Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Edoardo Sciatti | -- | 1594 | 2023-06-14 03:19:10 | | | |
| 2 | Rita Xu | Meta information modification | 1594 | 2023-06-14 03:22:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sciatti, E.; Merlo, A.; Scangiuzzi, C.; Limonta, R.; Gori, M.; D’elia, E.; Aimo, A.; Vergaro, G.; Emdin, M.; Senni, M. Soluble Suppression of Tumorigenicity-2 in Heart Failure. Encyclopedia. Available online: https://encyclopedia.pub/entry/45543 (accessed on 07 February 2026).
Sciatti E, Merlo A, Scangiuzzi C, Limonta R, Gori M, D’elia E, et al. Soluble Suppression of Tumorigenicity-2 in Heart Failure. Encyclopedia. Available at: https://encyclopedia.pub/entry/45543. Accessed February 07, 2026.
Sciatti, Edoardo, Anna Merlo, Claudio Scangiuzzi, Raul Limonta, Mauro Gori, Emilia D’elia, Alberto Aimo, Giuseppe Vergaro, Michele Emdin, Michele Senni. "Soluble Suppression of Tumorigenicity-2 in Heart Failure" Encyclopedia, https://encyclopedia.pub/entry/45543 (accessed February 07, 2026).
Sciatti, E., Merlo, A., Scangiuzzi, C., Limonta, R., Gori, M., D’elia, E., Aimo, A., Vergaro, G., Emdin, M., & Senni, M. (2023, June 14). Soluble Suppression of Tumorigenicity-2 in Heart Failure. In Encyclopedia. https://encyclopedia.pub/entry/45543
Sciatti, Edoardo, et al. "Soluble Suppression of Tumorigenicity-2 in Heart Failure." Encyclopedia. Web. 14 June, 2023.
Copy Citation
There has been growing interest in the risk stratification for heart failure, and the use of multiple biomarkers to identify different pathophysiological processes associated with this condition. One such biomarker is soluble suppression of tumorigenicity-2 (sST2), which has shown some potential for integration into clinical practice. sST2 is produced by both cardiac fibroblasts and cardiomyocytes in response to myocardial stress. Other sources of sST2 are endothelial cells of the aorta and coronary arteries and immune cells such as T cells.
ST2
heart failure
biomarker
1. Introduction
Heart failure (HF) is a complex clinical syndrome resulting from a diverse range of etiologies. The prevalence of HF appears to be 1–2% of adults, reaching more than 10% of those aged 70 years or more [1]. The prevalence and incidence of HF have been increasing in the last decades due to both better survival of many cardiac diseases and improved care of those patients already diagnosed with HF. Additionally, population aging and the emerging pandemic of cardiovascular (CV) disease in developing countries presage a rise in the incidence and prevalence of HF globally [2]. Nevertheless, mortality and morbidity associated with HF remain high. Recent European data show that the rate of all-cause mortality in 1 year is 8.1% and the rate of hospitalization in 1 year is 28.2% [3]. In this scenario, early diagnosis, accurate identification of disease severity, and risk stratification appear to be crucial to proper HF management.
The most commonly used HF biomarkers in clinical practice are B-type natriuretic peptides (NPs), which are useful in the diagnostic work-up, for risk stratification and in defining the best clinical management. NPs have well-known limitations, as their circulating levels are affected by renal dysfunction, age, obesity, atrial fibrillation, and several cardiac and non-cardiac conditions other than HF [4]. Moreover, although they are considered to be the gold-standard tests to diagnose HF in patients with acute dyspnea, their prognostic utility in the acute setting seems limited [5][6], and their role in guiding treatment has not yet been clearly established [7]. Indeed, the Guiding Evidence-based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT) trial showed that a therapeutic strategy guided by N-terminal pro-B-type NP (NT-proBNP) does not lead to better outcomes than a usual care strategy [8]. In recent years, there has been growing interest in the risk stratification for HF, and the use of multiple biomarkers to identify different pathophysiological processes associated with this condition [9]. One such biomarker is soluble suppression of tumorigenicity-2 (sST2), which has shown some potential for integration into clinical practice [10]. The Serial sST2 Testing in Chronic Heart Failure (STRONG) study demonstrated that serial dosing of sST2, in addition to NT-proBNP, could play a role in optimizing guideline-directed medical therapy (GDMT) for chronic HF patients. This study showed that renin–angiotensin system inhibitors and beta-blockers were associated with significantly lower values of this biomarker, especially when the latter two were introduced and up-titrated [11]. Contrary to the GUIDE-IT trial, these findings suggest that serial sST2 dosing could be useful to optimize GDMT for HF.
