You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Flavius-Alexandru Gherasie | -- | 2732 | 2023-06-13 08:31:41 | | | |
| 2 | Rita Xu | Meta information modification | 2732 | 2023-06-13 08:43:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gherasie, F.; Popescu, M.; Bartos, D. Pathophysiology and Mortality with Peripheral Artery Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/45479 (accessed on 25 December 2025).
Gherasie F, Popescu M, Bartos D. Pathophysiology and Mortality with Peripheral Artery Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/45479. Accessed December 25, 2025.
Gherasie, Flavius-Alexandru, Mihaela-Roxana Popescu, Daniela Bartos. "Pathophysiology and Mortality with Peripheral Artery Disease" Encyclopedia, https://encyclopedia.pub/entry/45479 (accessed December 25, 2025).
Gherasie, F., Popescu, M., & Bartos, D. (2023, June 13). Pathophysiology and Mortality with Peripheral Artery Disease. In Encyclopedia. https://encyclopedia.pub/entry/45479
Gherasie, Flavius-Alexandru, et al. "Pathophysiology and Mortality with Peripheral Artery Disease." Encyclopedia. Web. 13 June, 2023.
Copy Citation
There are a number of devastating complications associated with peripheral artery disease, including limb amputations and acute limb ischemia. In coronary atherosclerosis, thrombosis is often precipitated by rupture or erosion of fibrous caps around atheromatous plaques, which leads to acute coronary syndrome.
peripheral artery disease
chronic limb-threatening ischemia
acute limb ischemia
1. Introduction
More than 200 million adults suffer from peripheral artery disease in their lower extremities, which increases their risk of cardiovascular events (such as coronary heart disease, strokes, and leg amputations). Globally and in the United States, peripheral artery disease has gone underdiagnosed and undertreated due to a lack of awareness [1]. Generally speaking, lower-extremity peripheral artery disease refers to atherosclerotic diseases of the arteries supplying the limbs, from the aortoiliac segments to the pedal arteries. Even though this disease is associated with adverse clinical outcomes, impaired physical function, and reduced physical activity, it has been understudied and underrecognized compared with other atherosclerotic diseases such as myocardial infarction. In recent years, there has been mounting evidence that peripheral artery disease is significantly linked to mortality, primarily as a risk factor for future myocardial infarctions and strokes. Also, peripheral artery disease can cause devastating complications that result in limb amputations and acute limb ischemia. Despite the overlap, the causes of atherosclerotic diseases are not the same and the need for the appropriate diagnosis and treatment remains a major concern for medicine. Clinically, peripheral artery disease and coronary artery disease overlap due to their shared risk factors. In these patients, hyperlipidemia and type II diabetes mellitus were significant comorbidities. In addition to accelerating atherosclerosis development, diabetes mellitus affects the characteristic of atherosclerotic lesions in the lower extremities [2][3]. Compared with patients with PAD (peripheral artery disease) and without diabetes mellitus, patients who have PAD and coexisting diabetes mellitus have more arteries below the knee that are affected and more multilevel lesions [4][5][6]. Although patients with diabetes mellitus benefit from medical and endovascular procedures to manage atherosclerotic cardiovascular diseases, restenosis is a significant factor that may worsen the prognosis and may be associated with the necessity of reintervention [7]. The presence of dyslipidemia is associated with both changes in basic lipid parameters and modified lipoproteins, which are not routinely measured in clinical practice. Nitrated lipoproteins are oxidatively modified lipoproteins discussed in the context of cardiovascular dysfunction development. However, their role remains unclear at this time [8]. Many studies have found that coronary artery disease and peripheral artery disease are frequently coexisting conditions. Saleh et al. suggested that there was a significant increase in peripheral artery disease prevalence among those with coronary artery disease compared with those with normal coronaries [9]. Despite being under the care of a cardiovascular specialist, studies have shown that one out of six patients with coronary artery disease had an unrecognized peripheral artery disease [10].
The ankle-brachial index’s high positive predictive value and the high prevalence of peripheral artery disease with coronary artery disease suggest a high degree of suspicion for, and pretest probability for, coronary artery disease in a patient with peripheral artery disease. Coronary artery disease diagnosis is highly dependent on cardiac imaging tests such as electrocardiograms, stress electrocardiograms, myocardial perfusion imaging, and coronary angiographies. Kumar et al. say pretest probabilities can be increased through the ankle-brachial index, but it cannot replace the above testing methods [11].
Many factors contribute to this lack of knowledge about peripheral artery disease. Various nomenclatures and definitions have been used to describe peripheral artery disease, making effective communication challenging [12]. It is also likely that the clinical presentation of peripheral artery disease contributes to confusion regarding peripheral artery disease. A small percentage of patients are diagnosed with intermittent claudication, with either no exertional leg symptoms (up to 50%) or atypical leg symptoms (almost 50%) [13]. Patients may experience leg pain that begins at rest, or that does not interfere with walking, or that resolves with rest. These symptoms can be confused with arthritis or degenerative spinal disease. Various adverse outcomes can occur as a result of peripheral artery disease. To improve overall outcomes among this growing and undertreated population, increasing awareness about the definition, diagnosis, clinical manifestations, and complications of peripheral artery disease is critical.
