According to several studies carried out over the years, different cellular types produce little lipid particles that act as a mirror of the activities that take place inside the cells. Since extracellular vesicles (ECVs) are more complex than they first appear to be, they have been divided into groups based on their size, metabolic make-up, and cell of origin
[1]. Whatever their origins, the cargo of ECVs has emerged as the most intriguing subject. Among the cargo carried by ECVs are proteins, lipids, nucleic acids such as mRNA, and other bioactive materials. Later research confirmed the crucial role ECVs play in cell-cell communication
[2] and their connection to a variety of disorders, such as diabetes
[3], inflammatory conditions
[4], and cancer
[4]. ECVs are essential intermediaries of cell-cell interaction because they carry or deliver actionably active biological molecules, such as proteins, nucleic acids, and lipids in the DNA role, including RNA, into the adjoining tissues or to detached bearer cells, where they lead to a biologic answer
[5][6]. According to Maas et al., (2017)
[2] intercellular communication via ECVs appears to have a role in the control of a number of physiological processes as well as the pathogenesis of a number of diseases, including inflammation, cancer, neurological, cardiovascular, and autoimmune disorders. In fact, ECVs carry distinctive payloads that reflect the location and affliction of the benefactor cell and may also be applied as biomarkers for scanning for investigations, including prognosis purposes
[7][8]. In addition, ECVs may pass through biological barricades, breach impenetrable structural tissues, and move safely in extracellular fluids while distributing endogenous cargo to desired cells with high efficiency and selectivity
[5]. Due to these factors, ECVs have attracted a lot of attention as possible drug delivery systems for regenerative medicine, immunomodulation, and anti-tumor therapy
[9]. ECVs have advantages over organic or inorganic nanoparticles in that they have inherent biocompatibility, elevate physicochemical resilience, have low immunogenicity, and have long-distance information, including an inherent aiming potential to intermingle along cells through membrane fusion with signal transduction
[10][11]. The lack of knowledge of ECVs’ in vivo bio function in real stint, despite efforts and advancements in the field, poses a significant obstacle to their usage in diagnostic or therapeutic settings
[12]. For ECVs to be used effectively as a curative or drug deployment framework in bioscience utilizations, it is necessary to determine their circulation kinetics and biodistribution profile, as well as their aiming potency to exact cells or tissues and uptake route, including cargo transport reliability to inheritor cells
[13]. Exogenous in vivo comportment of ECVs can be accurately understood using a non-invasive molecular envisioning approach
[14][15]. Nuclear imaging stands out among the various preclinical research modalities, mainly because of its excellent profile regarding sensitivity and safety, as well as its huge potential for medical transformation.
2. Advantages of ECVs in Drug Delivery System
As a nominally aggressive synthetic drug delivery deployment approach has been devised to daze restrictions of the unrestricted therapeutics and overcome diverse biological blockades across patient diseases and populations, tailored clinical interventions are becoming more and more essential for therapeutic efficacy. Nanoparticles have been developed as a delivery method to enhance the issuance or spreading therapeutic model, with a focus on controlling the intervention through the attributes of the vehicle rather than the physicochemical properties of the drug molecule
[16]. The use of nanoparticles is still accompanied by a number of disadvantages despite the benefits they provide, such as increasing the solubility and steadiness of encapsulated consignments, encouraging passage across membranes, and delaying circulation times to increase the safety and efficacy for delivery of therapeutics
[17]. The target organ dose is notable, represented by the reticuloendothelial system’s quick clearance
[18], congregation in the spleen and liver, and immediate hypersensitive reaction
[19][20]. Extracellular vesicles (ECVs) are one of the areas of interest that is constantly rising in the field of bio-inspired drug delivery systems, or biologics. The comprehension of novel types of cell-cell communication has been enhanced by these assorted populations of naturally derived membrane vesicles of nano-to-micro-sized vesicles that can transfer biological molecules from manufacturer to receiver cells
[21]. These systems share a significant benefit in that they are derived from living cells, which makes them appealing for commercial product development. Cell-derived ECVs carry a biological payload that modulates immune responses while promoting angiogenesis and tissue repair
[22]. This has focused attention, in particular, on using ECVs for therapeutic delivery to get around problems with synthetic drug delivery methods. The circulation, central stability, and capacity of ECVs to deliver and shield a wide range of nucleic acids into inheritor cells while evading the mononuclear phagocytic system (MPS) through displaying the exterior protein CD47 are further noteworthy features of ECVs
[23]. Furthermore, it is important to note that ECVs may contain proteins which attach to and organize its RNA
[24]. Consequently, ECVs are a prospective source for building systems to deliver therapies in a variety of clinical settings, including in vivo gene editing, immunotherapy, and cancer treatment.
3. ECVs as Nanomedicines
The potential for ECVs or exosomes (200 nm) to serve as therapeutic nanomedicines has been studied among the many ECV subtypes
[25][26]. By more effectively targeting illness locations and/or lowering systemic harmful side effects, nanomedicine seeks to enhance the therapeutic effects of medications
[27]. Small extracellular vesicles (SECVs) are a desirable platform for nanomedicine due to a number of factors. Natural ECVs include antigens and, dependent on the cell of origin, may have particular tissue-approaching capabilities in comparison to other synthetic platforms (such as liposomes and polymers)
[28][29]. As drug delivery vehicles
[30], SECVs can be correspondingly fabricated to exhibit ligands for improved cell or tissue aiming, including steadiness. They have also been shown to accumulate in tumors as a result of the increased permeability and retention (EPR) effect
[31]. Their natural capacity to traverse the blood-brain barrier (BBB) i.e., a considerable biological barrier for various other nanomedicine drug delivery (DD) platforms, is another key characteristic that is frequently mentioned
[32].