More recently, a sub-analysis of the Prospective Comparison of ARNI With Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure (PIONEER-HF) trial demonstrated that sacubitril/valsartan was more effective than enalapril in reducing the aforementioned cardiac stress biomarkers in patients with acute decompensated HF who achieved hemodynamic stabilization, and this reduction was associated with an overall better prognosis [12]. Biomarkers such as NT-proBNP and sST2 could potentially be used as surrogates for clinical outcomes in patients with HF and may be useful in monitoring disease progression and assessing the response to therapy [13].
2. ST2 Biology
ST2 is a member of the interleukin-1 receptor family. The ST2 gene is placed on chromosome 2 and is part of the larger IL1 gene cluster [14]. Alternative promoter splicing and 3′-terminal processing of the same mRNA appear to be involved in the generation of two main isoforms: cellular (ST2 ligand or ST2L) and soluble or circulating (sST2) forms [15]. sST2 is a truncated soluble receptor that lacks the transmembrane and cytoplasmatic domains. ST2 is the receptor of IL-33, which is a cytokine secreted by living cells in response to cellular injury or necrosis. IL-33/ST2L signaling is a mechanically activated cardioprotective fibroblast–cardiomyocyte paracrine system, which seems to beneficially regulate the myocardial response to overload and injury [16][17]. Indeed, the interaction of IL-33 and ST2L prevents fibrosis and cardiomyocyte hypertrophy, reduces cellular apoptosis, and, ultimately, improves cardiac function. Such cardioprotective action occurs exclusively through the ST2L receptor and not through the soluble receptor [18]. The circulating isoform (sST2) acts as a decoy receptor and, by sequestrating IL-33, antagonizes the cardioprotective effects of IL-33/ST2L interactions. Both cardiac fibroblasts and cardiomyocytes release sST2 in response to myocardial stress [16][17][19]. Other sources of sST2 are endothelial cells of the aorta and coronary arteries and immune cells such as T cells [20]. Indeed, ST2 is also associated with inflammatory and immune processes, especially regarding the regulation of mast cells and type 2 CD4 T-helper cells [18] (Figure 1).
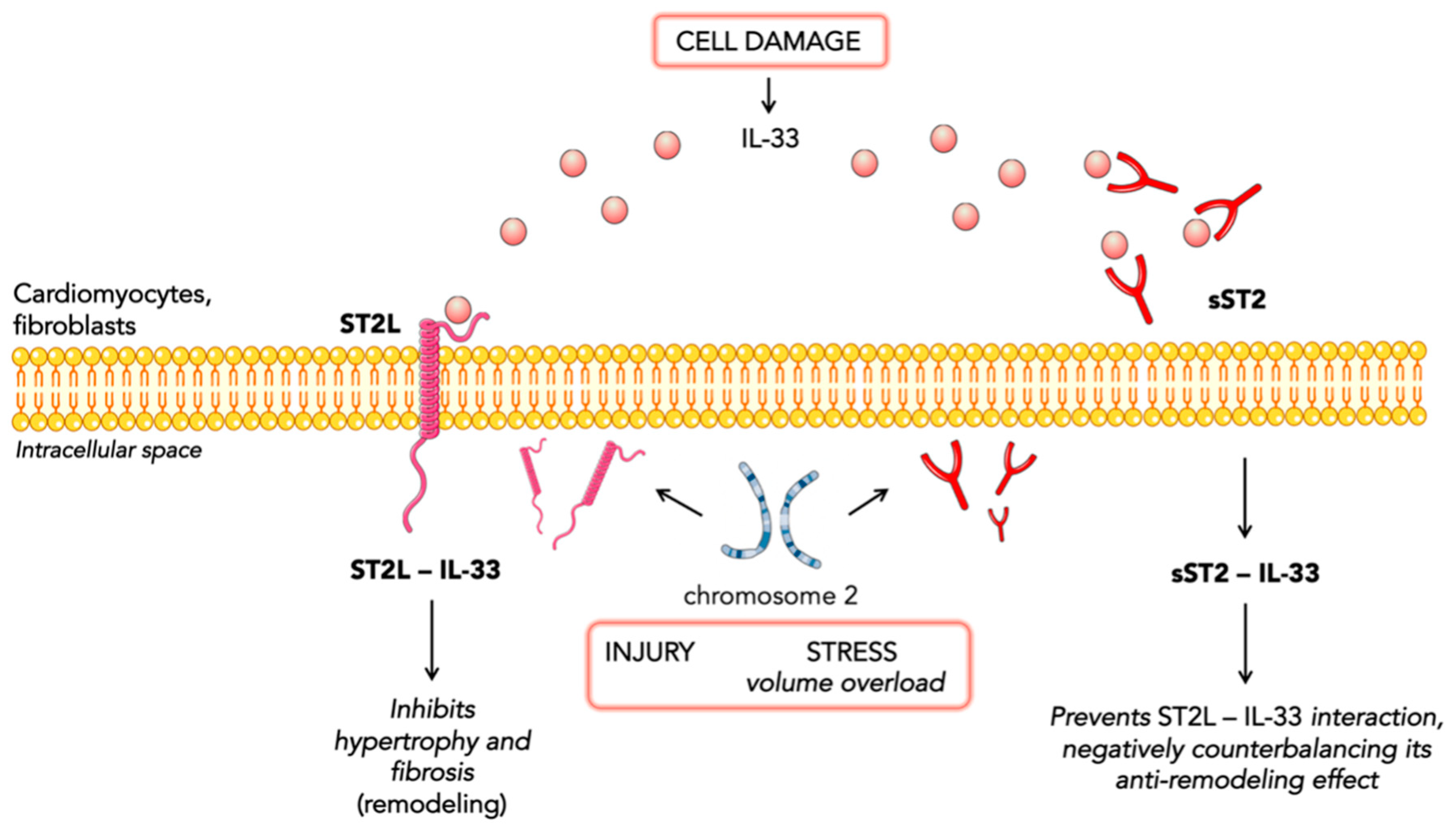
Figure 1. Representation of IL-33 signaling.
3. Prognostic Value of sST2 in Chronic Heart Failure
