
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nykia D Walker | -- | 3560 | 2023-06-13 22:37:28 | | | |
| 2 | Nykia D Walker | + 45 word(s) | 3605 | 2023-06-13 23:28:20 | | | | |
| 3 | Jessie Wu | Meta information modification | 3605 | 2023-06-14 04:39:34 | | |
Video Upload Options
In the endosome compartment, exosome synthesis occurs when multivesicular bodies mature into intraluminal vesicles. Upon fusion with the plasma membrane, intraluminal vesicles release exosomes into the extracellular space. Exosomes can be retrieved by endocytosis or receptor-mediated uptake, suggesting a selective intercellular communication between the donor and recipient cells. They are contain nucleic acids or proteins, which appear to be strategically used to modify the recipient cell’s function in a way that benefits the donor cell. This process is vital for cell-to-cell communication and for the transfer of genetic information. Uniquely positioning exosomes to serve as messengers of information which can be used to gain insight into a multitude of diseases. By isolating exosomes, researching their components and understanding their intended destination, these small vesicles of information become invaluable for diagnosing and treating conditions, making them a powerful tool in biomedical research.
1. Lymphatic-Induced Tumor Cell Dissemination by Exosome Secretion
1.1. Challenges of Managing Metastatic Disease
1.2. Exosome-Induced Cellular Dormancy
2. A Clinical Perspective on Exosomes
2.1. Exosomes as Biomarkers of Tumor Progression
2.2. Exosomes as Delivery Systems
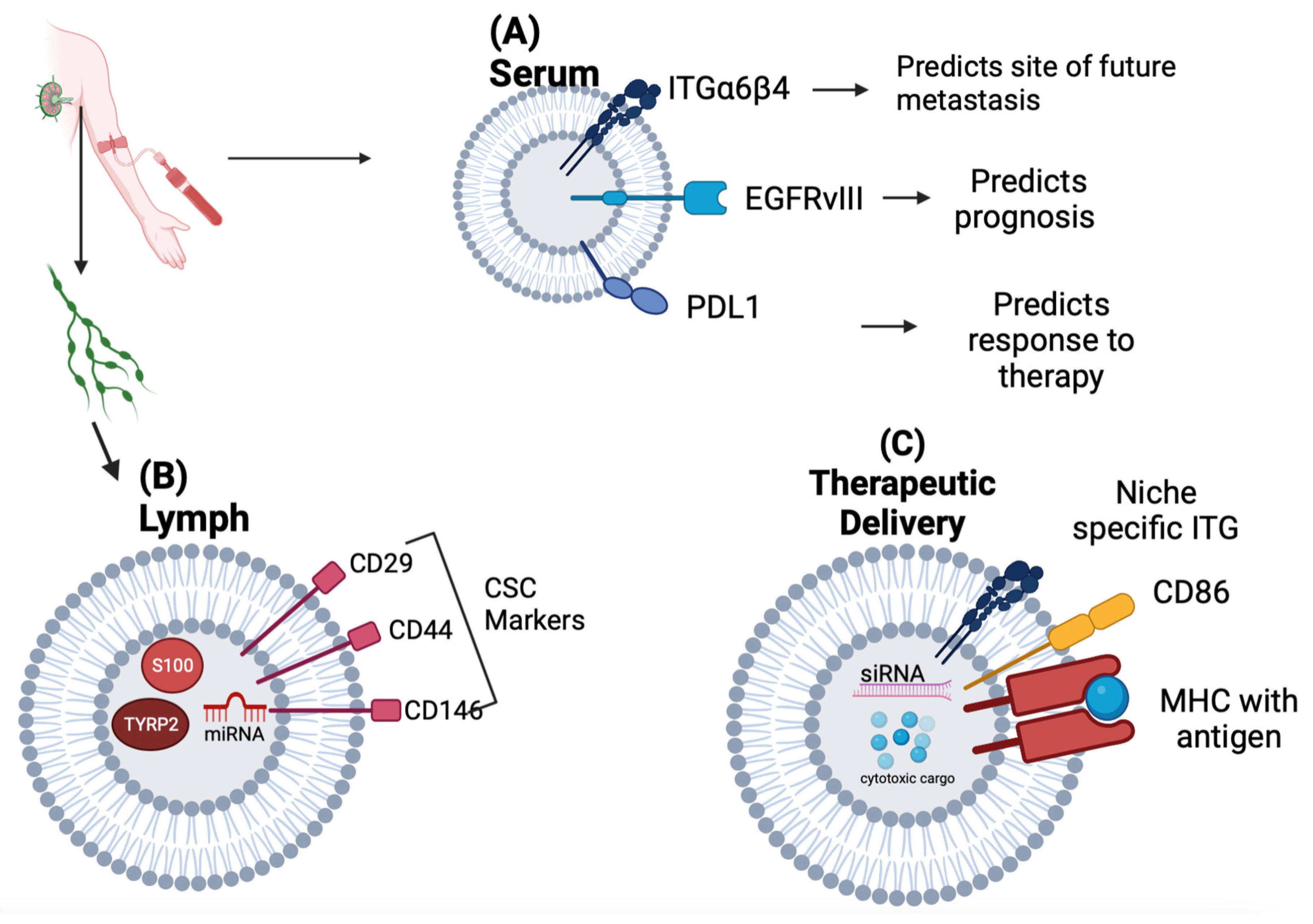
| Exosomal Biomarker | Significance | Reference | ||
|---|---|---|---|---|
| Serum | PDL1 | Correlation with Tumor PDL1 expression, Response to Immunotherapy, and Overall Survival in NSCLC | [9] | |