4. Radiolabelling of ECVs
4.1. Surface Radiolabelling
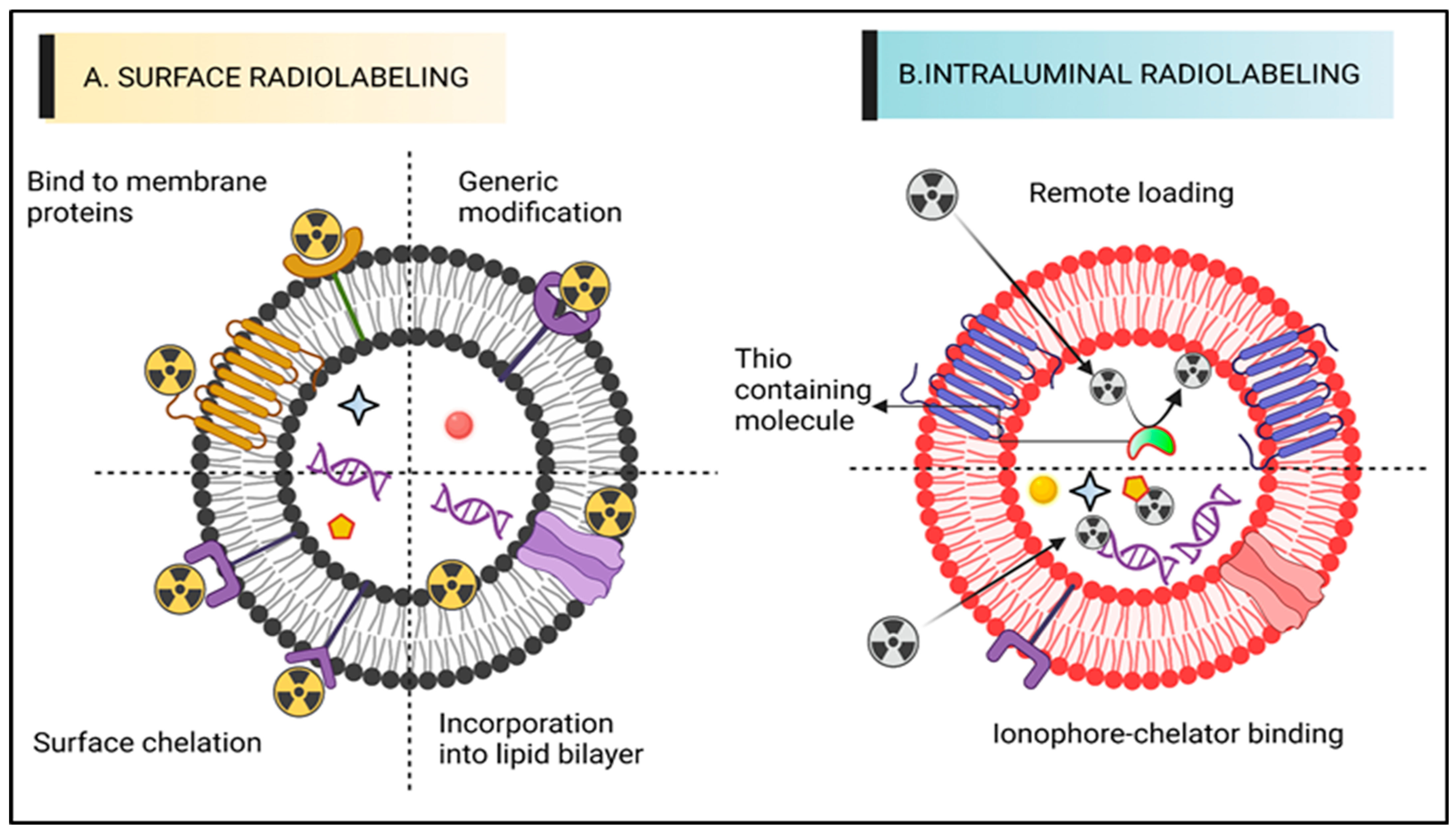
The most popular technique for radiolabelling ECVs is surface or membrane radiolabelling. This enables the radionuclide to be matched to the proteins of the membrane directly or indirectly via the formation of covalent chemical bonds. To accomplish this, the four tactics displayed below were applied. The four mechanisms of radioactive attachment are genetic adjustment, direct consolidation of radionuclides out of membrane proteins, direct integration into membrane proteins, or radionuclide adhesion via chelator (i.e., radiometals infringing chemical cohort) on the surfaces (
Figure 1A). Radionuclide assimilation, which involves the interaction between radionuclides and the elements of the membranes of extracellular vesicles (ECVs) without any specific targeting. In conventional bio-conjugate chemistry, the radiotracer is typically attached to the membrane using surface amine groups through surface chelation methods. The sections following, “Radiolabelling of ECVs using SPECT radionuclides” and “Radiolabelling of ECVs using SPECT radionuclides,” provide more thorough explanations of these techniques along with the relevant radiotracers (vide infra). The ECVs’ surface integrity may be compromised by surface radiolabelling procedures in general, especially if those approaches include chemically altering membrane proteins or significantly changing the makeup of the membrane. Numerous research studies
[33] have shown the significance of the proteins and lipids on the surface of ECVs in determining how they behave, and a recent study
[34] outlined the numerous techniques for changing the surface of ECVs. The physicochemical characteristics or biodistribution of those meant to permit radiolabelling could thus be significantly modified. These modifications are often implemented to better target tissues or understand ECV function.