sST2 was first evaluated as a HF biomarker in the Prospective Randomized Amlodipine Survival Evaluation 2 (PRAISE-2) trial. Changes in sST2, rather than the baseline value, were a significant independent predictor of mortality or cardiac transplantation in patients with severe HF [21]. The Penn Heart Failure Study on 1141 patients with chronic HF demonstrated that sST2 is a powerful indicator of prognosis and offers a moderate improvement in risk stratification when used in combination with conventional markers (BNP and pro-atrial natriuretic peptide). Higher sST2 levels were associated with a significantly increased risk of all-cause mortality or cardiac transplantation, and this risk was more pronounced in patients with nonischemic HF [22]. The utility of a panel of biomarkers reflecting diverse biologic pathways in HF was further investigated in the Barcelona Study. The authors examined the value of combining NT-proBNP (a marker of myocardial stretch), high-sensitivity cardiac troponin T (hs-cTnT) (a marker of myocyte injury), and sST2 (reflective of myocardial fibrosis and remodeling) (Figure 2). The combined addition of sST2 and hs-cTnT to the model with established risk factors showed a reclassification index of 14%. These findings suggest that the pathways identified by sST2 and hs-cTnT profoundly affect mortality in the context of chronic HF, whereas the information provided in their presence by NPs might be redundant [23]. Accordingly, Emdin and colleagues demonstrated that sST2 yielded a strong predictive value for all-cause and cardiovascular mortality and HF, as well as also improving risk reclassification over NT-proBNP and hs-TnT [24]. Notably, the prognostic value of sST2 was independent of the estimated glomerular filtration rate (eGFR). The inclusion of sST2 along with other biomarkers improved the prediction in patients with renal failure even more than in the global sample population [25]. Gruson and colleagues demonstrated the prognostic value of sST2 for cardiovascular (CV) death over a mean 4.2-year follow-up. sST2 was the strongest predictor of CV death among NPs, age, left ventricular ejection fraction, and eGFR [26]. Galectin-3 (Gal-3) is another biomarker reflective of myocardial fibrosis and remodeling. Higher Gal-3 circulating levels have been associated with the presence of myocardial fibrosis assessed by late gadolinium enhancement (LGE) at cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy [27]. A cohort study on 876 patients directly compared the two biomarkers of fibrosis (sST2 and Gal-3) and sST2 resulted to be superior over Gal-3 in risk stratification. In addition, both sST2 and Gal-3 were associated with an increased risk of all-cause mortality, but only sST2 with CV mortality. This could be due to the prominent role of Gal-3 in an earlier stage of fibrosis pathobiology and ventricular remodeling, whilst sST2 measurement provides a strong biohumoral overview of the cumulative myocardial fibrotic process [28]. Ky and colleagues identified a cut-off of 36 ng/mL to discriminate patients with chronic HF having a particularly high risk for all-cause death [22]: this cut-off was subsequently confirmed in a post hoc analysis of the PROTECT study [7]. Again, in the PROTECT study, sST2 levels identified patients who may benefit more from higher beta-blocker doses, exploring for the first time the possible interplay between anti-remodeling therapies and sST2 values [29]. The Co-ordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) showed that spironolactone treatment was significantly beneficial in groups with elevated sST2 [30]. The usefulness of serial monitoring of sST2 values over 12 months was evaluated in the Valsartan Heart Failure Trial (Val-HeFT). Increases in sST2 from baseline to 12 months were associated with an increased subsequent risk of poor outcomes and may be useful for monitoring patients. Treatment with valsartan significantly reduced the upward trend in sST2 levels seen in the placebo group [31]. A sub-analysis of the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) showed that sacubitril/valsartan significantly decreased many profibrotic biomarkers and changes from baseline to 8 months in sST2 alongside tissue inhibitor matrix metalloproteinase 1 (TIMP-1) were associated with a change in outcomes [32]. A systematic review of 11 studies with a total of 5121 participants confirmed that higher concentrations of sST2 predict long-term endpoints, such as all-cause mortality, CV mortality or HF-related hospitalization, and all-cause mortality or HF-related readmissions [33]. Furthermore, Vergaro and colleagues analyzed sex-related differences in heart failure biomarkers levels and showed that the optimal sST2 cut-off was approximately 10% lower in women than men. Therefore, risk prediction should consider gender-specific prognostic cut-offs [34].
