
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Filippos Triposkiadis | -- | 3429 | 2023-06-13 17:27:29 | | | |
| 2 | Lindsay Dong | Meta information modification | 3429 | 2023-06-15 03:25:04 | | |
Video Upload Options
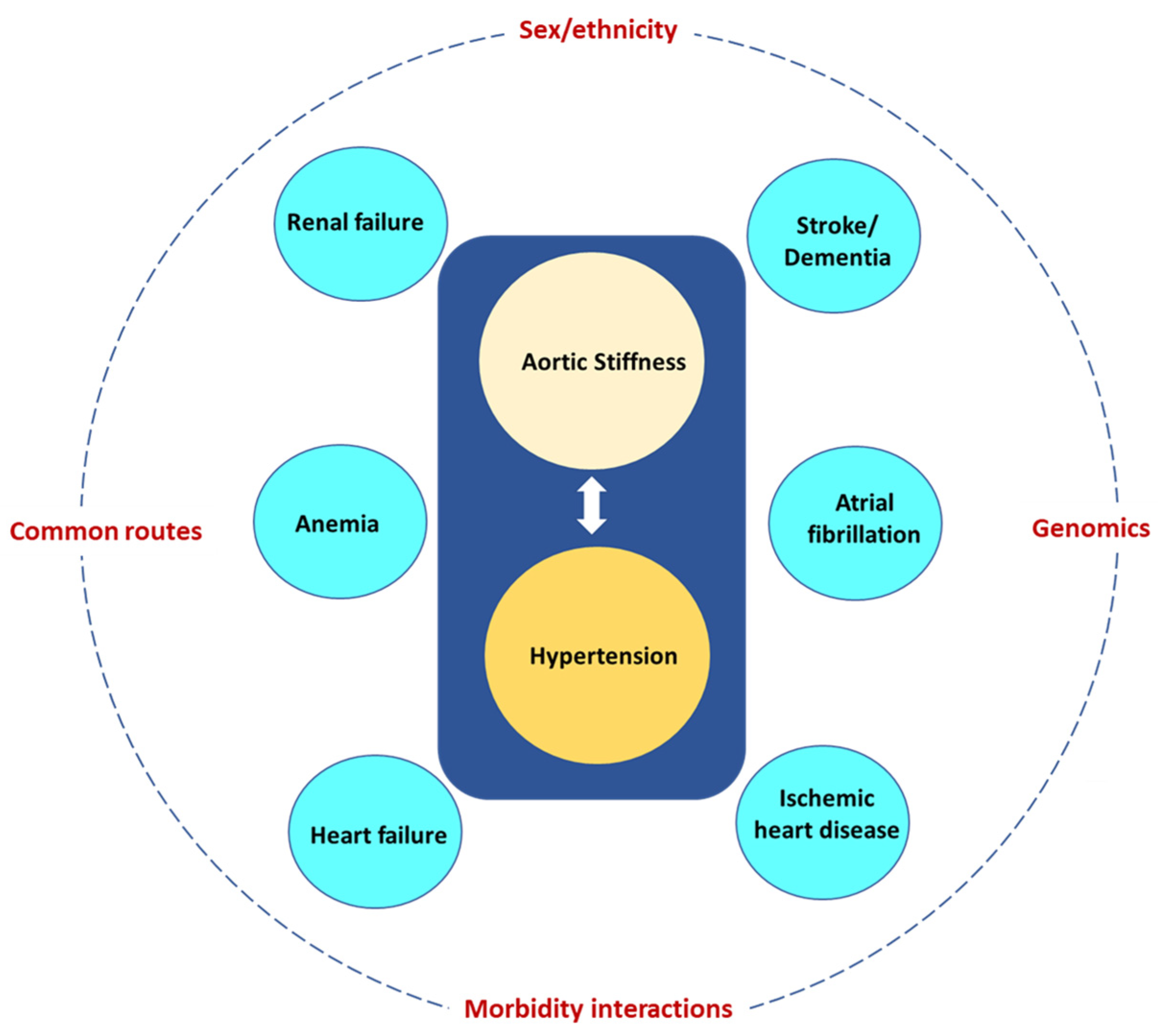
Multimorbidity, the coexistence of multiple health conditions in an individual, has emerged as one of the greatest challenges facing health services, and this crisis is partly driven by the aging population. Aging is associated with increased aortic stiffness (AoStiff), which in turn is linked with several morbidities frequently affecting and having disastrous consequences for the elderly. These include hypertension, ischemic heart disease, heart failure, atrial fibrillation, chronic kidney disease, anemia, ischemic stroke, and dementia. Two or more of these disorders (multimorbidity) often coexist in the same elderly patient and the specific multimorbidity pattern depends on several factors including sex, ethnicity, common morbidity routes, morbidity interactions, and genomics.