Figure 1. A diagram representing various ECV radiolabelling techniques. (
A) Surface radiolabelling: a chelator or a radionuclide can be directly introduced into the ECV membrane. (
B) Intraluminal radiolabelling: radionuclides can be trapped as their lipophilicity changes or bind to biomolecules that chelate metals thanks to ionophores, which allow radionuclides to pass the lipid barrier.
4.2. Intraluminal Radiolabelling
Entrapping the radiotracer inside the intravascular space is another way to radiolabel ECVs (
Figure 1B). Based on the limited information provided, it appears that the lipid bilayer barrier may act as a protective layer, preventing radionuclides from being trans-chelated by extracellular elements, such as serum proteins. As the radionuclide is exposed during surface radiolabelling, extra-ECV trans-chelation is more likely to take place. For intraluminal radiolabelling of ECVs, the radionuclide must traverse the lipid bilayer or reside inside the ECV. Ionophore-chelator binding and remote loading have both been investigated as ways to do this. The first technique makes use of endogenous intravesicular glutathione, which has the ability to change some complexes from lipophilic to hydrophilic, including Hexamethylpropyleneamine [
99mTc]-Tc-hexaoxime ([
99mTc]-Tc-HMPAO)
[35]. The lipophilic radiotracer complex is transformed into its hydrophilic form after crossing the lipid bilayer membrane and is thereafter confined in the aqueous core of most ECVs. The ionophore-chelator binding approach makes use of popular ionophore ligands, such as tropolone or 8-hydroxyquinolin (oxine), which mix with the radiometals to form a metastable, and thus neutral, complex or allow their transportation across the lipid membrane. Radiolabelling of either cells or liposomal nanomedicines is possible, thanks to the radiometals ability to entangle with metal chelating aggregates included in liposomal cargo
[36].
In the ECVs, the radiometal is seen to entangle intravesicular proteins, including nucleic acids. An inability to precisely pinpoint the intraluminal space of the ECVs that the radionuclide entangles will be the underlying flaw of intraluminal radiolabelling techniques, notably those centered on ionophore. Interpreting in vivo imaging can be challenging due to the fact that certain radionuclides, such as radiometals like 64Cu, tend to accumulate in organs that also contain extracellular vesicles (ECVs), such as the liver or spleen. This is particularly true at later time points when ECVs may experience significant lipid bilayer breakage
[37]. Both SPECT and PET radionuclides have been used along with the two primary radiolabelling categories, surface and intraluminal, with varying degrees of radiolabelling capability.
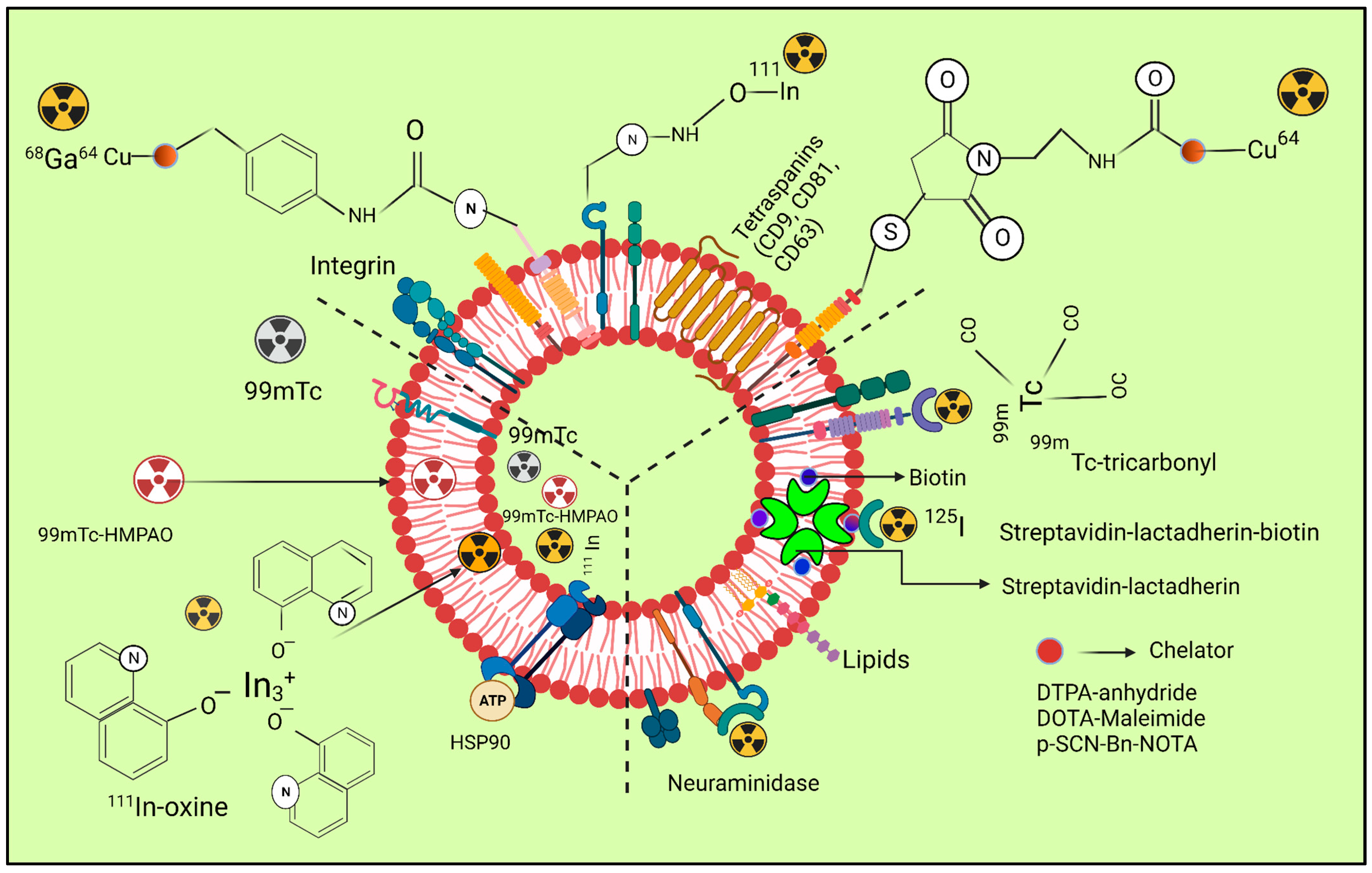
Figure 2 depicts the radiolabelling of exosomes by using different radio nuclei.
