengers, it is advantageous for these vesicles to contain cholesterol, sphingomyelin, and ganglioside GM3, which act as protective proteins against the complement system, preventing degradation while in circulation [17]. Regarding tumor progression, exosomes promote metastasis by coordinating communication between tumor cells and endothelial or immune cells [18]. Exosomes derived from colon cancer, for example, regulate the vascular volume by stimulating angiogenesis and altering the cellular permeability by targeting KLF2 and KLF4 [19]. Another example in renal carcinoma, CD105+ CSC-derived exosomes promoted endothelial cell vascular differentiation and proliferation through proangiogenic miRs and mRNA transfer [20].
TD-EVs Involvement in Metastatic Disease
Tumor-derived exosomes are a type of extracellular vesicle distinguished from apoptotic bodies, microvesicles, and oncosomes by their size, morphology, and protein markers [21][22][23][24]. Similar to exosomes, tumor cells secrete microvesicles into the extracellular space, but they are called ectosomes, since their formation involves the outward budding of the plasma membrane (Figure 1B) [25]. They are secreted from the plasma membrane and can transfer cytoskeletal and microtubule proteins by autologous communication with cells within the tumor microenvironment to facilitate proliferation [26]. In addition, they can also stimulate adjacent cells by transferring proteins that activate oncogenic signaling cascades to induce cell invasion.
Furthermore, microvesicles have been isolated from the peripheral blood of cancer patients, suggesting that they can promote long-distance communication to influence metastatic spread or aid in preparing the recruitment of the pre-metastatic niche [27][28]. According to Muralidharan-Chari et al., ARF6 regulates the sorting and shedding of microvesicles from tumor cells at specific regions of the plasma membrane. Microvesicles are directly transferred to the plasma membrane of the recipient cell, altering their function to promote cell growth [29]. In contrast, exosomes bind to their target cells and release their contents internally, activating signaling pathways and altering gene expressions in those cells [30]. Numerous studies have described overlapping markers that are shared between microvesicles and exosomes. In contrast, the proteomics analysis of microvesicle cargo was similar to that of the plasma membrane of the donor cell, whereas exosome biogenesis or cargo was synthesized in the endosomes (Figure 2) [27].
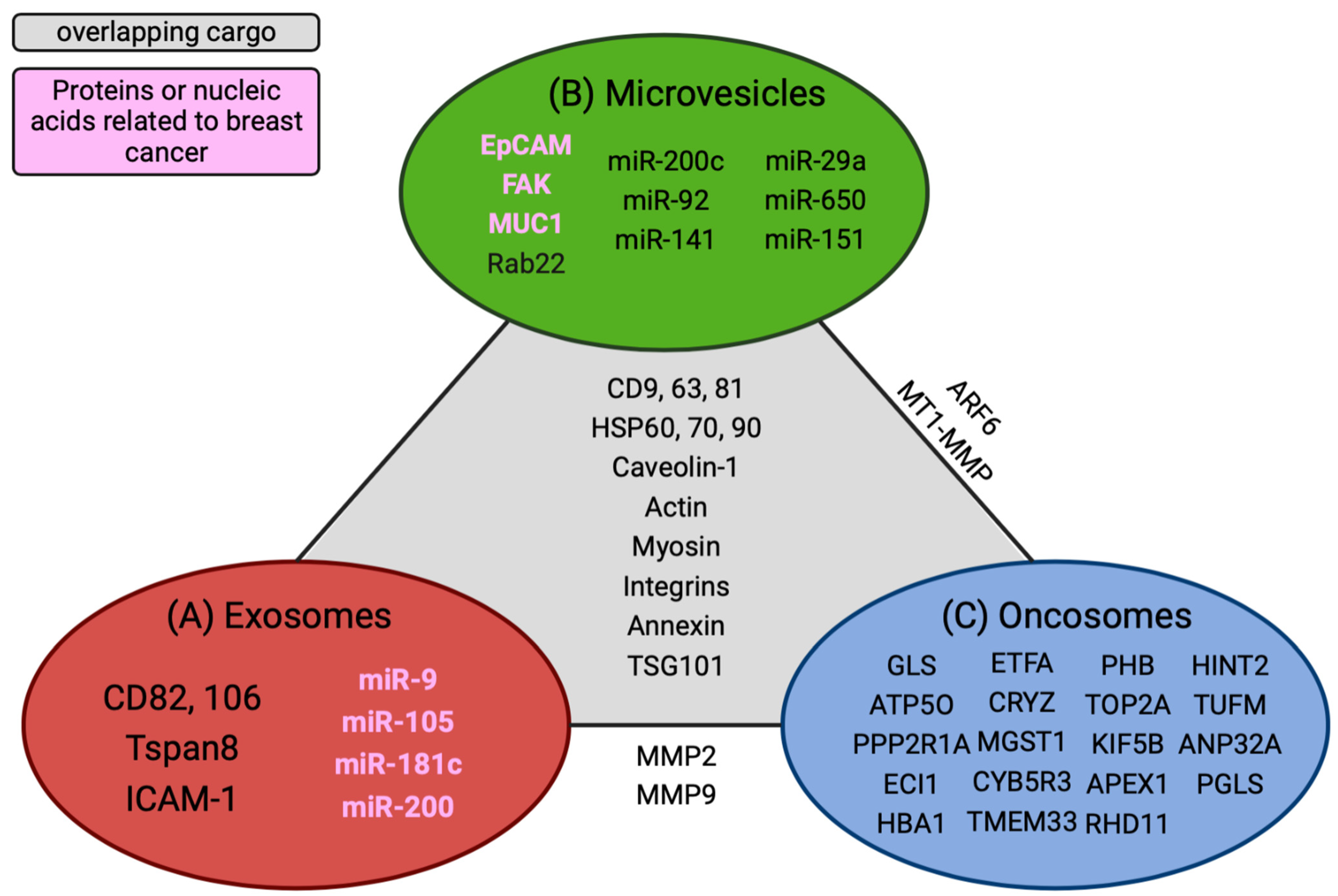
Figure 2. Overlapping cargo comparisons between extracellular vesicles. Unique nucleic acids and proteins are grouped within each specific vesicle type. (A) Identified exosome cargo. (B) Microvesicle cargo. (C) Common oncosome cargo. Overlapping cargos that are common among all EVs are delineated in the gray section of the diagram. Cargos found between two EVs are listed in tangent with the solid line that connects them. Biomarkers or nucleic acids highlighted in pink are associated with breast cancer. This figure was created using BioRender.com.