Coronary atherosclerosis often manifests as an acute coronary syndrome, where thrombosis is precipitated by rupture or erosion of the fibrous caps of atheromatous plaques. It is common for plaque ruptures to have large necrotic cores, as well as thin, inflamed fibrous caps. Regardless of the extent of atherosclerosis, peripheral artery disease manifests as thrombosis. In peripheral arteries with significant stenosis, approximately 75% of them are blocked by thrombi. Two-thirds of them also have thrombi associated with insignificant atherosclerosis. A local thrombogenic or remotely embolic basis of critical limb ischemia may be explained by obliterative thrombi in peripheral arteries of patients without coronary artery-like lesions [14].
2. Anatomy of Lower Extremity Arteries: Normal Aspects
To maintain the body’s mobility, lower extremity arteries supply oxygenated blood to muscles, tendons, and nerves. Several diseases can affect these arteries, which inhibit the optimal function of the limb. Lower-extremity arteries originate from the iliac artery at the point where the abdominal aorta is divided into the two common iliac arteries and the median sacral artery, approximately at the fourth lumbar vertebral body [15][16].
There are two branches of the common iliac artery, the internal and the external. A portion of the external iliac artery runs down into the lower limbs and becomes the common femoral artery [17]. Among the small branches of the common femoral artery, there are the superficial epigastric artery, external pudendal artery, and superficial circumflex artery. The common femoral artery divides into the superficial and deep femoral arteries.
Through Hunter’s canal, the superficial femoral artery travels along the medial side of the thigh. Once it exits the adductor canal, the superficial femoral artery courses posteriorly, where it is called the popliteal artery after it passes through the adductor hiatus. Approximately at the level of the proximal tibiofibular joint, the popliteal artery divides into the anterior tibial artery and the tibioperoneal trunk [18]. The anterior tibial artery runs along the anterior surface of the interosseus membrane, and there is a continuation of this artery in the foot as the dorsalis pedis artery. Located along the posteromedial aspect of the leg, the tibioperoneal trunk further divides into the peroneal artery and posterior tibial artery [15]. There are two arches on the medial and lateral surfaces of the plantar surface, formed by the posterior tibial artery. Besides giving rise to the metatarsals, the plantar arch is also responsible for producing the plantar digital arteries. Above the ankle joint, the peroneal artery divides into two calcaneal branches, one on the medial side and one on the lateral side. As a result of free communication with the dorsalis pedis artery and posterior tibial artery, these branches assist in the collateralization of the foot when it is sick or injured [19].
3. Atherosclerosis
Atherosclerosis is a chronic inflammatory disease with complex etiopathogenesis, which results in the development of atherosclerotic plaques, leading to the formation of narrowing and/or occlusion of the arteries [20][21]. This clinically may lead to the development of ischemic heart disease, cerebrovascular disease, or peripheral arterial disease [22][23][24][25]. In clinical practice, lowering the level of LDL cholesterol (low-density lipoprotein cholesterol) is the primary therapeutic objective of lipid-lowering therapy due to the role that oxidatively modified LDL particles play in atherosclerosis [26][27][28]. Additionally, researchers observe patients with advanced atherosclerosis and normal LDL concentrations in clinical practice, and such patients are also eligible for statin therapy. There are many pleiotropic effects associated with statins, which are mainly used for lowering cholesterol, and thus they are not only lipid-lowering drugs [27]. To achieve a target level for LDL cholesterol, statins and PCSK9 inhibitors (proprotein convertase subtilisin/kexin type 9) are effective [29][30][31]. There is no doubt that both of them are effective in controlling LDL cholesterol and reducing major adverse cardiovascular events by about 50% [32]. After controlling LDL cholesterol, the remaining risk for major adverse cardiovascular events is believed to be due to inflammation. As part of the CANTOS trial, it was shown that canakinumab, an antibody that blocks IL-1β (interleukin-1β), reduces major adverse cardiovascular events [33][34][35][35][36][37].
As a result of atherosclerosis, there is an autoimmune response to LDL and other antigens which could lead to an escalation or amelioration of the disease’s progression. There have been some recent advances in immunotherapy and vaccination that have shown promise in curbing atherosclerosis in animal models [38]. Based on the theory of the modulation of atherogenesis by adaptive immune responses, especially the CD4+ T cells that recognize self-antigens, a novel type of therapy that targets the adaptive immune response has been developed to address this phenomenon [39].
3.1. Pathogenesis of Atherosclerosis
Early-stage atherosclerosis is characterized by cell formation which is the result of lipid accumulation in the cells [40][41][42]. Inflammatory cytokines are released by damaged endothelial cells, which recruit monocytes through the endothelium and then differentiate into macrophages. Differentiated macrophages consume oxidized low-density lipoprotein (ox-LDL) through the scavenger receptors LOX-1, CD36, and SR-A1, which hydrolyze cholesteryl esters within lysosomes. By contrast, free cholesterol is esterified by acyl-CoA cholesterol acyltransferase 1 (ACAT1), while cholesteryl ester is hydrolyzed by neutral cholesteryl ester hydrolase (nCEH) and cholesteryl ester hydrolase (CEH) [41][43]. A large amount of free cholesterol is toxic to cells, so it must be efficiently removed from cells by ABCA1 and ABCG1 transporters [44][45]. When the above cholesterol homeostasis is disturbed, excessive cholesterol ester or free cholesterol will accumulate, causing foam cell formation or cell necrosis.