Figure 2. The three radiolabelling examples for extracellular vesicles are shown schematically (ECVs). Bifunctional chelators with a functional group for covalent attachment to reactive amine (-NH2) or thiol (-SH) groups on the surface of ECVs and a metal-binding moiety for radiometal sequestration, such as 64Cu, 68Ga, and o, can be used for membrane radiolabelling. Examples include diethylene triamine pentaacetic acid (DTPA)-anhydride, 1,4,7,10-tetraazacyclo. By intraluminal labelling with lipophilic 111In-oxine or ([99mTc]-Tc-HMPAO) complexes that breach the ECV membrane spontaneously and enter the vesicle lumen, radionuclides can be sealed off. Another method for getting 111In3+ into the exosomal lumen involves the tiny hydrophobic chemical tropolone. Covalent bonds between naturally reactive functional groups or previously added non-native binding groups in ECVs with imaging probes are the basis for covalent binding. Tyrosine residues in neuraminidase-treated ECVs, natural amino acids on the surface of ECVs, and the streptavidin-biotin-lactadherin fusion protein all come together to form stable complexes with radionuclides such as 124I and 99mTc.
5. Radiolabelling of Vesicles That Mimic Exosomes (EMVs)
ECVs’ fluctuation formation, isolation, and radiolabelling qualities can be minimized by utilizing exosome-mimetic vesicles (EMVs). Although they can be made in other ways as well, serial extrusion of cell membranes is the primary method used to make EMVs, and it has been employed for radiolabelling
[38]. EMVs’ primary advantage is that they can be designed in larger quantities than ECVs for the mass market. They are identical to ECVs in terms of size, lipid and biomarker expression, protein composition, and tissue aiming prowess. Utilizing mouse macrophage-generated EMVs, Hwang et al. were able to attain an RLY of >93% using [99mTc] Tc-HMPAO (t1/2 = 6 h) (218.8 nm)
[39]. The endogenous intra-vesicular thiol factions were employed in lieu of the superficial thiol groups that Banerjee et al. had previously used to transform lipophilic [99mTc]Tc-HMPAO intricate into a hydrophilic form, corralling the radionuclide in the intraluminal site. Mouse macrophage-derived SECVs’ and two distinct types of 99mTc-labelled EMVs’ in vivo biodistribution were studied, and although direct comparisons cannot be made from the photos provided, there seems to have been a emission of the [99mTc]-HMPAO intricate including [99mTcO4]
−.
6. Innovations in Imaging Techniques for Exosome Tracking In Vivo
Exosomes serve a wide range of nanocarriers for biological purposes for both short- or long-range intercellular communication. They enable the transfer of intricate information among cells, modulating a number of processes such as homeostasis, immunological response, and angiogenesis in both healthy and unhealthy settings. Their extraordinary abilities for motility and targeting, including selective internalization into particular cells, make them desirable delivery vectors. As a result, they offer a possible new area of diagnosis and therapy and could replace cell-based therapeutic strategies. However, this has become a significant obstacle to bringing exosome treatment into the clinic because it is not well understood. More research is needed to fully understand the function of endogenous vesicles within living organisms, as there is currently a lack of sufficient information available. Endogenous vesicles are tiny structures that are enclosed by a membrane. Exosomes can be monitored in vivo to learn more about their biodistribution, migratory potential, toxicity, biological function, communication potential, and mode of action. Therefore the development of exosome tagging and imaging methods that are effective, sensitive, and biocompatible is urgently necessary
[40].