1. Introduction
2. The Normal Young Aorta
3. The Aging Aorta
3.1. Biology
3.2. Stiffness
4. Cardiovascular Disorders
4.1. Hypertension
4.2. Atrial Fibrillation
4.3. Ischemic Heart Disease
4.4. Heart Failure
HF has been attributed to the interaction between cardiovascular aging and specific risk factors, comorbidities, and disease modifiers [4][39]. Wave reflections in the rigid aorta of the elderly tend to increase mid-to-late systolic LV load and myocardial wall stress [40]. When the LV pump function is preserved, the reflected wave induces a late systolic pressure peak in the pressure waveform, augmenting aortic pressure in mid-to-late systole. Conversely, when the LV pump function is depressed, wave reflection may exert more pronounced effects to decrease flow, with no apparent alteration in the appearance of the pressure waveform. In patients with severe LV systolic dysfunction, wave reflections truncate flow, reduce stroke volume and shorten the duration of LV ejection. In addition, forward waves may also be altered in patients with severely depressed LV function, as indicated by the decrease in the ratio of the first to second systolic peak, compared to individuals with preserved LV function [41].
5. Chronic Kidney Disease
6. Anemia
7. Ischemic Stroke
8. Dementia
9. Frailty
10. Determinants of Multimorbidity Pattern in the Elderly
1. Sex and ethnicity. Women are more often affected by multimorbidity than men. In the Rotterdam study (n = 6094, median years of follow-up 9.2) two-thirds of people over 45 developed multimorbidity in their remaining lifetime, with women manifesting nearly double the risk of multimorbidity compared to men [74]. MicroRNAs (miRNAs) are small, non-coding endogenous RNA molecules that regulate gene expression either by mRNA cleavage/destabilization or inhibition of translation. miR-181a, which acts as an inflamma-miRNA playing important roles in the aging process, has been identified as multimorbidity-associated miRNA [75].
11. Conclusions
References
- Johnston, M.C.; Crilly, M.; Black, C.; Prescott, G.J.; Mercer, S.W. Defining and measuring multimorbidity: A systematic review of systematic reviews. Eur. J. Public Health 2019, 29, 182–189.
- Pearson-Stuttard, J.; Ezzati, M.; Gregg, E.W. Multimorbidity-a defining challenge for health systems. Lancet Public Health 2019, 4, e599–e600.
- Salive, M.E. Multimorbidity in older adults. Epidemiol. Rev. 2013, 35, 75–83.
- Triposkiadis, F.; Xanthopoulos, A.; Butler, J. Cardiovascular Aging and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 804–813.
- Rodrigues, L.P.; de Oliveira Rezende, A.T.; Delpino, F.M.; Mendonca, C.R.; Noll, M.; Nunes, B.P.; de Oliviera, C.; Silveira, E.A. Association between multimorbidity and hospitalization in older adults: Systematic review and meta-analysis. Age Ageing 2022, 51, afac155.
- Vasan, R.S.; Pan, S.; Xanthakis, V.; Beiser, A.; Larson, M.G.; Seshadri, S.; Mitchell, G.F. Arterial Stiffness and Long-Term Risk of Health Outcomes: The Framingham Heart Study. Hypertension 2022, 79, 1045–1056.