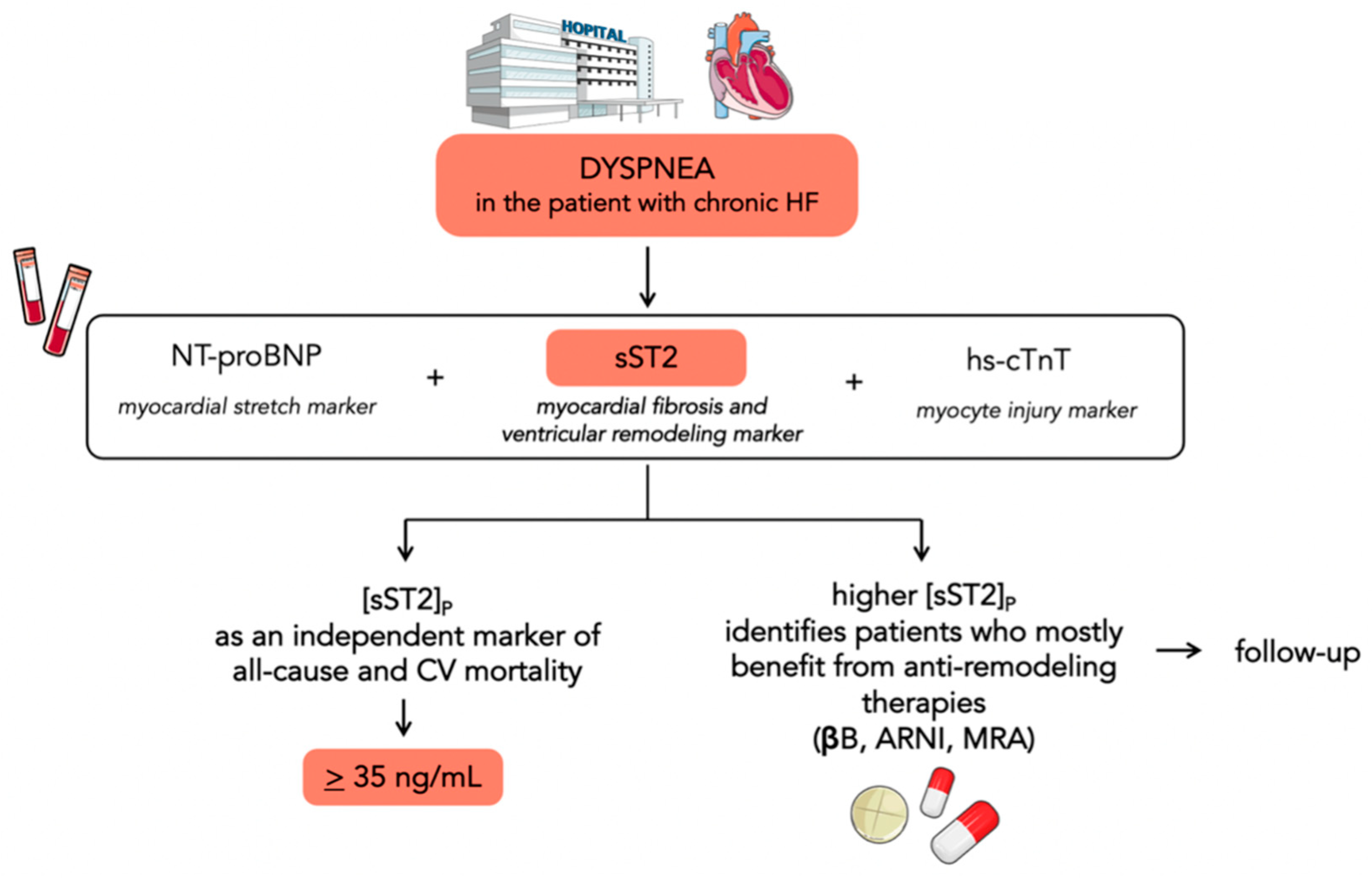
Figure 2. Flowchart showing the potential use of sST2 in chronic heart failure. sST2 is a biomarker of myocardial fibrosis and ventricular remodeling. It could be used in the risk stratification process in addition to NPs and hs-cTnT, as they reflect diverse biologic pathways in HF. Serial testing of sST2 could be helpful in identifying patients who most benefit from anti-remodeling therapies.
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Schocken, D.D.; Benjamin, E.J.; Fonarow, G.C.; Krumholz, H.M.; Levy, D.; Mensah, G.A.; Narula, J.; Shor, E.S.; Young, J.B.; Hong, Y. American Heart Association Council on Epidemiology and Prevention, American Heart Association Council on Clinical Cardiology, American Heart Association Council on Cardiovascular Nursing, American Heart Association Council on High Blood Pressure Research, Quality of Care and Outcomes Research Interdisciplinary Working Group, & Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 2008, 117, 2544–2565.
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.P.; Ros, E.; Fitó, M.; Estruch, R.; Lapetra, J.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585.
- Clerico, A.; Emdin, M. Diagnostic accuracy and prognostic relevance of the measurement of cardiac natriuretic peptides: A review. Clin. Chem. 2004, 50, 33–50.