3.2. Immune Response in Atherosclerosis
Atherosclerotic plaques can become unstable, rupture, or erode over time, causing major cardiovascular complications [45][46][47][48][49]. There is a direct correlation between plaque stability and the level of inflammatory cells as well as the thickness of the cap of the plaque. Plaques that have thin caps and are full of immune cells are referred to as soft or vulnerable plaques. To initiate immune cell infiltration, chemokines and adhesion molecules play a major role. An antigen-presenting cell is a cell that generates major histocompatibility complex molecules, costimulatory molecules, and cytokines upon the presence of antigens that are produced by pathogens, bacteria, or altered self to determine the polarization of the adaptive immune response.
In the arterial adventitia and neointima, there are macrophages and dendritic cells that are activated by TLR ligands (Toll-like receptors) and scavenger receptors [50]. As inflammation cytokines increase in amount and intensity, atherosclerosis gets worse, and more immune cells are attracted to the area. The inflammatory cytokine IL-1β is an effective target for the treatment of atherosclerosis and other vascular diseases [48].
Marchini et al. suggested that at the maturation stage of atherosclerotic plaque, atherosclerosis-antigen-specific T cells release cytokines, perpetuate inflammation, and help an immune cell infiltrate develop over time [51] (Figure 1).
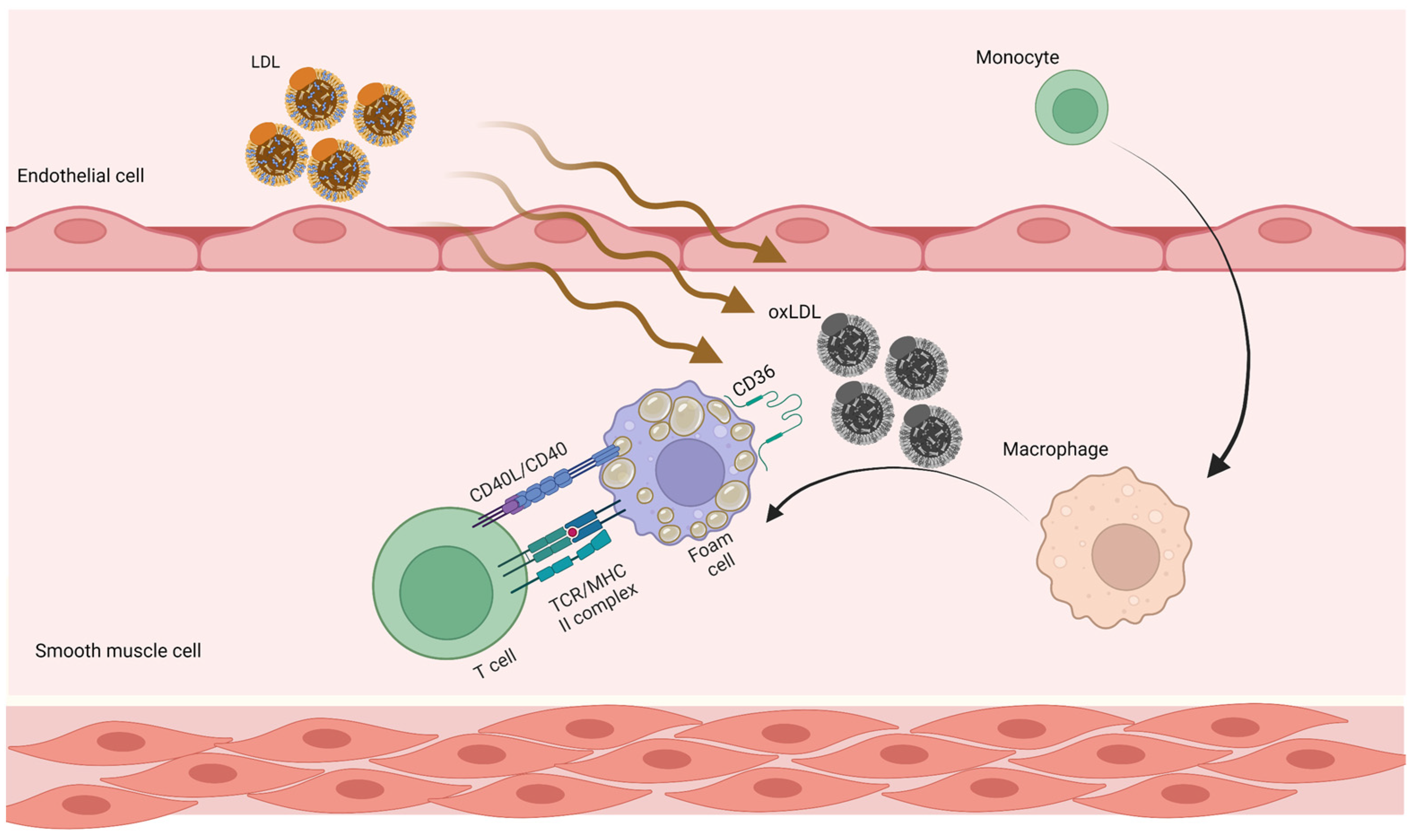
Figure 1. Adaptive immune responses in atherosclerosis. LDL penetrates the artery wall and experiences modification by oxidative and enzymatic processes. The modified LDL molecules promote the expression of leukocyte adhesion molecules. Monocytes invade the vascular wall and mature into macrophages, differentiating into foam cells after taking up large volumes of oxidized LDL. T cells become active throughout this process and release mediators, which subsequently increase the immune reaction and lead to atherogenesis. LDL, low-density lipoprotein; oxLDL, oxidized low-density lipoprotein.