- Boudoulas, K.D.; Vlachopoulos, C.; Raman, S.V.; Sparks, E.A.; Triposciadis, F.; Stefanadis, C.; Boudoulas, H. Aortic function: From the research laboratory to the clinic. Cardiology 2012, 121, 31–42.
- Fukamachi, K.; Horvath, D.J.; Karimov, J.H.; Kado, Y.; Miyamoto, T.; Kuban, B.D.; Starling, R.C. Left atrial assist device to treat patients with heart failure with preserved ejection fraction: Initial in vitro study. J. Thorac. Cardiovasc. Surg. 2021, 162, 120–126.
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol. Rev. 2017, 97, 1555–1617.
- Briet, M.; Boutouyrie, P.; Laurent, S.; London, G.M. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012, 82, 388–400.
- Belz, G.G. Elastic properties and Windkessel function of the human aorta. Cardiovasc. Drugs Ther. 1995, 9, 73–83.
- Wilkinson, I.B.; Maki-Petaja, K.M.; Mitchell, G.F. Uses of Arterial Stiffness in Clinical Practice. Arter. Thromb. Vasc. Biol. 2020, 40, 1063–1067.
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Mehdizadeh, M.; Aguilar, M.; Thorin, E.; Ferbeyre, G.; Nattel, S. The role of cellular senescence in cardiac disease: Basic biology and clinical relevance. Nat. Rev. Cardiol. 2022, 19, 250–264.
- Schmauck-Medina, T.; Moliere, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging 2022, 14, 6829–6839.
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Maki-Petaja, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular consequences of inflammation: A position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698.
- Nichols, W.W.; O’Rourke, M.F.; Avolio, A.P.; Yaginuma, T.; Murgo, J.P.; Pepine, C.J.; Conti, C.R. Effects of age on ventricular-vascular coupling. Am. J. Cardiol. 1985, 55, 1179–1184.
- Mitchell, G.F.; Wang, N.; Palmisano, J.N.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S. Hemodynamic correlates of blood pressure across the adult age spectrum: Noninvasive evaluation in the Framingham Heart Study. Circulation 2010, 122, 1379–1386.
- Burt, V.L.; Whelton, P.; Roccella, E.J.; Brown, C.; Cutler, J.A.; Higgins, M.; Horan, M.J.; Labarthe, D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension 1995, 25, 305–313.
- Franklin, S.S.; Gustin, W.t.; Wong, N.D.; Larson, M.G.; Weber, M.A.; Kannel, W.B.; Levy, D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997, 96, 308–315.
- Mitchell, G.F. Arterial stiffness and hypertension: Chicken or egg? Hypertension 2014, 64, 210–214.
- Mitchell, G.F.; Guo, C.Y.; Benjamin, E.J.; Larson, M.G.; Keyes, M.J.; Vita, J.A.; Vasan, R.S.; Levy, D. Cross-sectional correlates of increased aortic stiffness in the community: The Framingham Heart Study. Circulation 2007, 115, 2628–2636.
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012, 308, 875–881.
- Laurent, S.; Boutouyrie, P. The structural factor of hypertension: Large and small artery alterations. Circ. Res. 2015, 116, 1007–1021.
- Olsen, M.H.; Angell, S.Y.; Asma, S.; Boutouyrie, P.; Burger, D.; Chirinos, J.A.; Damasceno, A.; Delles, C.; Gimenez-Roqueplo, A.P.; Hering, D.; et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: The Lancet Commission on hypertension. Lancet 2016, 388, 2665–2712.
- Angoff, R.; Mosarla, R.C.; Tsao, C.W. Aortic Stiffness: Epidemiology, Risk Factors, and Relevant Biomarkers. Front. Cardiovasc. Med. 2021, 8, 709396.
- Mitchell, G.F. Arterial Stiffness in Aging: Does It Have a Place in Clinical Practice?: Recent Advances in Hypertension. Hypertension 2021, 77, 768–780.