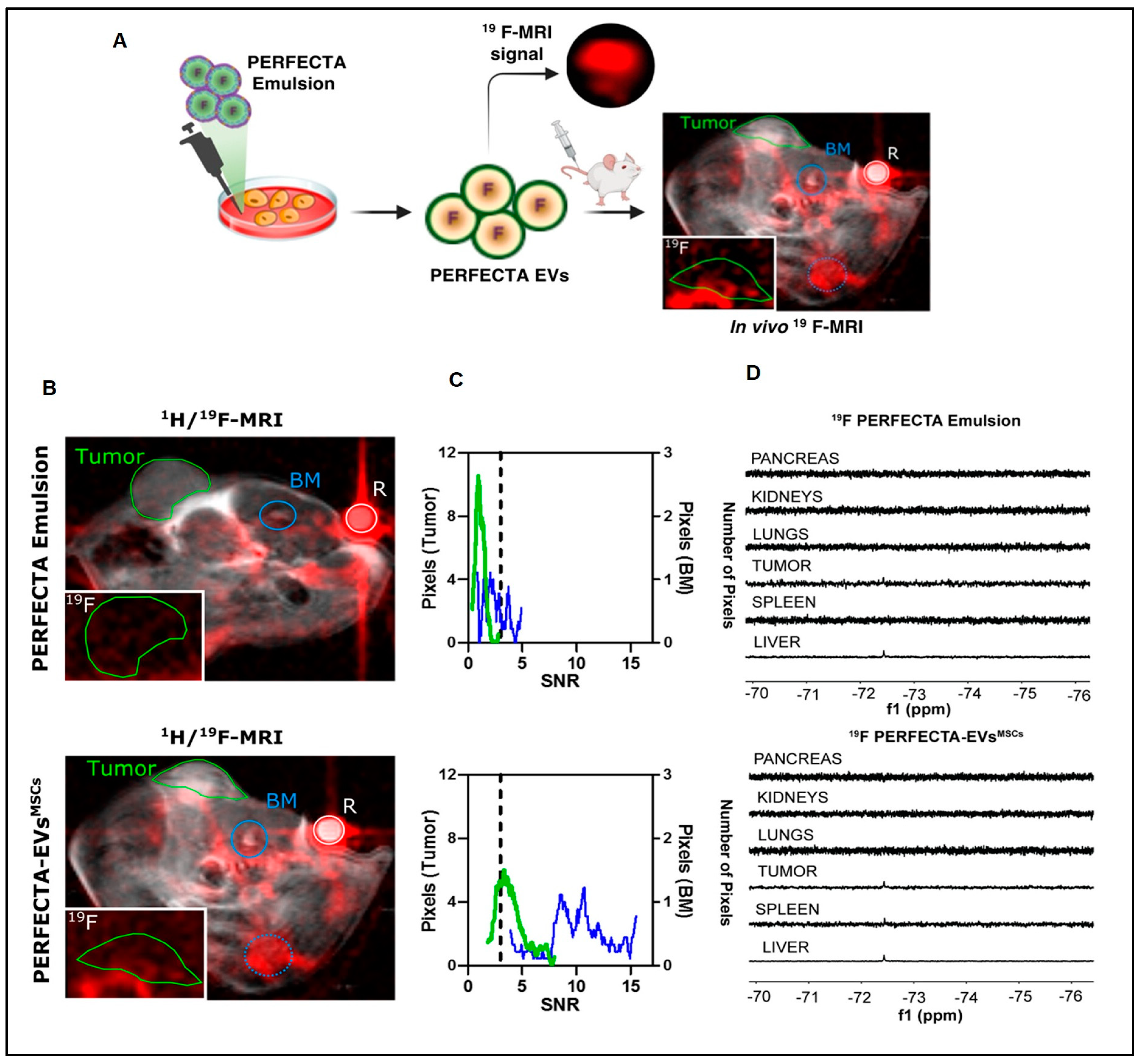
Figure 3 describes the in vivo tracking of super fluorinated extracellular vesicles.
Figure 3. Tumor homing of PERFECTA-ECVs-MSCs. (
A) In vivo 19F-MRI of BALB-nu mice bearing HeLa tumor: (
B) merged 1 H and 19F-MRI (red) images from mice after the introduction of PERFECTA emulsion (top) and PERFECTA-ECVs-MSCs (bottom). The green circle indicates the tumor site and the blue circles signify the bone marrow (BM) of the spinal cord (solid) and the knee (dotted). The white circle indicates the standard reference (R). In the white box (bottom-left), 19F-MR image magnification targeted on the tumor mass. (
C) Histograms of 19F signal-to-noise ratio (SNR) distribution in both tumor and bone marrow of the spinal cord are reported for each mouse. The dashed line identifies the SNR limit above which 19F signal is detectable (SNR > 3). (
D) 19F-NMR spectra of harvested organs from a representative mouse among those treated with PERFECTA emulsion and those treated with PERFECTA-ECVsMSCs
[41] attribution 4.0 International (CC BY 4.0).
7. Nuclear Imaging for Exosome Tracking
Cellular envisioning is frequently done via nuclear imaging, which uses radioactive materials to diagnose and cure disorders. Radiation that is emitted by radionuclides can be seen in real time with a specialized camera. Despite the fact that radionuclides have very short half-lives or require comparatively brief times of imaging or tracking, nuclear imaging is able to view deep structures of organs because of its higher sensitivity with tissue penetrating capacity
[42]. By incubating the vilifying agents explicitly into exosomes, this approach explicitly infuses the labelling agents into the exosomes. Smyth et al., (2015)
[43] state that 3D images are typically acquired using techniques such as single photon emission CT (SPECT) and positron emission tomography (PET). These imaging methods can be combined with anatomical imaging, including MRI, to improve the ability to pinpoint the location of exosomes. Exosomes were labeled with Indium-oxine (111In-oxine) and were infused intravenously. After 24 h, an ex vivo biodistribution examination was performed to observe the accumulation of the labeled exosomes in the body. This method of observation has been previously reported in studies that used fluorescence to compare the accumulation of doxorubicin-loaded exosomes with liposomes in tumors.
8. Use of Radiolabelled Exosome for Imaging and Quantitative Biodistribution
Hwang et al., (2015)
[39] developed a technique to label macrophage-derived exosome-mimetic nanovesicles (ENVs) with 99mTc-HMPAO under physiological conditions, in order to track the distribution of 99mTc-HMPAO-ENVs in live mice. The murine RAW264.7 macrophage cell line was used to create ENVs, which were then labelled with 99mTc-HMPAO for 1-h incubation before the free 99mTc-HMPAO was removed. “After being labelled with 99mTc-HMPAO, 99mTc-HMPAO-ENVs had radiochemical purity over 90%, while the utterance of the exosome explicit protein (CD63) was unaffected”.