There is always an autoimmune response associated with atherosclerosis. There are antibodies to oxidized (oxLDL) LDL that are produced by plasma cells derived from the B cell, and these antibodies can be detected in the serum of humans and animals suffering from atherosclerosis. The presence of T cells against antigens associated with atherosclerosis is also found. For some time now, regulating CD4+ T cells (Tregs) have been shown to protect mice from atherosclerosis in animal models [52]. The recent studies showed that CD4+ T cells specific for the core lipoprotein ApoB (apolipoprotein B), which is involved in LDL, very low-density lipoproteins, and chylomicron, are mostly Tregs in people without cardiovascular disease, but assume mixed and effector phenotypes in those with cardiovascular disease [39][53]. There is some evidence that atheroprotection may involve other T- and B-cell subsets, and this is an area of active research.
3.3. Atherosclerosis and Autoreactive CD4+ T cells
A majority of CD4+ T cells present in atherosclerotic lesions are memory T cells (CD45RO+) and are responsible for producing inflammatory cytokines in response to oxLDL. Several immunogenic epitopes have been identified and used as vaccine antigens against atherosclerosis in animal models derived from mouse ApoB (Apolipoprotein B).
Until recently, there has not been any direct proof that CD4+ T cells specific for ApoB epitopes exist in the body. To answer this question, scientists developed MHC-II tetramers for detecting such cells in mice as well as humans. ApoB epitope P18 is identical in mice (ApoB) and humans (APOB). P18 binds the mouse MHC-II allele I-Ab and DRB1*07:01 (presented in about 8% of humans). To detect APOB-specific CD4+ T cells, scientists created human APOB-peptide P18:DRB1*07:01 tetramers and found that P18-recognizing CD4+ T cells exist in human peripheral blood mononuclear cells. The presence of these cells was found in subjects both with and without subclinical cardiovascular disease [39]. The results of this study are the first in a series of studies to demonstrate that there are self-peptide-recognizing CD4+ T cells within human peripheral blood mononuclear cells. During the progression of atherosclerosis, the phenotypes of these cells seem to change [53][54][55][56][57][58].
3.4. Future Atherosclerosis Prevention
There is no doubt that vaccination is one of the most successful interventions in medicine. In recent years, vaccine development has moved from vaccine development for infectious diseases to vaccine development for non-communicable diseases, such as cancer, atherosclerosis, hypertension, Alzheimer’s disease, and diabetes mellitus. Identifying vaccine antigens is the first step in developing an atherosclerosis vaccine. PCSK9 (proprotein convertase subtilisin/kexin type 9), HSP65, and ApoB are some of the possible antigens that may be used as atherosclerosis vaccine antigens [59]. There are already antibodies against PCSK9 that are being used in clinical trials. As far as targeting PCSK9 is concerned, it is known to be safe because humans with null mutations in PCSK9 are asymptomatic except for the fact that they are resistant to atherosclerosis [60].
4. Mortality and Cardiovascular Outcomes
When it comes to mortality in patients with peripheral artery disease, The Ankle to Brachial Cohort Study found a strong association between low (0.90) and high (>1.40) ankle-to-brachial results and all-cause and cardiovascular mortality [61]. The mortality rate was doubled in those with an ankle-to-brachial index between 0.81 and 0.90, while the mortality rate was quadrupled in those with an ankle-to-brachial index between 0.70 and 0.80. There has been evidence in multiple studies from a variety of populations demonstrating that persons with peripheral artery disease are more likely to develop other cardiovascular diseases as well, such as coronary heart disease, strokes, and aneurysms of the abdominal aorta [62][63].
Recent research has shown that peripheral artery disease is becoming increasingly important in the context of polyvascular disease. An example of this is a subset of patients with multiple vascular beds affected by atherosclerosis, including peripheral arterial disease. According to the FOURIER trial, peripheral artery disease in combination with myocardial infarction or stroke had the highest risk of major adverse cardiovascular events (cardiovascular mortality, myocardial infarction, and stroke) over 2.5 years, with a risk of 14.9% [64][65][66][67][68]
There is an interesting finding in this study that peripheral artery disease without myocardial infarction/stroke was associated with a higher risk of major adverse cardiovascular events (10.3%) than myocardial infarction/stroke without peripheral artery disease (7.6%) [69][70][71][72][73].
Numerous studies have demonstrated a correlation between peripheral artery disease and increased mortality [64][74][75][76][77].
The mortality and adverse outcomes associated with acute coronary syndrome and peripheral artery disease are not only unfortunate consequences of the natural progression of these diseases but can also result from medical, endovascular, or surgical interventions [78]. While potentially beneficial, these interventions can have unintended consequences that may result in death or other adverse outcomes.
