Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tibor Fülöp | -- | 2252 | 2023-06-13 15:57:41 | | | |
| 2 | Lindsay Dong | Meta information modification | 2252 | 2023-06-15 03:22:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pethő, �.G.; Tapolyai, M.; Browne, M.; Fülöp, T.; Orosz, P.; Szabó, R.P. Importance of Nephrologist in Diuretic-Resistant Heart Failure Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/45515 (accessed on 08 February 2026).
Pethő �G, Tapolyai M, Browne M, Fülöp T, Orosz P, Szabó RP. Importance of Nephrologist in Diuretic-Resistant Heart Failure Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/45515. Accessed February 08, 2026.
Pethő, Ákos Géza, Mihály Tapolyai, Maria Browne, Tibor Fülöp, Petronella Orosz, Réka P. Szabó. "Importance of Nephrologist in Diuretic-Resistant Heart Failure Treatment" Encyclopedia, https://encyclopedia.pub/entry/45515 (accessed February 08, 2026).
Pethő, �.G., Tapolyai, M., Browne, M., Fülöp, T., Orosz, P., & Szabó, R.P. (2023, June 13). Importance of Nephrologist in Diuretic-Resistant Heart Failure Treatment. In Encyclopedia. https://encyclopedia.pub/entry/45515
Pethő, Ákos Géza, et al. "Importance of Nephrologist in Diuretic-Resistant Heart Failure Treatment." Encyclopedia. Web. 13 June, 2023.
Copy Citation
Heart failure is not only a global problem but also significantly limits the life prospects of these patients. The risk factors leading to heart failure are well known; however, the real challenge is to provide effective treatments. A vicious cycle develops in heart failure of all etiologies, sooner or later compromising both cardiac and kidney functions simultaneously. This can explain the repeated hospital admissions due to decompensation and the significantly reduced quality of life. Moreover, diuretic-refractory heart failure represents a distinct challenge due to repeated hospital admissions and increased mortality.
ascites
cardiorenal syndrome
diuretic resistance
mortality
nephrologist
heart failure
1. Introduction
Heart failure and cardiovascular diseases (CVDs) are the leading cause of death globally. The World Health Organization’s (WHO’s) declared goal is to reduce the number of premature deaths by 25% by 2025 through nine voluntary global targets [1]. The epidemiology and clinical manifestations of heart failure are intensively researched topics in cardiology. The risk factors leading to heart failure are well known; however, the current key challenge is the offer of effective treatments with sustained efficacy for these patients. A vicious cycle often develops in heart failure of different etiologies leading to progressive fluid accumulation and tissue hypoxia, ultimately being responsible for the disease’s poor prognosis through a simultaneous compromise of both kidney and heart functions. Repeated hospital admissions due to decompensation are the rule with a significantly reduced quality of life. Multidisciplinary treatment of patients with severe heart failure aims to stop or interrupt this downward spiral (Figure 1).