- Verdecchia, P.; Angeli, F.; Reboldi, G. Hypertension and Atrial Fibrillation: Doubts and Certainties From Basic and Clinical Studies. Circ. Res. 2018, 122, 352–368.
- Gumprecht, J.; Domek, M.; Lip, G.Y.H.; Shantsila, A. Invited review: Hypertension and atrial fibrillation: Epidemiology, pathophysiology, and implications for management. J. Hum. Hypertens. 2019, 33, 824–836.
- Roetker, N.S.; Chen, L.Y.; Heckbert, S.R.; Nazarian, S.; Soliman, E.Z.; Bluemke, D.A.; Lima, J.A.; Alonso, A. Relation of systolic, diastolic, and pulse pressures and aortic distensibility with atrial fibrillation (from the Multi-Ethnic Study of Atherosclerosis). Am. J. Cardiol. 2014, 114, 587–592.
- Izuhara, M.; Shioji, K.; Kadota, S.; Baba, O.; Takeuchi, Y.; Uegaito, T.; Mutsuo, S.; Matsuda, M. Relationship of cardio-ankle vascular index (CAVI) to carotid and coronary arteriosclerosis. Circ. J. 2008, 72, 1762–1767.
- Park, J.B.; Park, H.E.; Choi, S.Y.; Kim, M.K.; Oh, B.H. Relation between cardio-ankle vascular index and coronary artery calcification or stenosis in asymptomatic subjects. J. Atheroscler. Thromb. 2013, 20, 557–567.
- Birudaraju, D.; Cherukuri, L.; Kinninger, A.; Chaganti, B.T.; Haroun, P.; Pidikiti, S.; Lakshmanan, S.; Hamal, S.; Flores, F.; Dailing, C.; et al. Relationship between cardio-ankle vascular index and obstructive coronary artery disease. Coron. Artery Dis. 2020, 31, 550–555.
- Lonnebakken, M.T.; Eskerud, I.; Larsen, T.H.; Midtbo, H.B.; Kokorina, M.V.; Gerdts, E. Impact of aortic stiffness on myocardial ischaemia in non-obstructive coronary artery disease. Open Heart 2019, 6, e000981.
- Frak, W.; Wojtasinska, A.; Lisinska, W.; Mlynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938.
- Kim, H.-L. Arterial stiffness and coronary artery disease. EMJ Cardiol. 2016, 4, 84–89.
- Medina-Leyte, D.J.; Zepeda-Garcia, O.; Dominguez-Perez, M.; Gonzalez-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850.
- Back, M.; Yurdagul, A., Jr.; Tabas, I.; Oorni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406.
- Triposkiadis, F.; Xanthopoulos, A.; Parissis, J.; Butler, J.; Farmakis, D. Pathogenesis of chronic heart failure: Cardiovascular aging, risk factors, comorbidities, and disease modifiers. Heart Fail. Rev. 2022, 27, 337–344.
- Weber, T.; Chirinos, J.A. Pulsatile arterial haemodynamics in heart failure. Eur. Heart J. 2018, 39, 3847–3854.
- Curtis, S.L.; Zambanini, A.; Mayet, J.; Mc, G.T.S.A.; Foale, R.; Parker, K.H.; Hughes, A.D. Reduced systolic wave generation and increased peripheral wave reflection in chronic heart failure. Am. J. Physiol. Circ. Physiol. 2007, 293, H557–H562.
- Bidani, A.K.; Griffin, K.A.; Williamson, G.; Wang, X.; Loutzenhiser, R. Protective importance of the myogenic response in the renal circulation. Hypertension 2009, 54, 393–398.
- Mitchell, G.F. Aortic stiffness, pressure and flow pulsatility, and target organ damage. J. Appl. Physiol. (1985) 2018, 125, 1871–1880.
- Christensen, P.K.; Hansen, H.P.; Parving, H.H. Impaired autoregulation of GFR in hypertensive non-insulin dependent diabetic patients. Kidney Int. 1997, 52, 1369–1374.