5. Conclusions
Overall mortality rates for acute coronary syndromes and acute limb ischemia have declined in most developed countries by 24–50%. It is estimated that approximately half of this reduction in cardiovascular mortality can be attributed to changes in therapy, including secondary preventive measures following myocardial infarction and revascularization. Initial treatments, advancements in heart failure treatments, and revascularization for chronic angina contributed to this drop. The other half of the effect was caused by changes in risk factors, including reductions in total cholesterol, systolic blood pressure, smoking, and physical inactivity. In addition, detecting peripheral arterial disease and developing novel treatments can improve the therapeutic options.
Slowing the progression of peripheral artery disease is key to decreasing cardiovascular mortality. For now, lifestyle changes, dietary changes, and conventional therapies may seem like a winning combination, but vaccines might be the medicine of the future.
References
- Neumann, F.J.; Sechtem, U.; Banning, A.P.; Bonaros, N.; Bueno, H.; Bugiardini, R.; Chieffo, A.; Crea, F.; Czerny, M.; Delgado, V.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary SyndromesThe Task Force for the Diagnosis and Management of Chronic Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477.
- Verner, V.A.; Mel’Nik, M.V.; Knjazeva, S.A. Cardio-Ankle Vascular Index (CAVI) in Diagnostics, Risk and Severity Evaluation of Magistral Vessels Lesion in Patients with Cardio-Vascular Diseases and Type 2 Diabetes. Ter. Arkhiv 2021, 93, 87–93.
- Thiruvoipati, T.; Kielhorn, C.E.; Armstrong, E.J. Peripheral Artery Disease in Patients with Diabetes: Epidemiology, Mechanisms, and Outcomes. World J. Diabetes 2015, 6, 961–969.
- Foussard, N.; Dari, L.; Ducasse, E.; Rigalleau, V.; Mohammedi, K.; Caradu, C. Lower-Limb Peripheral Arterial Disease and Amputations in People with Diabetes: Risk Factors, Prognostic Value and Management. Presse Med. 2023, 52, 104164.
- Chun, D.I.; Kim, S.; Kim, J.; Yang, H.J.; Kim, J.H.; Cho, J.H.; Yi, Y.; Kim, W.J.; Won, S.H. Epidemiology and Burden of Diabetic Foot Ulcer and Peripheral Arterial Disease in Korea. J. Clin. Med. 2019, 8, 748.
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic Lower Extremity Ischemia and Its Association with the Frailty Syndrome in Patients with Diabetes. Int. J. Environ. Res. Public Health 2020, 17, 9339.
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970.
- Aday, A.W.; Everett, B.M. Dyslipidemia Profiles in Patients with Peripheral Artery Disease. Curr. Cardiol. Rep. 2019, 21, 42.
- Saleh, A.; Makhamreh, H.; Qoussoos, T.; Alawwa, I.; Alsmady, M.; Salah, Z.A.; Shakhatreh, A.; Alhazaymeh, L.; Jabber, M. Prevalence of Previously Unrecognized Peripheral Arterial Disease in Patients Undergoing Coronary Angiography. Medicine 2018, 97, 11519.
- Moussa, I.D.; Jaff, M.R.; Mehran, R.; Gray, W.; Dangas, G.; Lazic, Z.; Moses, J.W. Prevalence and Prediction of Previously Unrecognized Peripheral Arterial Disease in Patients with Coronary Artery Disease: The Peripheral Arterial Disease in Interventional Patients Study. Catheter. Cardiovasc. Interv. 2009, 73, 719–724.
- Kumar, A.; Bano, S.; Bhurgri, U.; Kumar, J.; Ali, A.; Dembra, S.; Kumar, L.; Shahid, S.; Khalid, D.; Rizwan, A.; et al. Peripheral Artery Disease as a Predictor of Coronary Artery Disease in Patients Undergoing Coronary Angiography. Cureus 2021, 13, 15094.
- Aursulesei Onofrei, V.; Ceasovschih, A.; Marcu, D.T.M.; Adam, C.A.; Mitu, O.; Mitu, F. Mortality Risk Assessment in Peripheral Arterial Disease—The Burden of Cardiovascular Risk Factors over the Years: A Single Center’s Experience. Diagnostics 2022, 12, 2499.
- Hirsch, A.T.; Criqui, M.H.; Treat-Jacobson, D.; Regensteiner, J.G.; Creager, M.A.; Olin, J.W.; Krook, S.H.; Hunninghake, D.B.; Comerota, A.J.; Walsh, M.E.; et al. Peripheral Arterial Disease Detection, Awareness, and Treatment in Primary Care. J. Am. Med. Assoc. 2001, 286, 1317–1324.
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.C.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic): Executive Summary a Collaborative Report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines f. J. Am. Coll. Cardiol. 2006, 47, 1239–1312.
- Qazi, E.; Wilting, J.; Patel, N.R.; Alenezi, A.O.; Kennedy, S.A.; Tan, K.T.; Jaberi, A.; Mafeld, S. Arteries of the Lower Limb-Embryology, Variations, and Clinical Significance. Can. Assoc. Radiol. J. 2022, 73, 259–270.
- Chen, Q.; Shi, Y.; Wang, Y.; Li, X. Patterns of Disease Distribution of Lower Extremity Peripheral Arterial Disease. Angiology 2015, 66, 211–218.