Large oncosomes (LO) are another type of extracellular vesicle worthy of attention for their involvement in tumor progression. They are larger (1–10 μm) compared to microvesicles or exosomes, are secreted by tumor cells, and transport proteins from the plasma membrane to recipient cells [25] (Figure 1C). As a result of membrane blebbing, membrane proteins from the donor cell are transferred to the recipient cell, mimicking the donor’s physiological state. Oncosomes are a source of oncogenic material excreted by metastatic cells and often have similar EV-associated markers, such as CD9, and CD81 is enriched in LOs but to a lesser degree than exosomes or microvesicles [31]. In addition, they contain signaling factors, RNA processing molecules, or growth factors related to tumor progression [32]. Anaglous to the other types of TD-EVs described above, they are more prevalent in metastatic than benign tumors, suggesting that the cargo are potential biomarkers [33]. Isolating these vesicles from the peripheral blood and analyzing their cargo will help researchers decipher their function throughout tumor dissemination to aid researchers in developing methods to inhibit their transition from the primary tumor site to distant tissue.
Oncosomes are secreted in large amounts by tumor cells, and the amount seems to correlate with aggressive tumors. Di Vizio et al. demonstrated that oncosomes are secreted in large amounts upon the silencing of a protein, Diaphanous-related formin-3 (DIAPH3), which is involved in cell motility [34]. Depending on the type of cargo, LOs can induce an amoeboid shape in the recipient cell, which is associated with cell invasion and migration [35]. For instance, prostate cancer cell overexpression of Akt also triggers the release of oncosomes, resulting in cells with amoeboid migration properties rather than mesenchymal shapes; this implies that the tumor cells likely dictate the invasive cells’ migration properties based on the type of matrix they will traverse. Conely et al. measured the mRNA expression from LOs compared to microvesicles and exosomes isolated from glioblastoma cell lines and the peripheral blood of breast cancer patients. They discovered that the mRNA cargo was the same between the TD-EVs and within the two sample sets, with 5% of the genes unique to Los associated with the plasma membrane, transporters, and receptors [36].
Overall, TD-EVs are secreted in higher amounts than normal cells. They are potential diagnostic markers based solely on the number of particles isolated in bodily fluids from cancer patients. Additionally, the three TD-EVs carry different cargos, which use other mechanisms to drive metastasis. The caveat is that tetraspanins, ALIX, and TSG101 are commonly shared proteins among TD-EVs, requiring additional markers to stratify the unique features associated with the different TD-EVs. An ideal approach would be quantifying the protein markers enriched in one type of TD-EV over another and the tumor markers associated with specific vesicles. Mincaicchi et al. studied used enrichment strategies in proteomics to isolate three different TD-EVs and compared the protein expressions among them in search of distinct proteins solely expressed in LOs (Figure 2). They classified LOs as a specific type of TD-EV that influences metabolic changes in prostate cancer patients via GOT1 uptake in recipient cells to promote cell proliferation, while exosome proteins are involved in driving cell motility and adhesion [31]. These studies provided insight into the mechanism used by tumor cells to alter naïve cell functions. Additionally, despite having similar membrane proteins, TD-EVs invoke different functions to perpetuate metastasis and should be further investigated as potential nanomedicine strategies to alter their communication with their recipient cells.