| EGFRvIII mRNA | Able to identify mutation status in serum via RT-PCR to predict prognosis and identify therapy options (vaccine vs tyrosine kinase inhibitor) in Glioblastoma Multiforme | [10] | ||
| ITGβ4 | Associated with Lung Metastasis in Breast Cancer | [31] | ||
| ITGαv | Associated with Liver Metastasis in Breast and Pancreatic Cancer Predictor of Liver Metastasis in Pancreatic Cancer |
|||
| Lymphatics | S100 TYRP2 |
Associated with extra-nodal spread in melanoma patients undergoing lymph node dissection | [14] | |
| CSC Markers (CD 29, CD44, CD146) |
Identified in Breast Cancer patients undergoing axillary lymph node dissection | [15] | ||
| Therapeutic Delivery | Approach | Stage | Reference | |
| Anti-Sense Oligonucleotides |
KRAS G12D siRNA For Pancreatic Ductal Adenocarcinoma |
Phase I Trial: NCT03608631 |
[16] | |
| Anti-miR-9 for drug resistant Glioblastoma Multiforme | In-vitro | [21] | ||
| Chemotherapeutic Loading | Paclitaxel-loaded macrophage-derived exosomes in G-Glycoprotein expressing Cancers | In-Vivo murine model | [17] | |
| Dendritic Cell Derived exosome (DEX) based Cancer Vaccination Strategies |
Autologous DEX-loaded with MAGE antigens in NSCLC | Phase I Trial | [19] | |
| Allogeneic Dex pulsed with INF-Y-loaded with MHC class I- and class II-restricted cancer antigens for maintenance immunotherapy in NSCLC | Phase II Trial: NCT01159288 |
[28] | ||
| Dex-derived from DCs pulsed with SART1 for advanced squamous cell carcinoma of the esophagus | Phase I/II Trial | [29] | ||
2.3. Nanotechnology-Based Approaches
References
- Swartz, M.A. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol. Res. 2014, 2, 701–707.
- Broggi, M.A.; Maillat, L.; Clement, C.C.; Bordry, N.; Corthésy, P.; Auger, A.; Matter, M.; Hamelin, R.; Potin, L.; Demurtas, D. Tumor-associated factors are enriched in lymphatic exudate compared to plasma in metastatic melanoma patients. J. Exp. Med. 2019, 216, 1091–1107.
