Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Loic Ah-Thiane | -- | 2627 | 2023-06-12 10:35:44 | | | |
| 2 | Conner Chen | Meta information modification | 2627 | 2023-06-13 10:32:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ah-Thiane, L.; Rousseau, C.; Aumont, M.; Cailleteau, A.; Doré, M.; Mervoyer, A.; Vaugier, L.; Supiot, S. The Sentinel Lymph Node in Treatment Planning. Encyclopedia. Available online: https://encyclopedia.pub/entry/45436 (accessed on 13 January 2026).
Ah-Thiane L, Rousseau C, Aumont M, Cailleteau A, Doré M, Mervoyer A, et al. The Sentinel Lymph Node in Treatment Planning. Encyclopedia. Available at: https://encyclopedia.pub/entry/45436. Accessed January 13, 2026.
Ah-Thiane, Loic, Caroline Rousseau, Maud Aumont, Axel Cailleteau, Mélanie Doré, Augustin Mervoyer, Loig Vaugier, Stéphane Supiot. "The Sentinel Lymph Node in Treatment Planning" Encyclopedia, https://encyclopedia.pub/entry/45436 (accessed January 13, 2026).
Ah-Thiane, L., Rousseau, C., Aumont, M., Cailleteau, A., Doré, M., Mervoyer, A., Vaugier, L., & Supiot, S. (2023, June 12). The Sentinel Lymph Node in Treatment Planning. In Encyclopedia. https://encyclopedia.pub/entry/45436
Ah-Thiane, Loic, et al. "The Sentinel Lymph Node in Treatment Planning." Encyclopedia. Web. 12 June, 2023.
Copy Citation
The sentinel lymph node is a surgical technique developed in oncological surgery to identify and analyze fewer lymph nodes than a conventional lymph node dissection in order to limit the morbidity and mortality of such an extensive procedure without compromising the patients’ outcomes. This concept seems to also be useful in radiation oncology that treats lymph node areas. This may help radiation oncologists to treat their patients more precisely by targeting more accurately pathological sites and sparing healthy tissues.
sentinel lymph node
lymphatic drainage
radiation therapy
lymph flow guided radiotherapy
1. Introduction
The lymphatic vasculature is a system draining the organs through vessels and lymph nodes that act as filters. Its complexity stems from the numerous anatomical variants described in studies during cadaver dissections or surgical explorations, but knowledge of these variations is becoming increasingly available [1]. The metastatic spreading of cancer was described in some models to start with the invasion of the first draining loco-regional nodes, which then became known as sentinel lymph nodes (SLNs) [2]. The idea of using these SLNs thus emerged, based on a sentinel lymph node mapping (SLNM) using colorimetric, fluorescent or radioisotope tracers to identify the area of drainage of an anatomical territory, followed by sentinel lymph node biopsies (SLNBs) to assess whether a node is pathological (pSLN) or not (nSLN) [3]. The concept of SLNs was a major breakthrough for surgeons, allowing for a decrease in the number of morbid extensive lymph node dissections (ELND). SLNB is a minimally invasive technique, used routinely in penile cancer, breast cancer and melanoma thanks to its reliable performance and proven safety, and plays a key role in cancer staging and treatment decisions [4][5].
2. Breast Cancer
2.1. Axillary Lymph Node Dissection (ALND) Can Be Avoided Thanks to SLNB
In patients with nSLN, ALND can be avoided, as no difference was observed in overall and disease-free survival, or in axillary failure, which was low and reported in 0.7–0.8% of patients [6][7].
In patients with pSLN presenting a tumor smaller than 5 cm and no palpable adenopathy (cT1-T2 cN0), ALND could be avoided in patients with micrometastasis (<2 mm), since the IBCSG 23-01 trial showed no inferiority in disease-free survival after 10 years [8], as well as in patients with up to two macrometastasis but no capsular effraction, since the ACOSOG Z0011 (Alliance) trial showed no inferiority in overall survival after 10 years [9]. ALND was also proposed to be replaced by axillary radiotherapy, since the AMAROS and OTOASOR trials showed no difference in overall and disease-free survival between ALND and axillary radiotherapy [10][11]. A retrospective study compared 260 patients who received axillary radiotherapy versus those who did not and found no significant difference: 5-year overall survival was 93.4% versus 96.8% (p = 0.19), respectively, and 5-year disease-free survival was 92.3% versus 100% (p = 1.06), respectively [12].