After being labeled with 99mTc-HMPAO, the 99mTc-HMPAO-ENVs demonstrated a radiochemical purity of over 90%. The expression of the exosome-specific protein CD63 was not impacted. The serum stability of 99mTc-HMPAOENVs was high (90%) and comparable to that of phosphate-buffered saline for the first five hours. In contrast to mice treated via 99mTc-HMPAO, which exhibited significant brain retention till 5 h after injection, animals treated with 99mTc-HMPAO-ENVs showed enhanced liver uptake with negligible uptake in the brain on SPECT/CT imaging. In order to enhance exosome fabrication, melanoma cells (B16F10) were cultivated in bioreactor vesicles.
Radiolabelled ExoB16 (1 × 1011 particles/mouse) was administered intravenously to melanoma incurring, C57BL/6 immuno-competent and immune-deficient NSG mice. The radiolabelling reliability or radiochemical resilience of membrane-labelled ExoB16 was higher than that of intraluminal denoted exosomes (4.73 ± 0.39% with 14.21 ± 2.76%, respectively), showing greater radiolabelling stability (80.4 ± 1.6%) or radiolabelling efficiency (19.2 ± 4.53%). Utilizing the membrane vilifying perspective, ExoB16 in vivo biodistribution in melanoma incurring C57Bl/6 mice was explored. ExoB16 was found to accumulate the most in the liver and spleen, accounting for 56% of the total accumulation with 38% ID/gT. The kidneys showed a lower accumulation of 3% ID/gT. Exosome membrane radiolabelling is a dependable method that enables quantitative biodistribution investigations and accurate live imaging on possibly all exosome kinds without modifying parent cells.
In this investigation, various types of cells were studied, including tumor cells that have or have not undergone treatment, myeloid-inferred suppressor cells, and progenitor cells of endothelial cells. The objective was to understand how to measure and visualize the in vivo distribution of exosomes marked with radioisotope 131I in these different cell types. The exosome-based targeted therapy and disease progression can be tracked using the implemented in vivo envisioning approach. Exosomes can be radiolabelled to examine their biodistribution in vivo; Faruqu et al., (2019)
[44] set out to create an innovative, trustworthy, and all-encompassing technique for this. With the use of electron microscopy, flow cytometry, nanoparticle tracking evaluation (NTA), and protein assays, exosomes (ExoB16) synthesized from B16F10 were isolated by ultracentrifugation onto a single cushion of sucrose. ExoB16 was radiolabelled via membrane labelling and intraluminal labelling (
111Indium was trapped through tropolone shuttling) (chelation of
111Indium utilizing DTPA-anhydride, a bifunctional chelator exhibiting covalent attachment), utilizing gel filtration and thin-layer chromatography, and labelling effectiveness and stability were evaluated. At 1, 4, and 24 h after intravenous injection of radiolabelled ExoB16 (1 × 1011 particles/mouse) into melanoma incurring immunocompetent (C57BL/6) and immunodeficient (NSG) mice, metabolic cage findings, complete body SPECT-CT envisioning, and ex vivo gamma tallying were carried out. The intraluminal marked exosomes (4.73 ± 0.39% including 14.21 and 2.76%, respectively), in contrast to the membrane-marked ExoB16, evidenced enhanced radiolabelling potency and radiochemical resilience (19.2 and 4.53% including 80.4 and 1.6%, respectively).
9. Radiolabelling Including In Vivo SPECT/PET Imaging Problems with ECVs
The ECVs’ volatility is one of the main obstacles to in vivo imaging. According to a study by Clayton et al., (2003)
[45], ECVs have a high concentration of CD55 and CD59, which may help them survive longer in-vivo. The ECV radiolabelling experiments, as well as those using non-radiolabelled ECVs, showed that the half-life of intravenously injected ECVs was very short, as brief as two minutes
[46]. Even after ECVs have been eliminated from the circulation, they are still persistent, primarily in the liver or spleen and in the reticuloendothelial system (RES) organs. The bladder accumulates the most imaging signals after the liver and spleen, which is only conceivable if the ECVs can get through the kidneys’ glomerular filtration. Choi et al., (2007)
[47] used quantum dots to show that kidneys often do not pass nanoparticles larger than 8 nm. These findings, including information on antibody clearance, imply that the kidney filtration threshold size may be akin to minute proteins. Clearance of renal preserved antibodies is presumed to be unimportant since the size of a reminiscent antibody is significantly larger than the threshold of glomerular filtration (55 kDa)
[48].