- Ekström, K.; Crescitelli, R.; Pétursson, H.I.; Johansson, J.; Lässer, C.; Bagge, R.O. Characterization of surface markers on extracellular vesicles isolated from lymphatic exudate from patients with breast cancer. BMC Cancer 2022, 22, 1–17.
- Walker, N.D.; Patel, J.; Munoz, J.L.; Hu, M.; Guiro, K.; Sinha, G.; Rameshwar, P. The bone marrow niche in support of breast cancer dormancy. Cancer Lett. 2016, 380, 263–271.
- Argentiero, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Pantano, F.; Iuliani, M.; Santini, D.; Silvestris, N.; Vacca, A. Skeletal metastases of unknown primary: Biological landscape and clinical overview. Cancers 2019, 11, 1270.
- Takagi, T.; Katagiri, H.; Kim, Y.; Suehara, Y.; Kubota, D.; Akaike, K.; Ishii, M.; Mukaihara, K.; Okubo, T.; Murata, H. Skeletal metastasis of unknown primary origin at the initial visit: A retrospective analysis of 286 cases. PLoS ONE 2015, 10, e0129428.
- Piccioli, A.; Maccauro, G.; Spinelli, M.S.; Biagini, R.; Rossi, B. Bone metastases of unknown origin: Epidemiology and principles of management. J. Orthop. Traumatol. 2015, 16, 81–86.
- Oskarsson, T.; Batlle, E.; Massagué, J. Metastatic stem cells: Sources, niches, and vital pathways. Cell Stem Cell 2014, 14, 306–321.
- Bainer, R.; Frankenberger, C.; Rabe, D.; An, G.; Gilad, Y.; Rosner, M.R. Gene expression in local stroma reflects breast tumor states and predicts patient outcome. Sci. Rep. 2016, 6, 39240.
- Corcoran, K.E.; Trzaska, K.A.; Fernandes, H.; Bryan, M.; Taborga, M.; Srinivas, V.; Packman, K.; Patel, P.S.; Rameshwar, P. Mesenchymal Stem Cells in Early Entry of Breast Cancer. PLoS ONE 2008, 3, e2563.
- Bliss, S.A.; Sinha, G.; Sandiford, O.A.; Williams, L.M.; Engelberth, D.J.; Guiro, K.; Isenalumhe, L.L.; Greco, S.J.; Ayer, S.; Bryan, M. Mesenchymal Stem Cell–Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer Res. 2016, 76, 5832–5844.
- Ono, M.; Kosaka, N.; Tominaga, N.; Yoshioka, Y.; Takeshita, F.; Takahashi, R.-u.; Yoshida, M.; Tsuda, H.; Tamura, K.; Ochiya, T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal 2014, 7, 63.
- Walker, N.D.; Elias, M.; Guiro, K.; Bhatia, R.; Greco, S.J.; Bryan, M.; Gergues, M.; Sandiford, O.A.; Ponzio, N.M.; Leibovich, S.J. Exosomes from differentially activated macrophages influence dormancy or resurgence of breast cancer cells within bone marrow stroma. Cell Death Dis. 2019, 10, 59.
- Dioufa, N.; Clark, A.M.; Ma, B.; Beckwitt, C.H.; Wells, A. Bi-directional exosome-driven intercommunication between the hepatic niche and cancer cells. Mol. Cancer 2017, 16, 172.
- Wen, J.; Yang, T.; Mallouk, N.; Zhang, Y.; Li, H.; Lambert, C.; Li, G. Urinary exosomal CA9 mRNA as a novel liquid biopsy for molecular diagnosis of bladder cancer. Int. J. Nanomed. 2021, 16, 4805.
- Keller, S.; Sanderson, M.P.; Stoeck, A.; Altevogt, P. Exosomes: From biogenesis and secretion to biological function. Immunol. Lett. 2006, 107, 102–108.