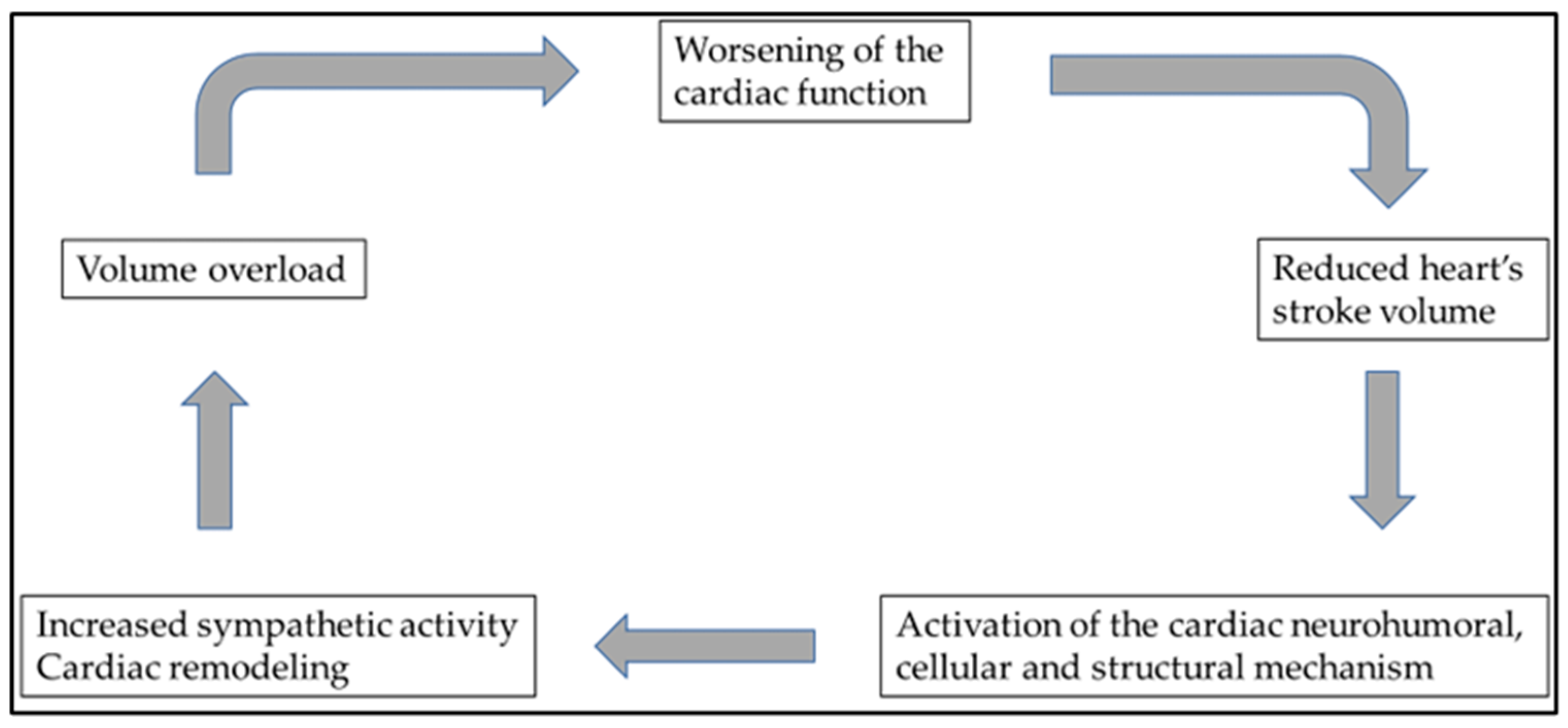
Figure 1. Multidisciplinary treatment of patients with severe heart failure aims to stop or interrupt this downward spiral. Unfortunately, heart failure of various etiologies eventually ends up in this vicious cycle, ultimately responsible for the disease’s poor prognosis.
In treating heart failure, an effective fluid management program is crucial to reduce hospital readmissions and overall mortality. This is based on optimal diuretic use and other procedures. Therefore, cooperation with other professions, e.g., nephrologists, is often necessary to maintain euvolemia, but patient education is crucial and contributes to treatment success [2]. According to the latest guidelines of the European Society of Cardiology (ESC), heart failure can be classified into three groups on the basis of the heart’s left-ventricular ejection fraction: HFrEF (“heart failure with reduced ejection fraction”; ≤40%), HFmrEF (“heart failure with mildly reduced ejection fraction”; 41–49%), and HFpEF (“heart failure with preserved ejection fraction”; ≥50%). This staging better defines therapeutic options and guidelines [3].
2. Diuretic Resistance to Heart Failure
Heart failure, including diuretic-resistant heart failure, can be responsible for the repeated hospitalization of such patients. Unfortunately, epidemiological data show that this number is regrettably increasing [4]. In treating fluid overload in severe heart failure, loop diuretics are the mainstay of diuretic treatment. Loop diuretics reversibly inhibit sodium reabsorption in the thick ascending limb of the loop of Henle. This is why loop diuretics are primarily used, since most sodium reabsorption occurs in the ascending limb, enabling a more significant diuresis.
Other diuretics, such as thiazides, act on the distal ascending limb, while potassium-sparing diuretics work on the collecting ducts. Sodium reabsorption is lower in those sections of the nephron [5]. The family of loop diuretics, such as the most used furosemide, torasemide, and bumetamide, theoretically inhibits the same symporter in the apical membrane of the macula densa cells. Reduced sodium reabsorption further inhibits tubuloglomerular feedback and, thus, stimulates renin secretion [6]. On the other hand, the renin–angiotensin–aldosterone system (RAAS) increased by loop diuretics increases the plasma renin level, which also causes an increase in angiotensin II.