10. Thiol-Michael Inclusion Loads Imaging Probes Including Drugs onto Exosomes
The dimensions and integrity of exosomes (EXOs) were unaffected by additional chemical engineering, with the exception of chemical transfection and the previously stated saponin-incorporated drug freight on the exosomes (EXOs). An a, b-unsaturated carbonyl reacts with an enolate-type nucleophile in the Michael addition. It has been used for a long time in organic synthesis to create highly selective compounds in a safe manner
[49]. Chemical reactions of this nature have evolved into highly efficient and adaptable procedures, and it appears to have emerged roughly concurrently with “click chemistry” in materials science. To produce a highly effective reaction, the thiol-Micheal addition is intended to proceed in conditions that are mild and solvent-free. Sulfhydryl groups (-SH) are widely distributed in the majority of membrane proteins found on exosomes (EXOs). These groups can be utilized as a binding link for drug loading through the use of maleimide-containing sulfhydryl strings. Numerous efforts have been made to date to put thiol-Michael addition on the exosomes (EXOs) in practice. For instance, Roberts-Dalton et al., (2017)
[50] used the maleimide/thiol progression to bind Mal-Alexa 633 and MalAlexa488 on the EXOs produced by prostate malignant cells in order to describe the endocytosis of EXOs by living cells. This chemical method undoubtedly gave the exosomes (EXOs) more versatility when it came to fluorescent labelling. The fluorescent signals allowed them to rapidly observe their endocytic traffic and interactions with cells
[50]. By using thiol-Michael addition, quantum dots (QDs) were affixed to the exosomes (EXOs), in addition to fluorophores, also enabling a gentle and biocompatible labelling method. The study used maleimide or biotin to functionalize a DNA hinge at each terminal. The chemical interaction between the DNA’s built-in maleimides and the thiols on the exosomes (EXOs) subsequently enabled the DNA fragment to be coupled on the M1 macrophage-originated exosomes (EXOs). The terminal biotin of the DNA hinge was then easily realized by the streptavidin-labelled QDs to combine to EXOs for tumor scanning. Another component of thiol-maleimide interaction focuses on its use in disease therapies in addition to in vivo imaging
[51]. Due to the availability of maleimide-thiol interactions, the addition of a novel group of attraction ligands on exosomes (EXOs) in this manner is getting a lot of consideration in focused therapy. Han et al. used thiol-Michael addition to bind aptamers to the HEK293T cell-derived exosomes (EXOs) in order to identify prostatic cancer cells
[52].
11. Luminescence-Based Aptasensor
11.1. Transfer of Luminescence Resonance Energy
Aptasensors depending on LRET display the same positive effects of high sensitivity, excellent operability, and resolution as the FRET mechanism. Therefore, utilizing rare earth incapacitated up-conversion nanoparticles (UCNPs) that are transformed into gold (Au) nanorods is essential. Chen and colleagues (2018)
[53] created a simple and user-friendly aptasensor based on paper-supported LRET using gold nanoparticles (NRs). The sensor employed two aptamer sections of CD63, one for capturing and the other for detecting. The aptamers allowed exosomes to recognize and conjugate with each other, leading to the bridging of paper upconversion nanoparticles (UCNPs) via the capture probe and Au NRs via the detection probe. This bridging induced LRET, resulting in quenching of green fluorescence.
11.2. Electrochemiluminescence
The ECL approach, in contrast to electrochemical exploration, does not rely on external parameters, including temperature, electrode surface roughness, pH, humidity, and many more. Qiao et al., (2019)
[54] created an aptasensor for electrochemiluminescence (ECL) by functionalizing an aptamer onto a glassy carbon electrode. The electrode was then covered with ECL emitters comprising of CdS nanocrystals incapacitated with Eu+++ and modified with mercaptopropionic acid. This approach was employed to avoid interference from background currents
[54].
11.3. Strip Aptasensor
On paper-based portable devices, the aptasensor, lateral flow-strip (LFS) is frequently used and has a simple user interface and color and signal change makes the outcome clear. LFS is frequently separated into 2 categories of pack strip, with either competitive strip grounded on various exchanges onto test lines [Cheng et al., 2019b]. Based on thermal signals, Cheng et al. created a sandwich strip for exosome identification.
11.4. Surface Enhanced Raman Scattering Aptasensor
SERS has a constrained spectral bandwidth and distinctive fingerprint properties for distinguishing different exosomes. MB-capture substrates by utilizing diverse aptamer probes. Wang et al. advanced a SERS aptasensor for simultaneous finding of multiple exosomes
[55]. To collect common exosomes, the CD63 aptamer was embellished with gold shell magnetic nanobead substrates. Targeting exosomes with specific aptamers modified AuNPs and a Raman correspondent as a readout signal foundation, establishing a pack of MB-exosome AuNPs, which were the foundations for the SERS identification.
11.5. Surface Plasmon Resonance Aptasensor
SPR is an approach to ocular biosensing that can be utilized to observe in-situ and real-time biomolecular interactions on metal surfaces. This technique doesn’t require any markers and doesn’t harm biomolecules in any way.
11.6. Giant Magnetoresistance Aptasensor
GMR sensors are gaining significant attention in the field of biosensors due to their compatibility with IC technology, including their ability to work seamlessly with superparamagnetic nanoparticles. This compatibility makes GMR sensors an attractive option for biosensor applications. Zhu et al., (2019)
[56] established a GMR aptasensor for exosome finding grounded on the magnetic signal reply of MoS
2-Fe
3O
4 nanostructures (MOFE). On the GMR sensor surface, the CD63 aptamer was modified for widespread exosome capture. To recognize exosomes, MOFE-labelled aptamer probes were used. This pack of “GMR/CD63 aptamer-exosome-MOFE/detection aptamer” greatly increased the signal of GMR.
12. Exosome Tracking In Vivo and In Vitro
Through in vivo and in vitro research, real time drug delivery systems must be watched to demonstrate the drug’s structure, concentration, and timing of arrival at the target site. Animal application experiments require the tagging and monitoring of exosomes. The exosomes of sick tissues were observed using computer tomography (CT), fluorescent bioluminescence imaging, microscopy, and magnetic resonance imaging (MRI)
[57]. Fluorescence microscopy is a crucial tool for understanding the physiological processes of living cells and, currently, optical envisioning is an arguably popular envisioning approach for studying cells including molecules. To determine the association between the exosomes and the cells examined, fluorescent probes are used to initially label the exosomes. When enticed, the specimens being assessed are examined based on fluorescence signals that are emitted, and the physiological evolution of specific cells may be followed. Exosome fluorescence tagging techniques fell into four groups that were widely used by many people. These include:
- (1)
-
Natural dyes: the surface of the exosome lipid bilayer receives the fat chain through the lipophilic organic dye. This characteristic makes it possible to prolong and stabilize the fluorescence of the fluorescent dye. In addition, the fluorescent dye’s emission spectrum, which extends from 502 from 734 nm, has a broad application window with great selectivity. However, the fluorescence quantum yield, half-life, light color, and other properties of this organic dye differ between dyes. Moreover, variations among dyes may involve the mutual reinforcement of other liposomes through dyeing, which will contribute to imprecise results
[58].