- Hill, G.S.; Heudes, D.; Bariety, J. Morphometric study of arterioles and glomeruli in the aging kidney suggests focal loss of autoregulation. Kidney Int. 2003, 63, 1027–1036.
- Georgianos, P.I.; Sarafidis, P.A.; Liakopoulos, V. Arterial Stiffness: A Novel Risk Factor for Kidney Injury Progression? Am. J. Hypertens. 2015, 28, 958–965.
- Sedaghat, S.; Mattace-Raso, F.U.; Hoorn, E.J.; Uitterlinden, A.G.; Hofman, A.; Ikram, M.A.; Franco, O.H.; Dehghan, A. Arterial Stiffness and Decline in Kidney Function. Clin. J. Am. Soc. Nephrol. 2015, 10, 2190–2197.
- van Varik, B.J.; Vossen, L.M.; Rennenberg, R.J.; Stoffers, H.E.; Kessels, A.G.; de Leeuw, P.W.; Kroon, A.A. Arterial stiffness and decline of renal function in a primary care population. Hypertens. Res. 2017, 40, 73–78.
- Bach, V.; Schruckmayer, G.; Sam, I.; Kemmler, G.; Stauder, R. Prevalence and possible causes of anemia in the elderly: A cross-sectional analysis of a large European university hospital cohort. Clin. Interv. Aging 2014, 9, 1187–1196.
- Stauder, R.; Valent, P.; Theurl, I. Anemia at older age: Etiologies, clinical implications, and management. Blood 2018, 131, 505–514.
- Montero, D.; Diaz-Canestro, C.; Keiser, S.; Lundby, C. Arterial stiffness is strongly and negatively associated with the total volume of red blood cells. Int. J. Cardiol. 2016, 221, 77–80.
- Tiwaskar, M.; Kalra, S.; Bantwal, G.; Bhattacharya, A.; Sahay, M.; Jadhav, U.; Joshi, A.; Das, A.K.; Khullar, D.; Baruah, M.; et al. SGLT2-inhibition and Vascular Euphoria a Reconciliation of Vascular Health and Disease Homeostasis. J. Assoc. Physicians India 2021, 69, 11–12.
- Montero, D.; Diaz-Canestro, C.; Flammer, A.; Lundby, C. Unexplained Anemia in the Elderly: Potential Role of Arterial Stiffness. Front. Physiol. 2016, 7, 485.
- Chen, Y.; Shen, F.; Liu, J.; Yang, G.Y. Arterial stiffness and stroke: De-stiffening strategy, a therapeutic target for stroke. Stroke Vasc. Neurol. 2017, 2, 65–72.
- Fico, B.G.; Miller, K.B.; Rivera-Rivera, L.A.; Corkery, A.T.; Pearson, A.G.; Eisenmann, N.A.; Howery, A.J.; Rowley, H.A.; Johnson, K.M.; Johnson, S.C.; et al. The Impact of Aging on the Association Between Aortic Stiffness and Cerebral Pulsatility Index. Front. Cardiovasc. Med. 2022, 9, 821151.
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int. J. Mol. Sci. 2019, 20, 5694.
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495.
- Am Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); Am Psychiatric Association: Washington, DC, USA, 2013.
- Decourt, B.; Noorda, K.; Noorda, K.; Shi, J.; Sabbagh, M.N. Review of Advanced Drug Trials Focusing on the Reduction of Brain Beta-Amyloid to Prevent and Treat Dementia. J. Exp. Pharm. 2022, 14, 331–352.
- Niotis, K.; Akiyoshi, K.; Carlton, C.; Isaacson, R. Dementia Prevention in Clinical Practice. Semin. Neurol. 2022, 42, 525–548.
- Biessels, G.J. Diagnosis and treatment of vascular damage in dementia. Biochim. Biophys. Acta. 2016, 1862, 869–877.
- Kalaria, R.N. The pathology and pathophysiology of vascular dementia. Neuropharmacology 2018, 134, 226–239.