Nevertheless, this increase in RAAS activity can further inhibit tubuloglomerular feedback, thus reducing the glomerular filtration rate (GFR), i.e., a narrowing of kidney function [6]. RAAS activated by loop diuretics increases systemic blood pressure, as well as sodium and water reabsorption. In addition, loop diuretics also increase the level of vasodilator prostaglandins, as a result of which an increase in pressure also occurs in the proximal tubule [7]. Due to the countervailing effects of these biochemical processes, high doses of intravenous (IV) loop diuretics can either decrease or increase arterial blood pressure. Furthermore, blood flow to the kidneys decreases due to centralizing circulation [8], and decreasing kidney function itself further reduces the effective fluid removal with diuretics.
Another reason for diuretic resistance, in addition to the activation of the RAAS, is that structural changes in the nephrons also occur, also known as the “braking phenomenon”. Chronic use of loop diuretics results in hypertrophy and hyperplasia of the cells of the distal tubules. Hypertrophy of distal tubular cells reduces the response to diuretics due to compensatory increased sodium reabsorption [9].
3. Cardiorenal Syndrome
As discussed above, elevated serum renin levels raise intraglomerular pressure due to efferent arteriolar constriction. In severe, decompensated heart failure, due to significantly increased renal venous pressure and reduced renal blood flow, the compensation that preserves GFR fails, thus resulting in a further decrease in GFR [10]. The progression of cardiorenal syndrome into more and more severe heart failure is, thus, the result of not only sodium accumulation but perhaps also an increase in the intra-abdominal compartment [11]. The fact that hypertonic saline infusion with furosemide may be more effective than diuretics only [12] and the phenomenon that a normal-sodium diet compared to a low-sodium diet improves outcome [13] all point the attention to reconsider sodium excess as the only instigator of cardiac decompensation. As a result of RAAS and neurohumoral activation, preglomerular vasoconstriction results in decreased intraglomerular pressure and reduced GFR. To maintain an adequate plasma volume, increased activation of the neurohumoral axis results in increased proximal tubular sodium and water reabsorption, ultimately resulting in oliguria and worsening congestion [14].
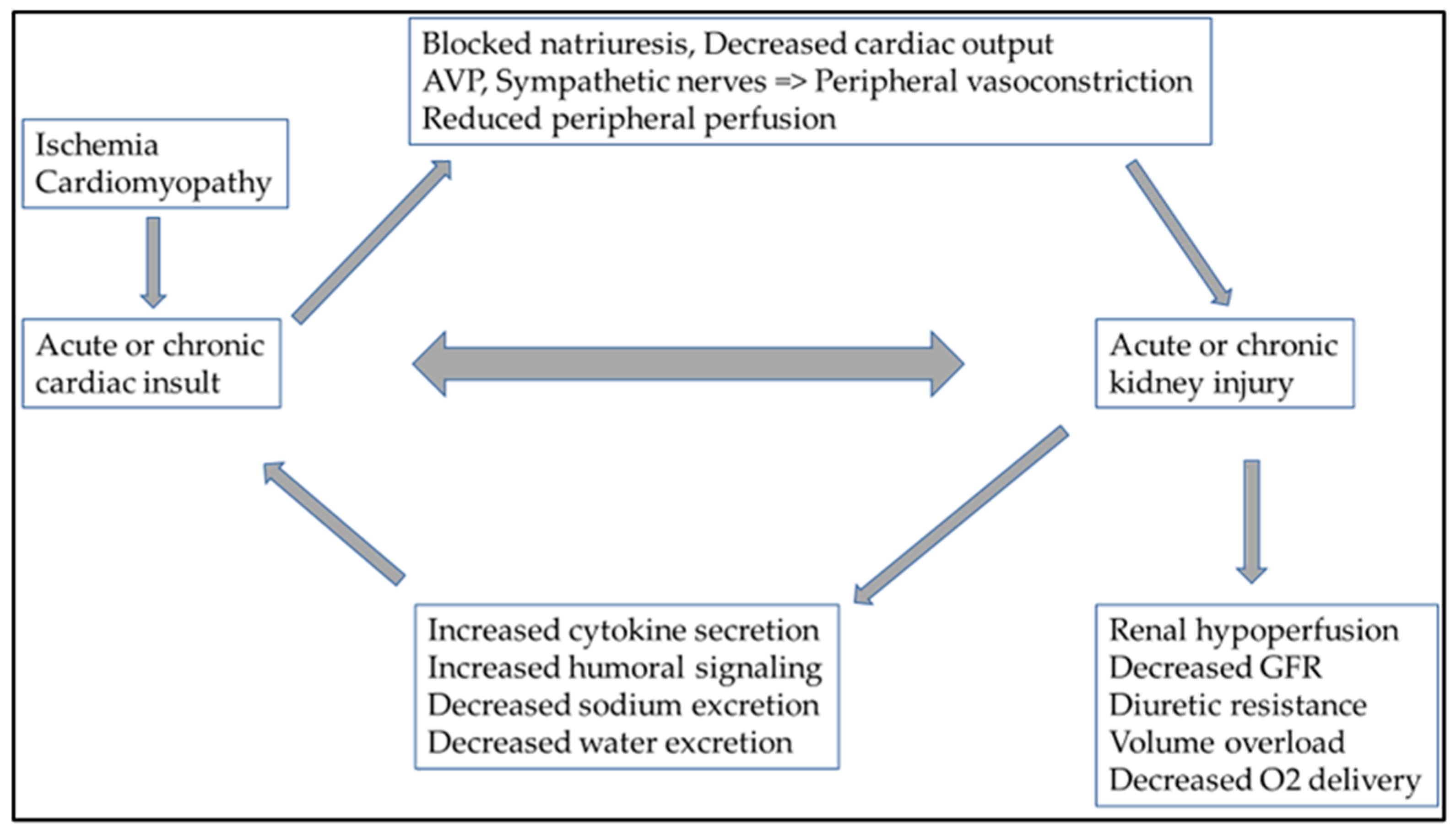
However, hemodynamic changes can lead to cardiorenal syndrome (CRS) and the activation of the sympathetic nervous system, through persistent RAAS activation. This also includes chronic inflammation and an imbalance in the ratio of reactive oxygen species/nitrogen monoxide production [15][16]. Specific circulating cytokines can be responsible for adverse cardiac side-effects in both acute and chronic kidney damage. In acute kidney injury, IL-6 (interleukin-6), TNF-α (tumor necrosis factor-α), and IL-1 (interleukin-1) have a cardio-depressant direct impact. The role of FGF-23 (fibroblast