A systematic review highlighted that ALND induced significantly more lymphedemas and shoulder dysfunctions in comparison with observation or axillary radiotherapy [13]. For most patients with nSLN or with pSLN (up to two metastasis) and cT1-T2 cN0 tumors, ALND should be avoided to decrease morbidity. Axillary radiotherapy is worth discussing in case of risk factors.
2.2. SLNM/SLNB Indicates Nodal Irradiation
Regional nodal irradiation, in addition to breast/chest wall irradiation, is currently indicated in case of clinical or pathological node involvement but deserves to be challenged. In fact, two phase III trials demonstrated that nodal irradiation (including axillary, infra/supraclavicular, internal mammary nodes) reduced breast cancer recurrence and specific mortality but did not significantly improve overall survival [14][15]. Moreover, a recent trial randomized 735 patients who received nodal irradiation, both including and excluding the internal mammary nodes, and found no benefit to irradiating this area, with the exception of a subgroup of patients with medial/central tumors [16], explained by their pattern of lymphatic drainage. Some authors suggested performing SLNM with the acquisition of SPECT/CT images to identify the drainage of each tumor, knowing that up to 50% of patients can present drainage in both axillary and internal mammary nodes, depending on the tumor’s location [17]. Figure 1 shows two examples of drainage in internal mammary nodes. However, in practice, only axillary nodes are noticed because they matter in surgery. Given recent findings, knowing the specific drainage of the cancer would help radiation oncologists delineate lymphatic areas, notably the internal mammary nodes, for relevant prophylactic irradiation [18].
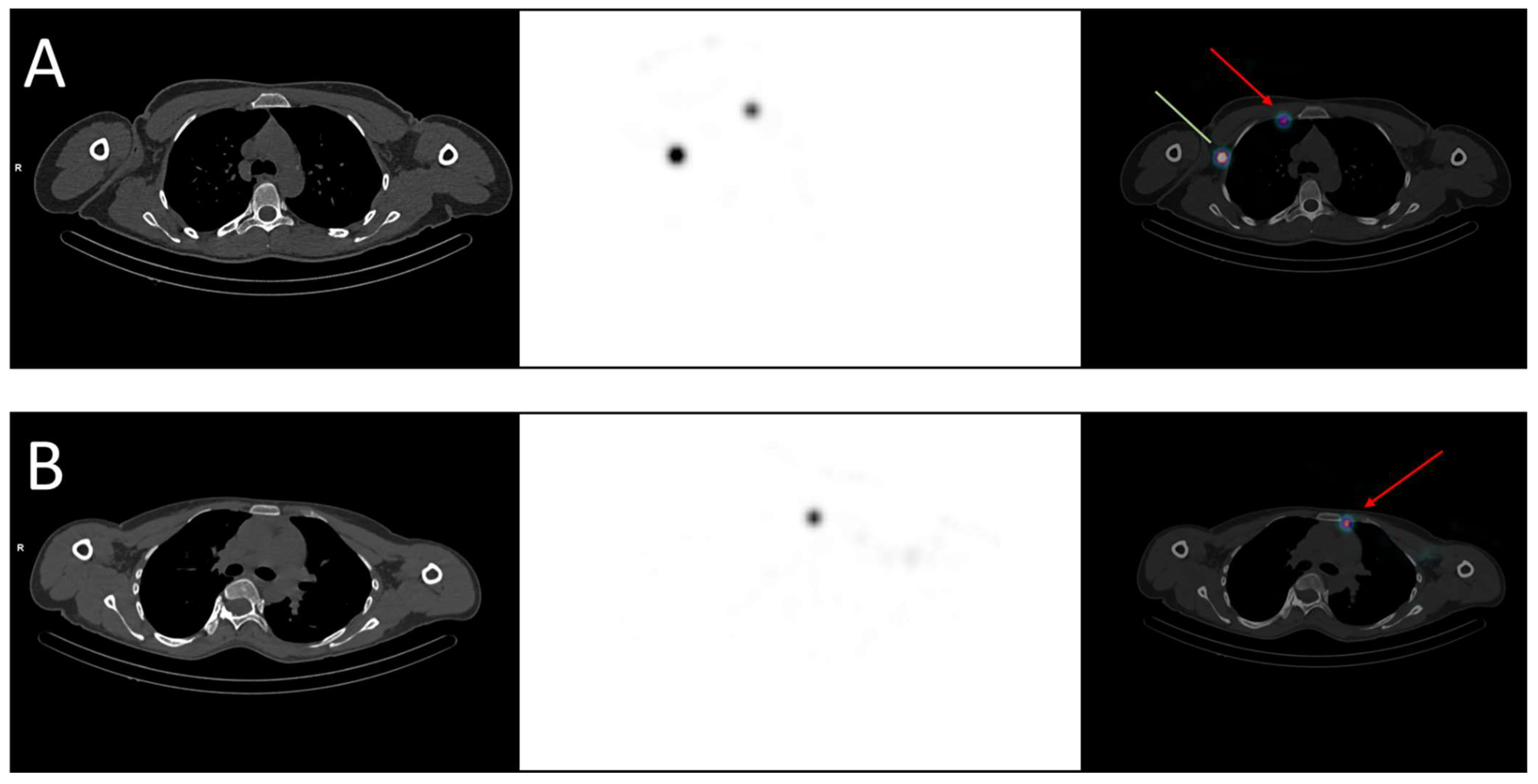
Figure 1. Examples of breast cancer drainage in the internal mammary nodes visualized in SPECT-CT. (A) A 36-year-old woman was diagnosed with two malignant nodules in the right breast, localized in the inner quadrants. The sentinel lymph node mapping revealed drainage in both the axillary (green line) and the internal mammary nodes (red arrow). (B) A 40-year-old woman was diagnosed with a malignant nodule in the left breast, localized in the upper-inner quadrant. The sentinel lymph node mapping revealed drainage in the internal mammary nodes (red arrow).