- Núñez, J.; Núñez, E.; Robles, R.; Bodí, V.; Sanchis, J.; Carratalá, A.; Aparici, M.; Llàcer, A. Prognostic value of brain natriuretic peptide in acute heart failure: Mortality and hospital readmission. Rev. Esp. Cardiol. 2008, 61, 1332–1337.
- Aimo, A.; Januzzi, J.L., Jr.; Mueller, C.; Mirò, O.; Pascual Figal, D.A.; Jacob, J.; Herrero-Puente, P.; Llorens, P.; Wussler, D.; Kozhuharov, N.; et al. Admission high-sensitivity troponin T and NT-proBNP for outcome prediction in acute heart failure. Int. J. Cardiol. 2019, 293, 137–142.
- Gaggin, H.K.; Szymonifka, J.; Bhardwaj, A.; Belcher, A.; De Berardinis, B.; Motiwala, S.; Wang, T.J.; Januzzi, J.L., Jr. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail. 2014, 2, 65–72.
- Felker, G.M.; Anstrom, K.J.; Adams, K.F.; Ezekowitz, J.A.; Fiuzat, M.; Houston-Miller, N.; Januzzi, J.L., Jr.; Mark, D.B.; Piña, I.L.; Passmore, G.; et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients with Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2017, 318, 713–720.
- Bayes-Genis, A.; Richards, A.M.; Maisel, A.S.; Mueller, C.; Ky, B. Multimarker testing with ST2 in chronic heart failure. Am. J. Cardiol. 2015, 115 (Suppl. S7), 76b–80b.
- Aimo, A.; Januzzi, J.L., Jr.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of sST2 in Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2193–2203.
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952.
- Morrow, D.A.; Velazquez, E.J.; DeVore, A.D.; Prescott, M.F.; Duffy, C.I.; Gurmu, Y.; McCague, K.; Rocha, R.; Braunwald, E. Cardiovascular biomarkers in patients with acute decompensated heart failure randomized to sacubitril-valsartan or enalapril in the PIONEER-HF trial. Eur. Heart J. 2019, 40, 3345–3352.
- Bayes-Genis, A.; Aimo, A.; Jhund, P.; Richards, M.; de Boer, R.A.; Arfsten, H.; Fabiani, I.; Lupón, J.; Anker, S.D.; González, A.; et al. Biomarkers in heart failure clinical trials. A review from the Biomarkers Working Group of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 1767–1777.
- Dale, M.; Nicklin, M.J. Interleukin-1 receptor cluster: Gene organization of IL1R2, IL1R1, IL1RL2 (IL-1Rrp2), IL1RL1 (T1/ST2), and IL18R1 (IL-1Rrp) on human chromosome 2q. Genomics 1999, 57, 177–179.
- Bergers, G.; Reikerstorfer, A.; Braselmann, S.; Graninger, P.; Busslinger, M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994, 13, 1176–1188.
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008, 7, 827–840.
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549.
- Villacorta, H.; Maisel, A.S. Soluble ST2 Testing: A Promising Biomarker in the Management of Heart Failure. Arq. Bras. Cardiol. 2016, 106, 145–152.
- Pascual-Figal, D.A.; Januzzi, J.L. The biology of ST2: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115 (Suppl. S7), 3b–7b.
- Sun, Y.; Pavey, H.; Wilkinson, I.; Fisk, M. Role of the IL-33/ST2 axis in cardiovascular disease: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0259026.
- Weinberg, E.O.; Shimpo, M.; Hurwitz, S.; Tominaga, S.; Rouleau, J.L.; Lee, R.T. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation 2003, 107, 721–726.
- Ky, B.; French, B.; McCloskey, K.; Rame, J.E.; McIntosh, E.; Shahi, P.; Dries, D.L.; Tang, W.H.; Wu, A.H.; Fang, J.C.; et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ. Heart Fail. 2011, 4, 180–187.