growth factor-23) in uremic cardiomyopathy (type 4 CRS) in chronic kidney damage is also known [17]. Fluid overload and consequent peripheral venous congestion in severe heart failure result in endothelial dysfunction and additional proinflammatory cytokines released by endothelial cells. This increased proinflammatory cytokine production also plays a role in developing CRS and changes in hemodynamic parameters [18]. The presence of abdominal ascites is known to pose an additional burden on net renal perfusion [19][20]. This also shows how complex the pathophysiology of CRS is and how closely related the heart and kidney functions are (Figure 2).

Figure 2. In cardiorenal syndrome, heart and kidney function are closely related, as are the pathophysiologies of several neurohumoral and inflammatory pathways. Ultimately, the deterioration of either the heart or kidney function causes consequential damage to the other organ. Abbreviations: AVP: arginine vasopressin; GFR: glomerular filtration ratio; O2: oxygen.
4. Peritoneal Dialysis in Diuretic-Resistant Heart Failure
Peritoneal dialysis (PD) treatment in severe diuretic-resistant heart failure can improve a patient’s quality of life and functional class stage of heart failure; however, cardiovascular mortality remains high [21]. The first published report of PD treatment offered for congestive heart failure is dated back to 1996 [22]. PD treatment is the gentlest procedure in such patients to reduce or eliminate fluid overload. These patients are most often readmitted to the hospital due to fluid overload, and PD treatment can effectively remove excess fluid and salt according to the details above [23]. Furthermore, it also prevents progressive ascites accumulation, known to impair net renal perfusion pressure. In the case of a cardiorenal syndrome associated with severe heart failure (CRS I-II), the degree of kidney damage does not affect survival [24]. However, improving left-ventricular systolic function in patients with PD treatment may result in a better quality of life [25][26]. It is also known that PD treatment can only effectively improve the quality of life of patients with severe LVEF who have markedly reduced left-ventricular systolic function (EF < 15%) [25]. The improvement in quality of life can be dramatic. PD therapy can improve the functional status of patients with severe diuretic-resistant heart failure whose vital functions can only be maintained by vasopressors [27].
In a retrospective study of nearly 11,000 patients, repeated hospitalizations of patients treated with hemodialysis (HD) and PD who required renal replacement therapy due to severe heart failure were compared. The study showed that the incidence of PD peritonitis and the resulting hospitalization rate were significantly higher in patients treated with PD. However, PD treatment still seemed more beneficial [28]. Despite a higher incidence of rehospitalization due to PD peritonitis, PD treatment is a more favorable renal replacement modality for severe diuretic-resistant heart failure than HD treatment.