2. Exosomal-Induced Metastasis via Organotropism
Metastatic sites are well established to be disease- and organ-dependent [37]; tissue tropism is likely the interactions between the cancer cells and microenvironment, especially at distant sites. Stephen Paget’s theory of seed and soil suggests that disseminating tumor cells (seed) must recognize specific organ cell entry and colonization. He speculated that breast cancer patients undergo metastasis, in which secondary outgrowths are organ-specific compared to other types of cancer [38]. Expanding on Paget’s observations, the metastatic niche model proposed by Psaila et al. suggested that organs are primed to mimic the primary tumor milieu in preparation for colonization by disseminating cancer cells to undergo colonization and fostering cellular dormancy [39][40]. A premetastatic niche consists of suppressive immune cells, a promiscuous extracellular matrix, and supporting stromal cells that attract disseminating cancer cells for colonization [39][41]. Premetastatic niches are crucial for successful tumor cell colonization and are mediated by growth factors, cytokines, and exosomes released from the primary tumor [37][42][43].
In a series of experiments, Hoshino et al. showed that breast cancer cell lines with specific tissue tropisms in the lung, liver, brain, or bone secrete exosomes with a matching biodistribution and preferential uptake at these sites [44][45]. Notably, mice pre-educated with exosomes derived from a lung trophic cell line displayed increased lung metastasis when injected with bone trophic cancer cells. These findings suggest that exosomes may be pivotal in establishing a pre-metastatic niche via organotropism. Integrins α6β1 and α6β4 were linked to lung metastasis, integrin αvβ5 to liver metastasis, and integrin β3 to brain metastasis. Altogether, the elegant work of Hoshino et al. suggested a profound phenomenon for exosome-driven cancer metastasis, suggesting that specific integrins may serve as a zip code for exosomes and directs them to the appropriate sites to deliver their cargo and reprogram the pre-metastatic niche to promote tumor cell colonization in distant sites. In addition to organotropism, exosome composition, such as its lipid moieties, might dictate their uptake and drive tumor progression in glioblastoma cells [46]. Changes in the pH within the tumor microenvironment have also been postulated to drive exosome secretion and uptake by metastatic melanoma cells, which alters the lipid composition of the cell. Additionally, an acidic tumor milieu correlates with an increased TD-EV caveloin-1 cargo, which is associated with malignancy in melanoma patients [47].
One approach executed by primary tumor exosomes to promote metastasis involves altering the pre-metastatic niche metabolic landscape by making it more hospitable to Warburg-like tumor cell colonization. Circulating miR-122, a regulator of pyruvate kinase expression, was identified as a marker for metastatic progression in early-stage breast cancer. Upon intravenous injection, breast cancer-derived exosomes containing miR-122 were taken up by lung fibroblasts and brain astrocytes, causing decreased pyruvate kinase and GLUT1 expression with diminished glucose uptake [48]. The subsequent intracardiac injection of metastatic breast cancer cells led to increased colonization compared to no colonization in mice without exosome treatment (Figure 3A,B). There is a possibility that primary tumor cells sense metabolic changes in the pre-metastatic niche as a trigger to begin metastatic dissemination. Alternatively, it is possible that metastatic tumor cells merely encounter a positive selection in metabolically favorable environments upon systemic dissemination. Further research is needed to tease out these hypotheses and the involvement of exosomes.