- Martinez, V.G.; O’Neill, S.; Salimu, J.; Breslin, S.; Clayton, A.; Crown, J.; O’Driscoll, L. Resistance to HER2-targeted anti-cancer drugs is associated with immune evasion in cancer cells and their derived extracellular vesicles. Oncoimmunology 2017, 6, e1362530.
- Edwardson, D.W.; Boudreau, J.; Mapletoft, J.; Lanner, C.; Kovala, A.T.; Parissenti, A.M. Inflammatory cytokine production in tumor cells upon chemotherapy drug exposure or upon selection for drug resistance. PLoS ONE 2017, 12, e0183662.
- Zhao, Y.; Chen, X.; Yin, J.; Qu, J. SNMFSMMA: Using symmetric nonnegative matrix factorization and Kronecker regularized least squares to predict potential small molecule-microRNA association. RNA Biol. 2020, 17, 281–291.
- Munoz, J.L.; Walker, N.D.; Mareedu, S.; Pamarthi, S.H.; Sinha, G.; Greco, S.J.; Rameshwar, P. Cycling quiescence in temozolomide resistant glioblastoma cells is partly explained by microRNA-93 and-193-mediated decrease of cyclin D. Front. Pharmacol. 2019, 10, 134.
- Shimada, Y.; Matsubayashi, J.; Kudo, Y.; Maehara, S.; Takeuchi, S.; Hagiwara, M.; Kakihana, M.; Ohira, T.; Nagao, T.; Ikeda, N. Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer. Sci. Rep. 2021, 11, 7830.
- Barbagallo, D.; Condorelli, A.; Ragusa, M.; Salito, L.; Sammito, M.; Banelli, B.; Caltabiano, R.; Barbagallo, G.; Zappalà, A.; Battaglia, R. Dysregulated miR-671-5p/CDR1-AS/CDR1/VSNL1 axis is involved in glioblastoma multiforme. Oncotarget 2016, 7, 4746.
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476.
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503.
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664.
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol. Cancer 2020, 19, 160.
- Pitt, J.M.; André, F.; Amigorena, S.; Soria, J.-C.; Eggermont, A.; Kroemer, G.; Zitvogel, L. Dendritic cell–derived exosomes for cancer therapy. J. Clin. Investig. 2016, 126, 1224–1232.
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9.
- Besse, B.; Charrier, M.; Lapierre, V.; Dansin, E.; Lantz, O.; Planchard, D.; Le Chevalier, T.; Livartoski, A.; Barlesi, F.; Laplanche, A. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016, 5, e1071008.
- Narita, M.; Kanda, T.; Abe, T.; Uchiyama, T.; Iwafuchi, M.; Zheng, Z.; Liu, A.; Kaifu, T.; Kosugi, S.; Minagawa, M. Immune responses in patients with esophageal cancer treated with SART1 peptide-pulsed dendritic cell vaccine. Int. J. Oncol. 2015, 46, 1699–1709.
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827.
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of functional anti-miR-9 by mesenchymal stem cell–derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol. Ther. Nucleic Acids 2013, 2, e126.
- Bastos, N.; Ruivo, C.F.; da Silva, S.; Melo, S.A. Exosomes in cancer: Use them or target them? Semin. Cell Dev. Biol. 2018, 78, 13–21.
- Ben, X.-Y.; Wang, Y.-R.; Zheng, H.-H.; Li, D.-X.; Ren, R.; Ni, P.-L.; Zhang, H.-Y.; Feng, R.-J.; Li, Y.-Q.; Li, Q.-F. Construction of Exosomes that Overexpress CD47 and Evaluation of Their Immune Escape. Front. Bioeng. Biotechnol. 2022, 10, 936951.
- Du, J.; Wan, Z.; Wang, C.; Lu, F.; Wei, M.; Wang, D.; Hao, Q. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics 2021, 11, 8185.
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal. Transduct. Target. Ther. 2021, 6, 225.