PD treatment in a group of patients suffering from severe diuretic-resistant heart failure has been known for several decades. However, it has not entered the everyday public consciousness, and no professional consensus has yet been formed. It has been shown to effectively reduce the need for hospital admissions and improve left-ventricular systolic function in several studies. In the case of associated kidney damage, the results were not favorable; in the CARESS HF study (Effectiveness of Ultrafiltration in Treating People with Acute Decompensated Heart Failure and Cardiorenal Syndrome), kidney function did not improve significantly, although, from the point of view of the outcome, the aim was not necessarily to improve kidney function [29].
The mortality of patients with diuretic-resistant heart failure is very high; thus, extracorporeal treatment (HD) performed with a central venous catheter (CVK) represents an additional increased risk due to bloodstream infection and death [30]. In the presence of other indwelling intravascular CV hardware, such as pacemakers or ventricular synchronizers, the risk of bacteremia is even higher with CVK [31][32]. If renal replacement therapy is justified, PD treatment can particularly benefit severe diuretic-resistant heart failure patients [33][34].
Atrial natriuretic peptide (ANP) and tumor necrosis factor-α (TNF-α) were removed during PD treatment in heart failure despite no improvement in renal function. In those patients with PD treatment, removed interleukin (IL)-1 and IL-6 also played a role in the functional phase and increased measurable ejection fraction [35]. The effectiveness of PD treatment depends not only on the transport of low-molecular-weight substances but also on uremic toxins secreted into the abdominal cavity. In this group of patients, the transperitoneal transport mechanism has additional potential benefits; by removing inflammatory and other cardiotoxic molecules, it contributes to an increase in myocardial contractility [36].
5. Urgent Start of the Peritoneal Dialysis in Diuretic Resistance Heart Failure
Minimally invasive percutaneous PD catheter insertion has been a procedure described for decades. Of course, there are cases where surgical PD catheter (PDC) insertion is indispensable, e.g., in overweight, obese patients or individuals who have undergone major abdominal surgery, in whom there is a possibility of intra-abdominal adhesions [37]. In the case of suspicion of abdominal adhesions, a laparoscopic intervention is recommended, during which any adhesions can be surgically dissolved, and the catheter can be inserted into the appropriate place; alternatively, if placement is not feasible, the procedure can be aborted. In the latest international recommendations (ISPD—International Society for Peritoneal Dialysis), the point of the passage of the percutaneous PD catheter through the abdominal wall is not strictly defined. When inserting a percutaneous PDC, the most important thing is the positioning of the catheter; therefore, the end of the catheter must be located in the pelvis, and the catheter exit site must be at least 2 cm in the abdominal wall from the outer Dacron ring [38]. The ISPD recommendation considers a waiting period of at least 2 weeks after the insertion of the PD catheter to be desirable. However, the recommendation also acknowledges that, in several studies, no significant difference was found with regard to PD peritonitis or modality survival in patient groups in which PD–fluid exchanges had to be started urgently after the intervention [39][40][41].
With the increasing acceptance of minimally invasive procedures, percutaneous PDC can be considered in patients with severe, critical circulatory failure under regional anesthesia. Most severely critically ill patients may already be sedated unconscious due to acute critical illness [42]. Percutaneous PD implantation and, in the case of emergency, the initiation of PD treatment are, in principle, comparable to the insertion of a tunneled dialysis catheter (TDC), with subsequent imminent use after position confirmation. Various abdominal injuries and early infections are possible complications, which are much lower when using the percutaneous technique [43]. According to some studies, no significant difference was found between the two procedures in terms of catheter dysfunction or leakage of PD solution. However, the incidence of PD peritonitis was significantly lower in the percutaneous group [44]. In the outcome of PD catheter implantation success, there was no statistical difference in survival between surgical and percutaneous placement [45].
6. Other Specific Nephrology Treatments in Diuretic-Resistant Heart Failure
One major comorbidity to modulate risk is the presence of anemia, which emerges with both heart failure and kidney diseases. In heart failure, anemia also correlates with the functional stage of severe diuretic-resistant heart failure and the degree of clinical symptoms [46]. It follows that treating anemia of clear renal origin can also be a therapeutic goal in such patients. Furthermore, treating the associated anemia clearly improves the heart’s pump function [47].
The presence of iron deficiency had a negative prognostic impact only in HFrEF but not in HFpEF. Persistent iron deficiency was strongly associated with mortality after a 6 month follow-up [48]. Several intravenous (IV) iron products have been used in HF research; however, ESC guidelines currently recommend only iron carboxymaltose [49][50]. This recommendation may change following the recent publication of the IRONMAN study, which showed benefits in HFrEF patients using ferric maltose [51].