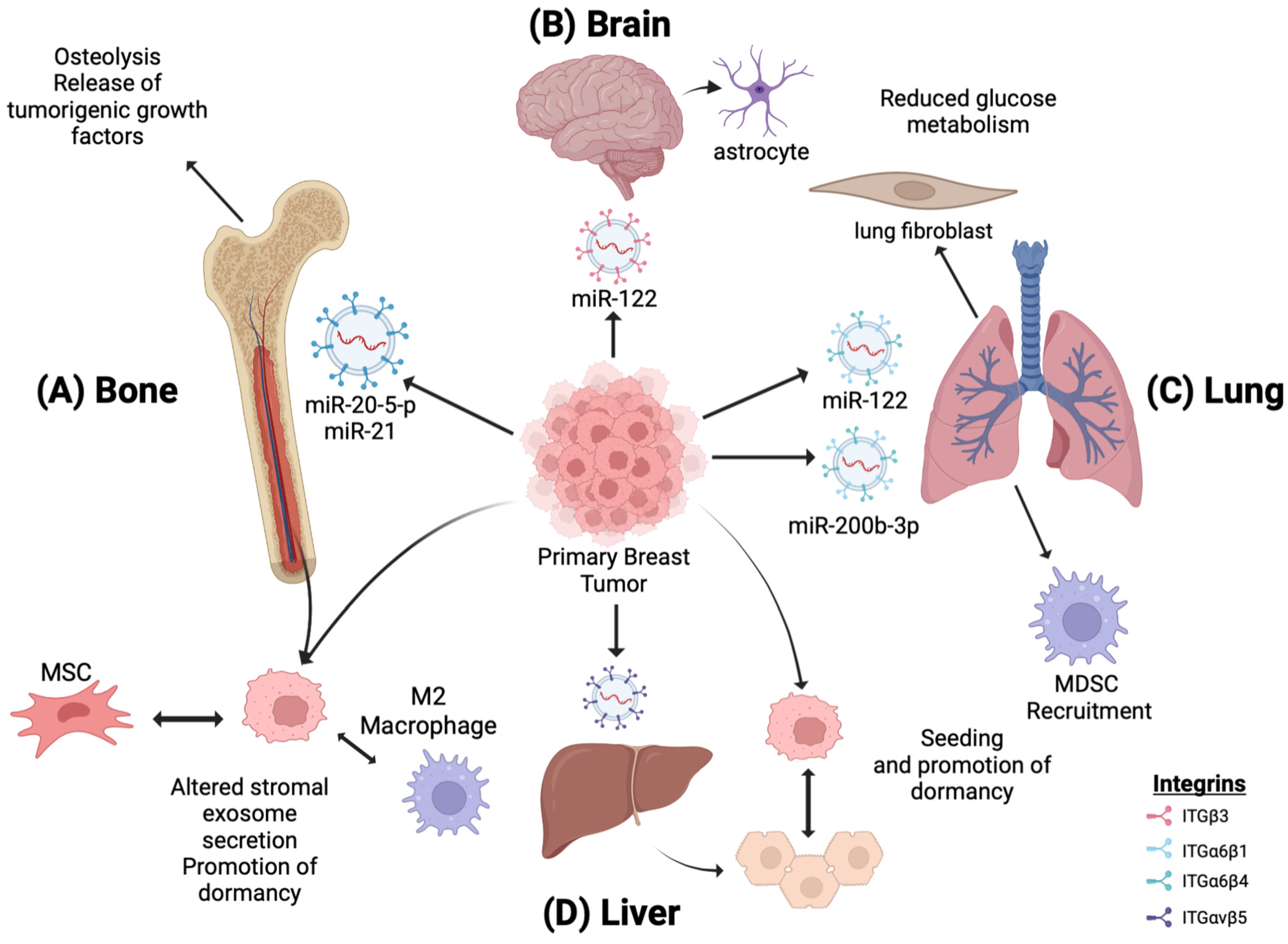
Figure 3. Tumor-derived exosome-induced tissue tropism by miR regulation. EVs secreted by primary breast tumors migrate to distal sites, priming them for breast cancer metastases. (A) miR-20-5-p and MiR-21-loaded EVs promote osteoclastogenesis, osteolysis, and metastatic bone invasion. (B,C) miR-122 downregulates the glucose uptake and metabolism in astrocytes and lung fibroblasts, creating a metabolically favorable environment for Warburg-induced tumor cells. (C) miR-200b-3p is taken up by alveolar epithelial type II cells in the lungs, leading to the recruitment of MDSCs and immunosuppressive macrophages and establishing an immunosuppressive premetastatic niche. (D) In the liver, primary breast EVs induce changes in the exosomal secretion of resident hepatocytes, promoting metastatic cancer seeding. (A,D) Additionally, disseminated breast cancer cells promote dormancy by altering the exosome secretion of residential bone marrow and liver cells, respectively. This figure was using BioRender.com
In another study involving metabolic changes in brain metastasis, disseminated breast cancer cells engulfed astrocyte-derived exosomes containing miR19a and subsequently lost the expression of the Phosphatase and tensin homolog (PTEN), a tumor-suppressor gene [49]. PTEN loss activated the PI3K signaling pathway, upregulating aerobic glycolysis over oxidative phosphorylation, creating an anabolic, pro-proliferative state characteristic of Warburg tumors. It remains unclear how astrocytes are initially reprogrammed to secrete these miR19a-containing exosomes by disseminated breast cancer cells or exosomes. Nonetheless, these two studies highlight the metabolic changes within the brain premetastatic niche, promoting Warburg tumor metabolism and facilitating breast cancer metastasis, suggesting that they may be particularly susceptible to Warburg-based therapeutic strategies such as:
1. Targeting glycolytic enzymes preferentially elevated in cancer cells (GLUT1, HKII, LDHA, and PKM2).
2. Stunting HIF-1α signaling.
3. Engineering chemo-prodrugs that become active under hypoxic and acidic conditions.
Other studies showed that primary breast cancer cells utilize exosomes to alter bone metabolism to a resorptive state and promote metastasis. Breast cancer cells use exosomes to stimulate bone resorption. Osteolysis enables the release of tumorigenic growth factors from the bone matrix, such as TGF-ꞵ, IGF1, and bone morphogenetic proteins, creating a more favorable environment for metastasis. Exosomes containing miR-20a-5p secreted by TNBC cells promote the proliferation and differentiation of pre-osteoclasts by inhibiting SRC kinase signaling [50]. Additionally, the orthotopic implantation of SCP28, a bone metastatic MDA-MB-231 subline, induces the loss of trabecular bone density in mice. A 21-day priming with SCP28 exosomes increased the tumor burden in the hind limbs of mice and accelerated bone metastasis. Mechanistically, miR-21 was identified within the SCP28 exosomes as an osteoclast promoter by inhibiting programmed cell death-4 protein function (Figure 3C). These findings suggest that distal exosome secretion from primary breast tumors can induce osteoclast activity in the BM, thereby driving osteolysis and creating a microenvironment that promotes metastasis [51].
In addition to driving metabolic changes that alter the secondary sites for tumor cell dissemination, breast cancer exosomes also modulate the immune landscape of the pre-metastatic niche. For example, Qi et al. showed that Lin28B-expressing tumors secrete exosomes with low let-7s expression, which induces the stemness and migratory capability of the primary tumor. However, upon migration to the lung, exosome-induced neutrophil recruitment and polarization towards an anti-inflammatory N2 phenotype occur, establishing an immunosuppressive niche that facilitates metastasis [52].
Gu et al. observed similar findings in a 4T1 primary breast line. Exosomes isolated from 4T1 primary breast cancer cells were intravenously injected into mice. Upon uptake in the lungs, alveolar epithelial type II cells upregulate the expression of C-C motif chemokine ligand 2 (CCL2), which leads to the recruitment of myeloid-derived suppressor cells and immunosuppressive macrophages, promoting an immunosuppressive, pre-metastatic niche that promoted 4T1 lung colonization. Specifically, miR-200b-3p was identified as the exosome cargo driving CCL2 expression through PTEN inhibition. In these studies, the recruitment of immunosuppressive cells by breast cancer exosomes was suggested as an alternative method of driving lung metastasis [53].
Tumor Secretome Fosters Metastasis
Cells that migrate from the primary tumor to metastatic sites have received attention, because they promote distant metastases, are used as prognostic markers, and are chemoresistant. Perhaps migratory cells should be considered a part of the tumor’s secretome, since they can efficiently communicate with the circulatory system and tumor microenvironment. Three subtypes of migratory cells are involved in fostering metastasis and relapse and are briefly described below. (A) Cancer stem cells (CSCs) have been isolated from the peripheral blood of cancer patients and are involved in promoting metastases, chemoresistance, and cellular dormancy [54][55]. Furthermore, CSCs can also be viewed as a type of tumor-derived secretome that facilitates metastasis within tumor microenvironments or pre-metastatic niches [56]. (B) Circulating tumor cells (CTCs) isolated from the peripheral blood of cancer patients share phenotypic and functional features similar with CSCs and are a prognostic marker of cancer relapse [57][58]. Some reports have suggested that CTCs leave the primary tumor site as individual cells but cluster together to colonize at metastatic sites in a cooperative manner [59]. However, the size and number of CTCs in the blood can vary, depending on the type of cancer, the stage of the cancer, and the patient’s individual immune system. (C) Disseminating tumor cells (DTCs) have similar characteristics to CSCs, such as the ability to evade the immune system and to resist treatment. However, DTCs also have some unique properties, such as the ability to travel through the bloodstream and to lodge in distant organs. For example, in bone marrow aspirates or lymphatics, DTCs have been isolated and are considered communicative cells that stimulate bone cells to gain entry and colonize [60]. The question of whether CTCs are analogous to CSCs in blood while DTCs are CSCs at metastatic sites remains unknown. Nonetheless, these cells have been implemented in promoting metastasis, and further studies are warranted to decipher the mechanism used by CSCs for colonization as a potential strategy to inhibit metastasis, as well as to elucidate if they secrete exosomes to aid them as they travel through the blood.