- Xie, D.; Na, J.; Zhang, M.; Dong, S.; Xiao, X. CT Angiography of the Lower Extremity and Coronary Arteries Using 256-Section CT: A Preliminary Study. Clin. Radiol. 2015, 70, 1281–1288.
- Kropman, R.H.J.; Kiela, G.; Moll, F.L.; De Vries, J.P.P.M. Variations in Anatomy of the Popliteal Artery and Its Side Branches. Vasc. Endovasc. Surg. 2011, 45, 536–540.
- Shah, S.; Fischman, A.; Marin, M.; Won, J. Spontaneous Tibioperoneal Trunk and Anterior Tibial Artery Pseudoaneurysms. Vasc. Med. 2012, 17, 164–167.
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Atherosclerosis and Inflammation. New Therapeutic Approaches. Med. Clin. 2020, 155, 256–262.
- Zárate, A.; Manuel-Apolinar, L.; Basurto, L.; De la Chesnaye, E.; Saldívar, I. Cholesterol and Atherosclerosis. Historical Considerations and Treatment. Arch. Cardiol. Mex. 2016, 86, 163–169.
- Sarmah, D.; Datta, A.; Raut, S.; Sarkar, A.; Shah, B.; Bohra, M.; Singh, U.; Jagtap, P.; Baidya, F.; Kalia, K.; et al. The Role of Inflammasomes in Atherosclerosis and Stroke Pathogenesis. Curr. Pharm. Des. 2020, 26, 4234–4245.
- Shi, Y.; Guo, L.; Chen, Y.; Xie, Q.; Yan, Z.; Liu, Y.; Kang, J.; Li, S. Risk Factors for Ischemic Stroke: Differences between Cerebral Small Vessel and Large Artery Atherosclerosis Aetiologies. Folia Neuropathol. 2021, 59, 378–385.
- Razavi, A.C.; Agatston, A.S.; Shaw, L.J.; De Cecco, C.N.; van Assen, M.; Sperling, L.S.; Bittencourt, M.S.; Daubert, M.A.; Nasir, K.; Blumenthal, R.S.; et al. Evolving Role of Calcium Density in Coronary Artery Calcium Scoring and Atherosclerotic Cardiovascular Disease Risk. Cardiovasc. Imaging 2022, 15, 1648–1662.
- Fioranelli, M.; Bottaccioli, A.G.; Bottaccioli, F.; Bianchi, M.; Rovesti, M.; Roccia, M.G. Stress and Inflammation in Coronary Artery Disease: A Review Psychoneuroendocrineimmunology-Based. Front. Immunol. 2018, 9, 2031.
- Weitgasser, R.; Ratzinger, M.; Hemetsberger, M.; Siostrzonek, P. LDL-Cholesterol and Cardiovascular Events: The Lower the Better? Wien. Med. Wochenschr. 2018, 168, 108–120.
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243.
- Kroon, A.A.; Stalenhoef, A.F.H. LDL-Cholesterol Lowering and Atherosclerosis—Clinical Benefit and Possible Mechanisms: An Update. Neth. J. Med. 1997, 51, 16–27.
- Gencer, B.; Marston, N.A.; Im, K.A.; Cannon, C.P.; Sever, P.; Keech, A.; Braunwald, E.; Giugliano, R.P.; Sabatine, M.S. Efficacy and Safety of Lowering LDL Cholesterol in Older Patients: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Lancet 2020, 396, 1637–1643.
- Stoekenbroek, R.M.; Lambert, G.; Cariou, B.; Hovingh, G.K. Inhibiting PCSK9—Biology beyond LDL Control. Nat. Rev. Endocrinol. 2018, 15, 52–62.
- Rosenson, R.S.; Hegele, R.A.; Fazio, S.; Cannon, C.P. The Evolving Future of PCSK9 Inhibitors. J. Am. Coll. Cardiol. 2018, 72, 314–329.
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Reduction in C-Reactive Protein and LDL Cholesterol and Cardiovascular Event Rates after Initiation of Rosuvastatin: A Prospective Study of the JUPITER Trial. Lancet 2009, 373, 1175–1182.
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131.
- Ridker, P.M. Closing the Loop on Inflammation and Atherothrombosis: Why Perform the CIRT and CANTOS Trials? Trans. Am. Clin. Climatol. Assoc. 2013, 124, 174–190.
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of Interleukin-1beta Decreases the Severity of Atherosclerosis in ApoE-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660.
- Merhi-Soussi, F.; Kwak, B.R.; Magne, D.; Chadjichristos, C.; Berti, M.; Pelli, G.; James, R.W.; MacH, F.; Gabay, C. Interleukin-1 Plays a Major Role in Vascular Inflammation and Atherosclerosis in Male Apolipoprotein E-Knockout Mice. Cardiovasc. Res. 2005, 66, 583–593.
- Isoda, K.; Sawada, S.; Ishigami, N.; Matsuki, T.; Miyazaki, K.; Kusuhara, M.; Iwakura, Y.; Ohsuzu, F. Lack of Interleukin-1 Receptor Antagonist Modulates Plaque Composition in Apolipoprotein E-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1068–1073.
- Kimura, T.; Tse, K.; Sette, A.; Ley, K. Vaccination to Modulate Atherosclerosis. Autoimmunity 2015, 48, 152–160.