References
- World Health Organization. Cardiovascular Diseases. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 20 April 2023).
- Back, M.; von Haehling, S.; Papp, Z.; Piepoli, M.F. A year in heart failure: Updates of clinical and preclinical findings. ESC Heart Fail 2023.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Rahman, R.; Paz, P.; Elmassry, M.; Mantilla, B.; Dobbe, L.; Shurmur, S.; Nugent, K. Diuretic Resistance in Heart Failure. Cardiol. Rev. 2021, 29, 73–81.
- Casu, G.; Merella, P. Diuretic Therapy in Heart Failure—Current Approaches. Eur. Cardiol. 2015, 10, 42–47.
- Palmer, L.G.; Schnermann, J. Integrated control of Na transport along the nephron. Clin. J. Am. Soc. Nephrol. 2015, 10, 676–687.
- Oppermann, M.; Hansen, P.B.; Castrop, H.; Schnermann, J. Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. Am. J. Physiol.-Ren. Physiol. 2007, 293, F279–F287.
- Francis, G.S.; Siegel, R.M.; Goldsmith, S.R.; Olivari, M.T.; Levine, T.B.; Cohn, J.N. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann. Intern. Med. 1985, 103, 1–6.
- Kaissling, B.; Stanton, B.A. Adaptation of distal tubule and collecting duct to increased sodium delivery. I. Ultrastructure. Am. J. Physiol. 1988, 255, F1256–F1268.
- Ljungman, S.; Laragh, J.H.; Cody, R.J. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs 1990, 39 (Suppl. 4), 10–21; discussion 22–14.
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbrain, M.; Tang, W.H.; Mullens, W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 2013, 62, 485–495.
- Licata, G.; Di Pasquale, P.; Parrinello, G.; Cardinale, A.; Scandurra, A.; Follone, G.; Argano, C.; Tuttolomondo, A.; Paterna, S. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: Long-term effects. Am. Heart J. 2003, 145, 459–466.
- Paterna, S.; Gaspare, P.; Fasullo, S.; Sarullo, F.M.; Di Pasquale, P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: Is sodium an old enemy or a new friend? Clin. Sci. 2008, 114, 221–230.
- Ichikawa, I.; Pfeffer, J.M.; Pfeffer, M.A.; Hostetter, T.H.; Brenner, B.M. Role of angiotensin II in the altered renal function of congestive heart failure. Circ. Res. 1984, 55, 669–675.
- Haase, M.; Muller, C.; Damman, K.; Murray, P.T.; Kellum, J.A.; Ronco, C.; McCullough, P.A. Pathogenesis of cardiorenal syndrome type 1 in acute decompensated heart failure: Workgroup statements from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib. Nephrol. 2013, 182, 99–116.
- Kopitko, C.; Medve, L.; Gondos, T.; Soliman, K.M.M.; Fulop, T. Mediators of Regional Kidney Perfusion during Surgical Pneumo-Peritoneum Creation and the Risk of Acute Kidney Injury-A Review of Basic Physiology. J. Clin. Med. 2022, 11, 2728.
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutierrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408.
- Ganda, A.; Onat, D.; Demmer, R.T.; Wan, E.; Vittorio, T.J.; Sabbah, H.N.; Colombo, P.C. Venous congestion and endothelial cell activation in acute decompensated heart failure. Curr. Heart Fail. Rep. 2010, 7, 66–74.
- Nguyen, V.Q.; Gadiraju, T.V.; Patel, H.; Park, M.; Le Jemtel, T.H.; Jaiswal, A. Intra-abdominal Hypertension: An Important Consideration for Diuretic Resistance in Acute Decompensated Heart Failure. Clin. Cardiol. 2016, 39, 37–40.
- Kopitko, C.; Gondos, T.; Fulop, T.; Medve, L. Reinterpreting Renal Hemodynamics: The Importance of Venous Congestion and Effective Organ Perfusion in Acute Kidney Injury. Am. J. Med. Sci. 2020, 359, 193–205.
- Wojtaszek, E.; Grzejszczak, A.; Niemczyk, S.; Malyszko, J.; Matuszkiewicz-Rowinska, J. Peritoneal Ultrafiltration in the Long-Term Treatment of Chronic Heart Failure Refractory to Pharmacological Therapy. Front. Physiol. 2019, 10, 310.