- (2)
-
Fluorescent proteins that are genetically encoded: red and green fluorescent proteina, as well as other fluorescent proteins fall under this category. Through the use of gene editing technologies, they are alluded to as marker proteins found on the superficial exosome. To assess the in vivo and in vitro movement and metabolic transfer of exosomes and prevent other liposomes from being mislabelled, one of the exosome markers, CD63, was frequently fused with them
[59].
- (3)
-
Reporter molecules that are immunofluorescent: the highly specific immunofluorescence labelling technique involves colouring the exosome first, then the antibody with an organic dye. The need for microscopy equipment is quite high because exosomes are so tiny. Due to their increased resolution, stochastic optical reconstruction microscopy and photoactivated localization microscopy were better suited for observing exosomes
[60].
- (4)
-
Nanomaterials that glow: due to their tiny size and superior optical qualities, fluorescent nanoparticles are uncomplicatedly bound and don’t affect the physiological activity of exosomes in any significant way. However, there is a great mandate for near infrared (IR) fluorescent nanoprobes including deep tissue dissemination and negligible autofluorescence background tracking
[61].
13. Radiopharmaceuticals for Theranostic Applications
13.1. Radiolabelled Metallic Nanoparticles
Metallic nanoparticles are very small metal particles that range in size from 1 to 100 nanometers. Due to their size and surface characteristics, they can function effectively as catalysts. The large surface area-to-volume ratio, quantum confinement, increased number of kinks, and plasmon excitation is some of the unique features of metallic nanoparticles that make them useful in various applications. Metallic nanoparticles have several benefits, such as their ability to enhance Rayleigh and Raman scattering, strong plasma adsorption, and imaging of biological systems. However, their commercial use is limited due to their instability, potential impurities, toxicity, and carcinogenicity. Nanoparticles have emerged as promising tools for both imaging and therapy of cancer cells.
13.2. Radiolabelled Gold Nanoparticles for Theranostic Application
Gold nanoparticles possess unique physicochemical properties that make them excellent candidates for use as nanocarriers in a variety of biomedical applications. They have a low reactivity, good binding affinity, high surface area, optimal size, good stability, and biocompatibility. Due to their chemical inertness, biocompatibility, and ease of modification, they can be used for both therapeutic and diagnostic purposes. Furthermore, these nanoparticles are non-toxic and can achieve passive as well as active targeting
[62].
13.3. Radiolabelled Magnetic Nanoparticles for Theranostic Application
The field of nanotechnology has made significant strides in the synthesis and physicochemical properties of magnetic nanoparticles, which have proven to be attractive candidates for various biomedical applications. Magnetic nanoparticles offer high surface area and improved quantum efficiencies, making them useful as nanocarriers for molecular imaging and therapeutic applications. Moreover, the release of drugs from these nanoparticles can be readily modified using external fields such as magnetic or NIR radiation. Another promising application of nanoparticles is in Surface Enhanced Resonance Raman Scattering (SERRS), which enables accurate detection of malignant or premalignant lesions and non-invasive imaging of the whole body. In light of these advantages, researchers have developed SERRS nanoparticles labelled with
68Ga using a chelator-free method for imaging pre-operative and intraoperative profiles in lymph node disease using PET imaging. This technique shows promise for improving the accuracy and sensitivity of imaging in lymph node disease, thereby aiding in more effective treatment planning and monitoring.
13.4. Radiolabelled Silica Nanoparticles for Theranostic Application
Silica nanoparticles are popular for drug delivery and bio-imaging due to their advantages, but they have some drawbacks. In a recent study, researchers developed mesoporous silica nanoparticles with MUC1 aptamer capping, loaded with safranin O, and functionalized with aminopropyl groups for controlled drug delivery and tumor imaging. The system demonstrated higher tumor uptake of nanoparticles in mice, showing a promising approach for simultaneous drug delivery and imaging
[63]. The study investigated the use of peanut-shaped silica-coated graphene oxide nanoparticles loaded with gambogic acid and radiolabelled with
188Re for tumor targeting. The results demonstrated that
188Re provided radiotherapy and SPECT/CT imaging in a VX2 tumor model.
13.5. Radiolabelled Polymeric Nanoparticles for Theranostic Application
Polymeric nanoparticles have become popular for radiotheranostic applications in diseases such as cancer, diabetes, and neurological disorders. Radiolabelling can be done through pre- or post-labelling strategies using chelators or cross-linking agents. A recent study used radiolabelled HPMA nanoparticles loaded with antitumor drugs to evaluate their efficacy in an in vivo tumor model, showing improved accumulation in tumor cells, longer circulation time, and a higher therapeutic index with the combination of radiotherapy and chemotherapy
[64]. The study investigated the use of radiolabelled nanoparticles for tumor angiogenesis therapy. Poly (HPMA) nanoparticles were radiolabelled with
64Cu using DOTA as a chelating agent and conjugated with target-specific peptide RGDyK for tumor localization using PET imaging. Additionally,
89Zr-radiolabelled HPMA polymeric conjugates were prepared for therapeutic efficacy on a
4T1 cancer cell model. The study found that the disparity and molecular weights of the polymer played a significant role in the pharmacokinetic profile of polymeric conjugates.