3. Challenges and Drawbacks to Clinical Application of Exosomes
While exosomes hold promise for various therapeutic applications, they also present several disadvantages when treating cancer patients. Here are some of the key drawbacks:
1. Exosome heterogeneity is thought to be a key factor in determining their function and effectiveness in intercellular communication. By displaying different properties and compositions, exosomes can interact with different target cells and activate different signaling pathways [61]. Moreover, the cells that secrete exosomes can also be different between individuals, making it difficult to identify the source of exosome heterogeneity, especially in cancer, where cancer cells themselves are heterogenous [62][63]. Efforts have been made to develop standardized protocols for exosome isolation, purification, and characterization. These protocols aim to ensure the consistent quality and purity of exosome preparations, reducing the heterogeneity and allowing for more reliable and reproducible therapeutic applications. However, the complexity of exosome heterogeneity, removal of undesirable contaminants, and different isolation methods make it difficult to standardize the procedures.
2. A consensus is needed for acceptable purification standards that are well tolerated with a maximum payload. Comparisons of various isolation steps across different cell lines or ex vivo samples such as urine and blood are currently underway [64][65][66]; however, the samples are normally collected in a laboratory setting without consideration for good manufacturing practices or differences in storage conditions, which also need to be standardized.
3. Exosomes have limited cargo capacity due to their size, which affects how much and what type of therapeutic agents can be loaded into them, as well as their delivery speed and clearance rate [67]. Furthermore, because exosomes are released from cells in a variety of sizes, shapes, and compositions, it is difficult to ensure a consistent therapeutic effect when using them as targets. Coating exosomes with stealth materials such as polyethylene glycol (PEG) or using a nanocarrier can increase an exosome’s circulation time in the bloodstream.
4. Targeting exosomes at breast cancer cells specifically can be challenging without proper selective markers, since exosomes are distributed throughout the body and potentially interact with healthy tissues, leading to off-target effects and reduced therapeutic efficiency. Additionally, exosome instability can also be problematic for cancer treatment, as their components can undergo rapid changes when exposed to different environmental conditions. Exosomes can degrade quickly, making them difficult to transport and store for long periods of time. Perhaps synthesizing exosomes from the same cell type will have the same surface proteins that are recognized and engulfed by the recipient cell. Additionally, using nanocarrier systems will protect exosomes from degradation and enhance their delivery to specific target cells.
5. The large-scale production of exosomes for clinical use can be technically challenging and costly. Standardized manufacturing processes need to be established to ensure the consistent quality, purity, and potency of exosome-based therapeutics. Scaling up production while maintaining batch-to-batch consistency remains a significant hurdle. Moreover, there is a need for standardized methods for the isolation, characterization, and quantification of exosomes to ensure consistency and reproducibility in their use as therapeutic agents.
6. As with any novel therapeutic approach, exosome-based treatments must undergo rigorous testing and regulatory approval processes. Regulatory challenges associated with exosome clinical implementation that include characterization, standardization, and administration are currently under review [68]. Ensuring exosome-based delivery system safety and efficacy, along with addressing concerns regarding biodistribution and bioaccumulation as potential long-term side effects, is crucial to elucidate before widespread clinical implementation can occur. Further, regulatory agencies, including the FDA and European Medicine Agency, are actively working to establish guidelines and frameworks specifically tailored to exosome-based therapies [69][70]. Although these advances are promising in addressing exosome therapy limitations, additional research and clinical trials are needed to validate their effectiveness and safety.
References
- Edgar, J.R. Q&A: What are exosomes, exactly? BMC Biol. 2016, 14, 1–7.
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 1–19.
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514.
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Keller, S.; Ridinger, J.; Rupp, A.-K.; Janssen, J.W.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86.
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015, 6, 203.
- Bebelman, M.P.; Janssen, E.; Pegtel, D.M.; Crudden, C. The forces driving cancer extracellular vesicle secretion. Neoplasia 2021, 23, 149–157.
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome mediated communication within the tumor microenvironment. J. Control. Release 2015, 219, 278–294.
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977.
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061.e18.
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145.
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Mathivanan, S.; Ji, H.; Simpson, R.J. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell Proteom. 2013, 12, 587–598.
- Zhang, H.-G.; Grizzle, W.E. Exosomes: A novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am. J. Pathol. 2014, 184, 28–41.
- Bliss, S.A.; Sinha, G.; Sandiford, O.A.; Williams, L.M.; Engelberth, D.J.; Guiro, K.; Isenalumhe, L.L.; Greco, S.J.; Ayer, S.; Bryan, M. Mesenchymal Stem Cell–Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer Res. 2016, 76, 5832–5844.