- Stegmayr, B.G.; Banga, R.; Lundberg, L.; Wikdahl, A.M.; Plum-Wirell, M. PD treatment for severe congestive heart failure. Perit. Dial. Int. 1996, 16 (Suppl. 1), S231–S235.
- Bertoli, S.V.; Musetti, C.; Ciurlino, D.; Basile, C.; Galli, E.; Gambaro, G.; Iadarola, G.; Guastoni, C.; Carlini, A.; Fasciolo, F.; et al. Peritoneal ultrafiltration in refractory heart failure: A cohort study. Perit. Dial. Int. 2014, 34, 64–70.
- Husain-Syed, F.; Grone, H.J.; Assmus, B.; Bauer, P.; Gall, H.; Seeger, W.; Ghofrani, A.; Ronco, C.; Birk, H.W. Congestive nephropathy: A neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail. 2021, 8, 183–203.
- Courivaud, C.; Kazory, A.; Crepin, T.; Azar, R.; Bresson-Vautrin, C.; Chalopin, J.M.; Ducloux, D. Peritoneal dialysis reduces the number of hospitalization days in heart failure patients refractory to diuretics. Perit. Dial. Int. 2014, 34, 100–108.
- Scurt, F.G.; Kuczera, T.; Mertens, P.R.; Chatzikyrkou, C. The Cardiorenal Syndrome. Dtsch. Med. Wochenschr. 2019, 144, 910–916.
- Sugiura, E.; Dohi, K.; Tanimura, M.; Kumagai, N.; Ishikawa, E.; Ito, M. Successful Peritoneal Dialysis for the Treatment of Inotrope-Dependent End-Stage Heart Failure. Int. Heart J. 2019, 60, 1211–1218.
- Sahlie, A.; Jaar, B.G.; Paez, L.G.; Masud, T.; Lea, J.P.; Burkart, J.M.; Plantinga, L.C. Burden and Correlates of Hospital Readmissions among U.S. Peritoneal Dialysis Patients. Perit. Dial. Int. 2019, 39, 261–267.
- Chionh, C.Y.; Clementi, A.; Poh, C.B.; Finkelstein, F.O.; Cruz, D.N. The use of peritoneal dialysis in heart failure: A systematic review. Perit. Dial. Int. 2020, 40, 527–539.
- Schalk, E.; Tolle, D.; Schulz, S.; Teschner, D.; Hentrich, M.; Fischer, T. Risk of central venous catheter (CVC)-related bloodstream infections (CRBSI) in cancer patients (pts) with or without neutropenia at time of CVC insertion. Oncol. Res. Treat. 2018, 41, 145.
- Hickson, L.J.; Gooden, J.Y.; Le, K.Y.; Baddour, L.M.; Friedman, P.A.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Sohail, M.R. Clinical presentation and outcomes of cardiovascular implantable electronic device infections in hemodialysis patients. Am. J. Kidney Dis. 2014, 64, 104–110.
- Carrillo, R.G.; Garisto, J.D.; Salman, L.; Merrill, D.; Asif, A. Contamination of transvenous pacemaker leads due to tunneled hemodialysis catheter infection: A report of 2 cases. Am. J. Kidney Dis. 2010, 55, 1097–1101.
- Thomas, B.A.; Logar, C.M.; Anderson, A.E. Renal replacement therapy in congestive heart failure requiring left ventricular assist device augmentation. Perit. Dial. Int. 2012, 32, 386–392.
- Dukka, H.; Kalra, P.A.; Wilkie, M.; Bhandari, S.; Davies, S.J.; Barratt, J.; Squire, I.; Odudu, A.; Selby, N.M.; McIntyre, C.; et al. Peritoneal Ultrafiltration for Heart Failure: Lessons from a Randomized Controlled Trial. Perit. Dial. Int. 2019, 39, 486–489.
- Gotloib, L.; Fudin, R.; Yakubovich, M.; Vienken, J. Peritoneal dialysis in refractory end-stage congestive heart failure: A challenge facing a no-win situation. Nephrol. Dial. Transplant. 2005, 20 (Suppl. 7), vii32–vii36.
- Wojtaszek, E.; Malyszko, J.; Matuszkiewicz-Rowinska, J. Peritoneal ultrafiltration in end-stage congestive heart failure. Cardiol. J. 2014, 21, 115–120.
- Akula, Y.V.; Fulop, T.; Dixit, M.P. Peritoneal Dialysis in Class 2-3 Obesity-A Single-Center Experience. Am. J. Med. Sci. 2017, 353, 70–75.