- Iulita, M.F.; Noriega de la Colina, A.; Girouard, H. Arterial stiffness, cognitive impairment and dementia: Confounding factor or real risk? J. Neurochem. 2018, 144, 527–548.
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762.
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104.
- Piotrowicz, K.; Gasowski, J. Risk Factors for Frailty and Cardiovascular Diseases: Are They the Same? Adv. Exp. Med. Biol. 2020, 1216, 39–50.
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, Frailty and Cardiovascular Disease. Adv. Exp. Med. Biol. 2020, 1216, 55–64.
- Schaller, M.S.; Ramirez, J.L.; Gasper, W.J.; Zahner, G.J.; Hills, N.K.; Grenon, S.M. Frailty Is Associated with an Increased Risk of Major Adverse Cardiac Events in Patients with Stable Claudication. Ann. Vasc. Surg. 2018, 50, 38–45.
- Ewe, S.H.; Ajmone Marsan, N.; Pepi, M.; Delgado, V.; Tamborini, G.; Muratori, M.; Ng, A.C.; van der Kley, F.; de Weger, A.; Schalij, M.J.; et al. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am. Heart J. 2010, 160, 1113–1120.
- Arnold, S.V.; Zhao, Y.; Leon, M.B.; Sathananthan, J.; Alu, M.; Thourani, V.H.; Smith, C.R.; Mack, M.J.; Cohen, D.J. Impact of Frailty and Prefrailty on Outcomes of Transcatheter or Surgical Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2022, 15, e011375.
- Campo, G.; Maietti, E.; Tonet, E.; Biscaglia, S.; Ariza-Sole, A.; Pavasini, R.; Tebaldi, M.; Cimaglia, P.; Bugani, G.; Serenelli, M.; et al. The Assessment of Scales of Frailty and Physical Performance Improves Prediction of Major Adverse Cardiac Events in Older Adults with Acute Coronary Syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1113–1119.
- Damluji, A.A.; Chung, S.E.; Xue, Q.L.; Hasan, R.K.; Moscucci, M.; Forman, D.E.; Bandeen-Roche, K.; Batchelor, W.; Walston, J.D.; Resar, J.R.; et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur. Heart J. 2021, 42, 3856–3865.
- Piotrowicz, K.; Gryglewska, B.; Grodzicki, T.; Gasowski, J. Arterial stiffness and frailty—A systematic review and metaanalysis. Exp. Gerontol. 2021, 153, 111480.
- Velek, P.; Luik, A.I.; Brusselle, G.G.O.; Stricker, B.C.; Bindels, P.J.E.; Kavousi, M.; Kieboom, B.C.T.; Voortman, T.; Ruiter, R.; Ikram, M.A.; et al. Sex-specific patterns and lifetime risk of multimorbidity in the general population: A 23-year prospective cohort study. BMC Med. 2022, 20, 304.
- Rippo, M.R.; Olivieri, F.; Monsurro, V.; Prattichizzo, F.; Albertini, M.C.; Procopio, A.D. MitomiRs in human inflamm-aging: A hypothesis involving miR-181a, miR-34a and miR-146a. Exp. Gerontol. 2014, 56, 154–163.
- Barnes, P.J. Mechanisms of development of multimorbidity in the elderly. Eur. Respir. J. 2015, 45, 790–806.
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420.
- Lacolley, P.; Regnault, V.; Laurent, S. Mechanisms of Arterial Stiffening: From Mechanotransduction to Epigenetics. Arter. Thromb. Vasc. Biol. 2020, 40, 1055–1062.
- Sheridan, P.E.; Mair, C.A.; Quinones, A.R. Associations between prevalent multimorbidity combinations and prospective disability and self-rated health among older adults in Europe. BMC Geriatr. 2019, 19, 198.
- Masoli, J.A.H.; Pilling, L.C.; Frayling, T.M. Genomics and multimorbidity. Age Ageing 2022, 51, afac285.
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 2020, 21, 88–101.