- Kimura, T.; Kobiyama, K.; Winkels, H.; Tse, K.; Miller, J.; Vassallo, M.; Wolf, D.; Ryden, C.; Orecchioni, M.; Dileepan, T.; et al. Regulatory CD4+ T Cells Recognize Major Histocompatibility Complex Class II Molecule–Restricted Peptide Epitopes of Apolipoprotein B. Circulation 2018, 138, 1130–1143.
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III-27–III-32.
- Sekiya, M.; Osuga, J.; Nagashima, S.; Ohshiro, T.; Igarashi, M.; Okazaki, H.; Takahashi, M.; Tazoe, F.; Wada, T.; Ohta, K.; et al. Ablation of Neutral Cholesterol Ester Hydrolase 1 Accelerates Atherosclerosis. Cell Metab. 2009, 10, 219–228.
- Sukhorukov, V.N.; Khotina, V.A.; Chegodaev, Y.S.; Ivanova, E.; Sobenin, I.A.; Orekhov, A.N. Lipid Metabolism in Macrophages: Focus on Atherosclerosis. Biomedicines 2020, 8, 262.
- Yu, X.; Fu, Y.; Zhang, D.; Yin, K.; Tang, C. Foam Cells in Atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252.
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Macrophage-mediated Cholesterol Handling in Atherosclerosis. J. Cell. Mol. Med. 2016, 20, 17–28.
- Cagnina, A.; Chabot, O.; Davin, L.; Lempereur, M.; Maréchal, P.; Oury, C.; Lancellotti, P. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 302–309.
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque Hemorrhage and Progression of Coronary Atheroma. N. Engl. J. Med. 2003, 349, 2316–2325.
- Ma, J.; Luo, J.; Sun, Y.; Zhao, Z. Cytokines Associated with Immune Response in Atherosclerosis. Am. J. Transl. Res. 2022, 14, 6424–6444.
- Tsioufis, P.; Theofilis, P.; Tsioufis, K.; Tousoulis, D. The Impact of Cytokines in Coronary Atherosclerotic Plaque: Current Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 15937.
- Creager, M.A.; Belkin, M.; Bluth, E.I.; Casey, D.E.; Chaturvedi, S.; Dake, M.D.; Fleg, J.L.; Hirsch, A.T.; Jaff, M.R.; Kern, J.A.; et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS Key Data Elements and Definitions for Peripheral Atherosclerotic Vascular Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Peripheral Atherosclerotic Vascular Disease). Circulation 2012, 125, 395–467.
- Lundberg, A.M.; Hansson, G.K. Innate Immune Signals in Atherosclerosis. Clin. Immunol. 2010, 134, 5–24.
- Marchini, T.; Mitre, L.S.; Wolf, D. Inflammatory Cell Recruitment in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 207.
- Ait-Oufella, H.; Salomon, B.L.; Potteaux, S.; Robertson, A.K.L.; Gourdy, P.; Zoll, J.; Merval, R.; Esposito, B.; Cohen, J.L.; Fisson, S.; et al. Natural Regulatory T Cells Control the Development of Atherosclerosis in Mice. Nat. Med. 2006, 12, 178–180.
- Saleheen, D.; Haycock, P.C.; Zhao, W.; Rasheed, A.; Taleb, A.; Imran, A.; Abbas, S.; Majeed, F.; Akhtar, S.; Qamar, N.; et al. Apolipoprotein(a) Isoform Size, Lipoprotein(a) Concentration, and Coronary Artery Disease: A Mendelian Randomisation Analysis. Lancet Diabetes Endocrinol. 2017, 5, 524–533.
- Sarwar, N.; Butterworth, A.S.; Freitag, D.F.; Gregson, J.; Willeit, P.; Gorman, D.N.; Gao, P.; Saleheen, D.; Rendon, A.; Nelson, C.P.; et al. Interleukin-6 Receptor Pathways in Coronary Heart Disease: A Collaborative Meta-Analysis of 82 Studies. Lancet 2012, 379, 1205–1213.
- Swerdlow, D.I.; Holmes, M.V.; Kuchenbaecker, K.B.; Engmann, J.E.L.; Shah, T.; Sofat, R.; Guo, Y.; Chung, C.; Peasey, A.; Pfister, R.; et al. The Interleukin-6 Receptor as a Target for Prevention of Coronary Heart Disease: A Mendelian Randomisation Analysis. Lancet 2012, 379, 1214–1224.
- Cai, T.; Zhang, Y.; Ho, Y.L.; Link, N.; Sun, J.; Huang, J.; Cai, T.A.; Damrauer, S.; Ahuja, Y.; Honerlaw, J.; et al. Association of Interleukin 6 Receptor Variant with Cardiovascular Disease Effects of Interleukin 6 Receptor Blocking Therapy: A Phenome-Wide Association Study. JAMA Cardiol. 2018, 3, 849–857.
- Kang, S.; Kishimoto, T. Interplay between Interleukin-6 Signaling and the Vascular Endothelium in Cytokine Storms. Exp. Mol. Med. 2021, 53, 1116–1123.
- Shuvalova, Y.A.; Kaminnaya, V.; Kaminnyi, A.I. Contribution of Interleukin-6 System Genes Polymorphisms to the Development of Coronary Atherosclerosis. Gene 2023, 861, 147253.