- Crabtree, J.H.; Shrestha, B.M.; Chow, K.M.; Figueiredo, A.E.; Povlsen, J.V.; Wilkie, M.; Abdel-Aal, A.; Cullis, B.; Goh, B.L.; Briggs, V.R.; et al. Creating and Maintaining Optimal Peritoneal Dialysis Access in the Adult Patient: 2019 Update. Perit. Dial. Int. 2019, 39, 414–436.
- Yeter, H.H.; Izgi, A.; Yildirim, S.; Akcay, O.F.; Derici, U. Outcomes of early-start peritoneal dialysis (PD) and the comparison with urgent-start hemodialysis and conventional-start PD. Ther. Apher. Dial. 2023, 27, 480–487.
- Cheng, S.; Yang, L.; Sun, Z.; Zhang, X.; Zhu, X.; Meng, L.; Guo, S.; Zhuang, X.; Luo, P.; Cui, W. Safety of a 24-h-or-less break-in period in elderly patients undergoing urgent-start peritoneal dialysis: A multicenter retrospective cohort study. Ther. Apher. Dial. 2023, 27, 304–313.
- Karpinski, S.; Sibbel, S.; Cohen, D.E.; Colson, C.; Van Wyck, D.B.; Ghaffari, A.; Schreiber, M.J.; Brunelli, S.M.; Tentori, F. Urgent-start peritoneal dialysis: Association with outcomes. Perit. Dial. Int. 2023, 43, 186–189.
- Szabó, R.P.; Pethő, Á.; Fedor, R.; Kertész, A.; Bódi, A.; Szegedi, A.; Balla, J. A diuretikum-refrakter szívelégtelen betegek körében alkalmazott peritonealis dialízissel szerzett tapasztalataink. Cardiol. Hung. 2018, 48, 179–183.
- Ponce, D.; Banin, V.B.; Bueloni, T.N.; Barretti, P.; Caramori, J.; Balbi, A.L. Different outcomes of peritoneal catheter percutaneous placement by nephrologists using a trocar versus the Seldinger technique: The experience of two Brazilian centers. Int. Urol. Nephrol. 2014, 46, 2029–2034.
- Boujelbane, L.; Fu, N.; Chapla, K.; Melnick, D.; Redfield, R.R.; Waheed, S.; Yevzlin, A.S.; Shin, J.I.; Astor, B.C.; Chan, M.R. Percutaneous versus surgical insertion of PD catheters in dialysis patients: A meta-analysis. J. Vasc. Access 2015, 16, 498–505.
- Medani, S.; Hussein, W.; Shantier, M.; Flynn, R.; Wall, C.; Mellotte, G. Comparison of Percutaneous and Open Surgical Techniques for First-Time Peritoneal Dialysis Catheter Placement in the Unbreached Peritoneum. Perit. Dial. Int. 2015, 35, 576–585.
- Tominaga, M.; Kawai, M.; Minai, K.; Ogawa, K.; Inoue, Y.; Morimoto, S.; Tanaka, T.; Nagoshi, T.; Ogawa, T.; Yoshimura, M. Association between plasma B-type natriuretic peptide and anaemia in heart failure with or without ischaemic heart disease: A retrospective study. BMJ Open 2019, 9, e024194.
- Sharma, R.; Francis, D.P.; Pitt, B.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Haemoglobin predicts survival in patients with chronic heart failure: A substudy of the ELITE II trial. Eur. Heart J. 2004, 25, 1021–1028.
- Loncar, G.; Obradovic, D.; Thiele, H.; von Haehling, S.; Lainscak, M. Iron deficiency in heart failure. ESC Heart Fail. 2021, 8, 2368–2379.
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Luscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448.
- Sindone, A.; Doehner, W.; Comin-Colet, J. Systematic review and meta-analysis of intravenous iron-carbohydrate complexes in HFrEF patients with iron deficiency. ESC Heart Fail. 2023, 10, 44–56.
- Kalra, P.R.; Cleland, J.G.F.; Petrie, M.C.; Thomson, E.A.; Kalra, P.A.; Squire, I.B.; Ahmed, F.Z.; Al-Mohammad, A.; Cowburn, P.J.; Foley, P.W.X.; et al. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): An investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. Lancet 2022, 400, 2199–2209.
More
Information
Subjects:
Urology & Nephrology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
561
Revisions:
2 times
(View History)
Update Date:
15 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