- Park, J.M.; Munoz, J.L.; Won, B.W.; Bliss, S.A.; Greco, S.J.; Patel, S.A.; Kandouz, M.; Rameshwar, P. Exogenous CXCL12 activates protein kinase C to phosphorylate connexin 43 for gap junctional intercellular communication among confluent breast cancer cells. Cancer Lett. 2013, 331, 84–91.
- Marleau, A.M.; Chen, C.-S.; Joyce, J.A.; Tullis, R.H. Exosome removal as a therapeutic adjuvant in cancer. J. Transl. Med. 2012, 10, 134.
- Ge, R.; Tan, E.; Sharghi-Namini, S.; Asada, H.H. Exosomes in cancer microenvironment and beyond: Have we overlooked these extracellular messengers? Cancer Microenviron. 2012, 5, 323–332.
- Lu, S.; Ding, X.; Wang, Y.; Hu, X.; Sun, T.; Wei, M.; Wang, X.; Wu, H. The relationship between the network of non-coding RNAs-molecular targets and N6-methyladenosine modification in colorectal cancer. Front. Cell Dev. Biol. 2021, 9, 3393.
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011, 71, 5346–5356.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell. Biol. 2013, 200, 373–383.
- Zhang, X.; Yuan, X.; Shi, H.; Wu, L.; Qian, H.; Xu, W. Exosomes in cancer: Small particle, big player. J. Hematol. Oncol. 2015, 8, 83.
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727.
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053.
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51.
- Di Vizio, D.; Kim, J.; Hager, M.H.; Morello, M.; Yang, W.; Lafargue, C.J.; True, L.D.; Rubin, M.A.; Adam, R.M.; Beroukhim, R. Oncosome formation in prostate cancer: Association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 2009, 69, 5601–5609.
- Menck, K.; Sivaloganathan, S.; Bleckmann, A.; Binder, C. Microvesicles in cancer: Small size, large potential. Int. J. Mol. Sci. 2020, 21, 5373.
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of extracellular communication during cancer progression. J. Cell. Sci. 2010, 123, 1603–1611.
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885.
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261.
- Minciacchi, V.R.; You, S.; Spinelli, C.; Morley, S.; Zandian, M.; Aspuria, P.-J.; Cavallini, L.; Ciardiello, C.; Sobreiro, M.R.; Morello, M. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 2015, 6, 11327.
- Ciardiello, C.; Migliorino, R.; Leone, A.; Budillon, A. Large extracellular vesicles: Size matters in tumor progression. Cytokine Growth Factor Rev. 2020, 51, 69–74.
- Morello, M.; Minciacchi, V.; De Candia, P.; Yang, J.; Posadas, E.; Kim, H.; Griffiths, D.; Bhowmick, N.; Chung, L.; Gandellini, P. Large oncosomes mediate intercellular transfer of functional microRNA. Cell Cycle 2013, 12, 3526–3536.
- Di Vizio, D.; Morello, M.; Dudley, A.C.; Schow, P.W.; Adam, R.M.; Morley, S.; Mulholland, D.; Rotinen, M.; Hager, M.H.; Insabato, L. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 2012, 181, 1573–1584.
- Graziani, V.; Rodriguez-Hernandez, I.; Maiques, O.; Sanz-Moreno, V. The amoeboid state as part of the epithelial-to-mesenchymal transition programme. Trends Cell Biol. 2022, 32, 228–242.
- Conley, A.; Minciacchi, V.R.; Lee, D.H.; Knudsen, B.S.; Karlan, B.Y.; Citrigno, L.; Viglietto, G.; Tewari, M.; Freeman, M.R.; Demichelis, F. High-throughput sequencing of two populations of extracellular vesicles provides an mRNA signature that can be detected in the circulation of breast cancer patients. RNA Biol. 2017, 14, 305–316.
- Aguado, B.A.; Bushnell, G.G.; Rao, S.S.; Jeruss, J.S.; Shea, L.D. Engineering the pre-metastatic niche. Nat. Biomed. Eng. 2017, 1, 0077.
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573.
- Psaila, B.; Lyden, D. The metastatic niche: Adapting the foreign soil. Nat. Rev. Cancer 2009, 9, 285–293.
- Ghajar, C.M. Metastasis prevention by targeting the dormant niche. Nature Reviews. Cancer 2015, 15, 238.
- Doglioni, G.; Parik, S.; Fendt, S.-M. Interactions in the (pre) metastatic niche support metastasis formation. Front. Oncol. 2019, 9, 219.
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827.
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317.
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335.
- Pantel, K. Blood-based analysis of circulating cell-free DNA and tumor cells for early cancer detection. PLoS Med. 2016, 13, e1002205.