- Liang, Y.; Iqbal, Z.; Lu, J.; Wang, J.; Zhang, H.; Chen, X.; Duan, L.; Xia, J. Cell-Derived Nanovesicle-Mediated Drug Delivery to the Brain: Principles and Strategies for Vesicle Engineering. Mol. Ther. 2022, 31, 1207–1224.
- Zhang, L.; Yang, X.; Lv, Y.; Xin, X.; Qin, C.; Han, X.; Yang, L.; He, W.; Yin, L. Cytosolic co-delivery of miRNA-34a and docetaxel with core-shell nanocarriers via caveolae-mediated pathway for the treatment of metastatic breast cancer. Sci. Rep. 2017, 7, 46186.
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A review on polymer and lipid-based nanocarriers and its application to nano-pharmaceutical and food-based systems. Front. Nutr. 2021, 8, 783831.
- JC Bose, R.; Uday Kumar, S.; Zeng, Y.; Afjei, R.; Robinson, E.; Lau, K.; Bermudez, A.; Habte, F.; Pitteri, S.J.; Sinclair, R. Tumor cell-derived extracellular vesicle-coated nanocarriers: An efficient theranostic platform for the cancer-specific delivery of anti-miR-21 and imaging agents. ACS Nano 2018, 12, 10817–10832.
- Hosseini, N.F.; Amini, R.; Ramezani, M.; Saidijam, M.; Hashemi, S.M.; Najafi, R. AS1411 aptamer-functionalized exosomes in the targeted delivery of doxorubicin in fighting colorectal cancer. Biomed. Pharmacother. 2022, 155, 113690.
- Wu, L.; Xie, W.; Li, Y.; Ni, Q.; Timashev, P.; Lyu, M.; Xia, L.; Zhang, Y.; Liu, L.; Yuan, Y. Biomimetic nanocarriers guide extracellular ATP homeostasis to remodel energy metabolism for activating innate and adaptive immunity system. Adv. Sci. 2022, 9, 2105376.
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637.
- Grimaldi, A.M.; Salvatore, M.; Incoronato, M. miRNA-based therapeutics in breast cancer: A systematic review. Front. Oncol. 2021, 11, 668464.
- Desantis, V.; Saltarella, I.; Lamanuzzi, A.; Melaccio, A.; Solimando, A.G.; Mariggiò, M.A.; Racanelli, V.; Paradiso, A.; Vacca, A.; Frassanito, M.A. MicroRNAs-based nano-strategies as new therapeutic approach in multiple myeloma to overcome disease progression and drug resistance. Int. J. Mol. Sci. 2020, 21, 3084.
- de Lázaro, I.; Mooney, D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021, 20, 1469–1479.
- Wu, W.; Pu, Y.; Shi, J. Nanomedicine-enabled chemotherapy-based synergetic cancer treatments. J. Nanobiotechnol. 2022, 20, 1–21.
- Gao, X.; Ran, N.; Dong, X.; Zuo, B.; Yang, R.; Zhou, Q.; Moulton, H.M.; Seow, Y.; Yin, H. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci. Transl. Med. 2018, 10, eaat0195.
- Diep, Y.N.; Kim, T.J.; Cho, H.; Lee, L.P. Nanomedicine for advanced cancer immunotherapy. J. Control. Release 2022, 351, 1017–1037.
- Zhang, M.; Hu, S.; Liu, L.; Dang, P.; Liu, Y.; Sun, Z.; Qiao, B.; Wang, C. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 124.
- Nair, A.; Javius-Jones, K.; Bugno, J.; Poellmann, M.J.; Mamidi, N.; Kim, I.-S.; Kwon, I.C.; Hong, H.; Hong, S. Hybrid Nanoparticle System Integrating Tumor-Derived Exosomes and Poly (amidoamine) Dendrimers: Implications for an Effective Gene Delivery Platform. Chem. Mater. 2023, 35, 3138–3150.