- Ortega-Rivera, O.A.; Shin, M.D.; Moreno-Gonzalez, M.A.; Pokorski, J.K.; Steinmetz, N.F. A Single-Dose Qβ VLP Vaccine against S100A9 Protein Reduces Atherosclerosis in a Preclinical Model. Adv. Ther. 2022, 5, 2200092.
- Ahamad, S.; Bhat, S.A. Recent Update on the Development of PCSK9 Inhibitors for Hypercholesterolemia Treatment. J. Med. Chem. 2022, 65, 15513–15539.
- Fowkes, G.; Fowkes, F.G.R.; Murray, G.D.; Butcher, I.; Heald, C.L.; Lee, R.J.; Chambless, L.E.; Folsom, A.R.; Hirsch, A.T.; Dramaix, M.; et al. Ankle Brachial Index Combined With Framingham Risk Score to Predict Cardiovascular Events and Mortality: A Meta-Analysis. JAMA 2008, 300, 197–208.
- Grenon, S.M.; Hiramoto, J.; Smolderen, K.G.; Vittinghoff, E.; Whooley, M.A.; Cohen, B.E. Association between Depression and Peripheral Artery Disease: Insights from the Heart and Soul Study. J. Am. Heart Assoc. 2012, 1, e002667.
- Wickström, J.E.; Laivuori, M.; Aro, E.; Sund, R.T.; Hautero, O.; Venermo, M.; Jalkanen, J.; Hakovirta, H. Toe Pressure and Toe Brachial Index Are Predictive of Cardiovascular Mortality, Overall Mortality, and Amputation Free Survival in Patients with Peripheral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 696–703.
- Bonaca, M.P.; Nault, P.; Giugliano, R.P.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Low-Density Lipoprotein Cholesterol Lowering with Evolocumab and Outcomes in Patients with Peripheral Artery Disease: Insights from the FOURIER Trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk). Circulation 2018, 137, 338–350.
- Sabatine, M.S.; Giugliano, R.P.; Wiviott, S.D.; Raal, F.J.; Blom, D.J.; Robinson, J.; Ballantyne, C.M.; Somaratne, R.; Legg, J.; Wasserman, S.M.; et al. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1500–1509.
- Sacks, C.A.; Avorn, J.; Kesselheim, A.S. The Failure of Solanezumab—How the FDA Saved Taxpayers Billions. N. Engl. J. Med. 2017, 376, 1706–1708.
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Skene, A.M. Intensive versus Moderate Lipid Lowering with Statins after Acute Coronary Syndromes. N. Engl. J. Med. 2004, 350, 1495–1504.
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N. Engl. J. Med. 2015, 372, 1489–1499.
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H., Jr.; Hobbs, H.H. Sequence Variations in PCSK9, Low LDL, and Protection against Coronary Heart Disease. N. Engl. J. Med. 2006, 354, 1264–1272.
- Dullaart, R.P.F. PCSK9 Inhibition to Reduce Cardiovascular Events. N. Engl. J. Med. 2017, 376, 1790–1791.
- Kathiresan, S. A PCSK9 Missense Variant Associated with a Reduced Risk of Early-Onset Myocardial Infarction. N. Engl. J. Med. 2008, 358, 2299–2300.
- La Rosa, J.C.; Conti, C.R. Intensive Lipid Lowering with Atorvastatin in Patients with Stable Coronary Disease. ACC Cardiosource Rev. J. 2006, 15, 97–99.
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722.
- Hiatt, W.R.; Fowkes, F.G.R.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Held, P.; Katona, B.G.; Mahaffey, K.W.; Norgren, L.; Jones, W.S.; et al. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N. Engl. J. Med. 2017, 376, 32–40.
- Bundó Vidiella, M.; Pérez Pérez, C.; Montero Alia, J.J.; Cobos Solórzano, M.D.; Aubà Llambrich, J.; Cabezas Peña, C. Peripheral Artery Disease of the Lower Limbs and Morbidity/Mortality in Type 2 Diabetics. Atención Primaria 2006, 38, 139–144.
- Mueller, T.; Hinterreiter, F.; Poelz, W.; Haltmayer, M.; Dieplinger, B. Mortality Rates at 10 Years Are Higher in Diabetic than in Non-Diabetic Patients with Chronic Lower Extremity Peripheral Arterial Disease. Vasc. Med. 2016, 21, 445–452.
- Schuyler Jones, W.; Patel, M.R.; Dai, D.; Vemulapalli, S.; Subherwal, S.; Stafford, J.; Peterson, E.D. High Mortality Risks after Major Lower Extremity Amputation in Medicare Patients with Peripheral Artery Disease. Am. Heart J. 2013, 165, 809–815.e1.
- Voci, D.; Fedeli, U.; Valerio, L.; Schievano, E.; Righini, M.; Kucher, N.; Spirk, D.; Barco, S. Mortality Rate Related to Peripheral Arterial Disease: A Retrospective Analysis of Epidemiological Data (Years 2008–2019). Nutr. Metab. Cardiovasc. Dis. 2023, 33, 516–522.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
745
Revisions:
2 times
(View History)
Update Date:
13 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