- Toda, Y.; Takata, K.; Nakagawa, Y.; Kawakami, H.; Fujioka, S.; Kobayashi, K.; Hattori, Y.; Kitamura, Y.; Akaji, K.; Ashihara, E. Effective internalization of U251-MG-secreted exosomes into cancer cells and characterization of their lipid components. Biochem. Biophys. Res. Commun. 2015, 456, 768–773.
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222.
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.; Chin, A.R. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194.
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.-C.; Li, P.; Li, M.; Wang, X.; Zhang, C. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104.
- Guo, L.; Zhu, Y.; Li, L.; Zhou, S.; Yin, G.; Yu, G.; Cui, H. Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1. Cancer medicine 2019, 8, 5687–5701.
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11, 1429.
- Qi, M.; Xia, Y.; Wu, Y.; Zhang, Z.; Wang, X.; Lu, L.; Dai, C.; Song, Y.; Xu, K.; Ji, W. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat. Commun. 2022, 13, 897.
- Gu, P.; Sun, M.; Li, L.; Yang, Y.; Jiang, Z.; Ge, Y.; Wang, W.; Mu, W.; Wang, H. Breast Tumor-Derived Exosomal MicroRNA-200b-3p Promotes Specific Organ Metastasis Through Regulating CCL2 Expression in Lung Epithelial Cells. Front. Cell Dev. Biol. 2021, 9, 1572.
- Ross, A.A.; Cooper, B.W.; Lazarus, H.M.; Mackay, W.; Moss, T.J.; Ciobanu, N.; Tallman, M.S.; Kennedy, M.J.; Davidson, N.E.; Sweet, D. Detection and viability of tumor cells in peripheral blood stem cell collections from breast cancer patients using immunocytochemical and clonogenic assay techniques. Blood 1993, 82, 2605–2610.
- Schulenburg, A.; Blatt, K.; Cerny-Reiterer, S.; Sadovnik, I.; Herrmann, H.; Marian, B.; Grunt, T.W.; Zielinski, C.C.; Valent, P. Cancer stem cells in basic science and in translational oncology: Can we translate into clinical application? J. Hematol. Oncol. 2015, 8, 1–21.
- Bliss, S.A.; Paul, S.; Pobiarzyn, P.W.; Ayer, S.; Sinha, G.; Pant, S.; Hilton, H.; Sharma, N.; Cunha, M.F.; Engelberth, D.J. Evaluation of a developmental hierarchy for breast cancer cells to assess risk-based patient selection for targeted treatment. Sci. Rep. 2018, 8, 367.
- Mohme, M.; Riethdorf, S.; Pantel, K. Circulating and disseminated tumour cells—Mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 2017, 14, 155–167.
- Matikas, A.; Kotsakis, A.; Apostolaki, S.; Politaki, H.; Perraki, M.; Kalbakis, K.; Nikolaou, M.; Economopoulou, P.; Hatzidaki, D.; Georgoulias, V. Detection of circulating tumour cells before and following adjuvant chemotherapy and long-term prognosis of early breast cancer. Br. J. Cancer 2022, 126, 1563–1569.
- Massagué, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306.
- Braun, S.; Vogl, F.D.; Naume, B.; Janni, W.; Osborne, M.P.; Coombes, R.C.; Schlimok, G.; Diel, I.J.; Gerber, B.; Gebauer, G. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 2005, 353, 793–802.
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.E.; Lehtiö, J.; El Andaloussi, S. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519.
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738.
- Yakubovich, E.; Polischouk, A.; Evtushenko, V. Principles and Problems of Exosome Isolation from Biological Fluids. Biochem. Suppl. Ser. A Membr. Cell Biol. 2022, 16, 115–126.
- Alvarez, M.L.; Khosroheidari, M.; Ravi, R.K.; DiStefano, J.K. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012, 82, 1024–1032.
- Lobb, R.J.; Becker, M.; Wen Wen, S.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031.
- Muller, L.; Hong, C.-S.; Stolz, D.B.; Watkins, S.C.; Whiteside, T.L. Isolation of biologically-active exosomes from human plasma. J. Immunol. Methods 2014, 411, 55–65.
- Song, J.; Song, B.; Yuan, L.; Yang, G. Multiplexed strategies toward clinical translation of extracellular vesicles. Theranostics 2022, 12, 6740.
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: Insights from drug delivery to clinical perspectives. Nanomaterials 2021, 11, 1481.
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal. 2022, 20, 145.
- Li, G.; Chen, T.; Dahlman, J.; Eniola-Adefeso, L.; Ghiran, I.C.; Kurre, P.; Lam, W.A.; Lang, J.K.; Marbán, E.; Martín, P. Current challenges and future directions for engineering extracellular vesicles for heart, lung, blood and sleep diseases. J. Extracell. Vesicles 2023, 12, 12305.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
854
Revisions:
3 times
(View History)
Update Date:
14 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