- Lupón, J.; de Antonio, M.; Galán, A.; Vila, J.; Zamora, E.; Urrutia, A.; Bayes-Genis, A. Combined use of the novel biomarkers high-sensitivity troponin T and ST2 for heart failure risk stratification vs conventional assessment. Mayo Clin. Proc. 2013, 88, 234–243.
- Emdin, M.; Aimo, A.; Vergaro, G.; Bayes-Genis, A.; Lupón, J.; Latini, R.; Meessen, J.; Anand, I.S.; Cohn, J.N.; Gravning, J.; et al. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT-proBNP and High-Sensitivity Troponin T. J. Am. Coll. Cardiol. 2018, 72, 2309–2320.
- Bayes-Genis, A.; Zamora, E.; de Antonio, M.; Galán, A.; Vila, J.; Urrutia, A.; Díez, C.; Coll, R.; Altimir, S.; Lupón, J. Soluble ST2 serum concentration and renal function in heart failure. J. Card. Fail. 2013, 19, 768–775.
- Gruson, D.; Lepoutre, T.; Ahn, S.A.; Rousseau, M.F. Increased soluble ST2 is a stronger predictor of long-term cardiovascular death than natriuretic peptides in heart failure patients with reduced ejection fraction. Int. J. Cardiol. 2014, 172, e250–e252.
- Vergaro, G.; Del Franco, A.; Giannoni, A.; Prontera, C.; Ripoli, A.; Barison, A.; Masci, P.G.; Aquaro, G.D.; Cohen Solal, A.; Padeletti, L.; et al. Galectin-3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int. J. Cardiol. 2015, 184, 96–100.
- Bayes-Genis, A.; de Antonio, M.; Vila, J.; Peñafiel, J.; Galán, A.; Barallat, J.; Zamora, E.; Urrutia, A.; Lupón, J. Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. J. Am. Coll. Cardiol. 2014, 63, 158–166.
- Gaggin, H.K.; Motiwala, S.; Bhardwaj, A.; Parks, K.A.; Januzzi, J.L., Jr. Soluble concentrations of the interleukin receptor family member ST2 and β-blocker therapy in chronic heart failure. Circ. Heart Fail. 2013, 6, 1206–1213.
- Maisel, A.; Xue, Y.; van Veldhuisen, D.J.; Voors, A.A.; Jaarsma, T.; Pang, P.S.; Butler, J.; Pitt, B.; Clopton, P.; de Boer, R.A. Effect of spironolactone on 30-day death and heart failure rehospitalization (from the COACH Study). Am. J. Cardiol. 2014, 114, 737–742.
- Anand, I.S.; Rector, T.S.; Kuskowski, M.; Snider, J.; Cohn, J.N. Prognostic value of soluble ST2 in the Valsartan Heart Failure Trial. Circ. Heart Fail. 2014, 7, 418–426.
- Zile, M.R.; O’Meara, E.; Claggett, B.; Prescott, M.F.; Solomon, S.D.; Swedberg, K.; Packer, M.; McMurray, J.J.V.; Shi, V.; Lefkowitz, M.; et al. Effects of Sacubitril/Valsartan on Biomarkers of Extracellular Matrix Regulation in Patients With HFrEF. J. Am. Coll. Cardiol. 2019, 73, 795–806.
- Dong, G.; Chen, H.; Zhang, H.; Gu, Y. Long-Term and Short-Term Prognostic Value of Circulating Soluble Suppression of Tumorigenicity-2 Concentration in Chronic Heart Failure: A Systematic Review and Meta-Analysis. Cardiology 2021, 146, 433–440.
- Vergaro, G.; Gentile, F.; Aimo, A.; Januzzi, J.L., Jr.; Richards, A.M.; Lam, C.S.P.; de Boer, R.A.; Meems, L.M.G.; Latini, R.; Staszewsky, L.; et al. Circulating levels and prognostic cut-offs of sST2, hs-cTnT, and NT-proBNP in women vs. men with chronic heart failure. ESC Heart Fail. 2022, 9, 2084–2095.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
563
Revisions:
2 times
(View History)
Update Date:
14 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