While SLNM identifies lymphatic drainage of the breast tumor after peritumoral tracer injection, axillary reverse mapping (ARM) identifies drainage of the upper limb after arm injection. ARM was initially developed for surgeons to preserve the main nodes draining the arm and not the tumor during ALND 6, but ARM seemed to be applicable for axillary irradiation too [19]. A dosimetric evaluation pointed out that all the nodes identified by ARM received the prescribed dose during standard axillary radiotherapy, explaining the rate of arm lymphedema [20]. A pilot study showed the feasibility of combining SLNM and ARM to preserve the main nodes, draining the arm while conserving the good coverage of the SLN sites in 5/6 of the patients. In the remaining patient, it was not possible to preserve these nodes because the SLNM and ARM overlapped [21]. The next step is to conduct trials to evaluate the oncological outcomes and their impact on lymphedema when reducing axillary irradiation volumes.
2.3. The Role of SLNB Needs to Be Redefined in a Neoadjuvant Setting
In cN0 patients, SLNB after neoadjuvant chemotherapy demonstrated a comparable performance to SLNB in upfront surgery and reduced the need to perform an ALND [22].
In patients with nodes confirmed by histology, the SN FNAC trial validated SLNB after neoadjuvant chemotherapy [23]. In case of residual nodal disease (ypN+), the guidelines recommend treating the axillary nodes [24]. For these patients, the ongoing ALLIANCE A011202 trial aims to determine the optimal treatment by comparing ALND and axillary radiotherapy with axillary radiotherapy alone (ClinicalTrials.gov number: NCT01901094). In case of complete nodal response (ypNO), the need for adjuvant nodal treatment is more debatable; hence, the ongoing NSAPB B-51 trial compares nodal irradiation with observation (ClinicalTrials.gov number: NCT01872975).
In conclusion, there is a clear decrease in ALND in breast cancer thanks to SLNB, in cases of nSLN but also in selected cases of pSLN. The results of SLNB indicate nodal irradiation, but SLNM may also provide some information on specific tumor drainage (especially internal mammary drainage) to help define which volumes should be targeted in radiotherapy. Moreover, ARM identifies the lymphatic nodes that drain the arm instead of the tumor and is worth exploring to reduce radiation-induced lymphedema. LFGRT is thus appealing as an effective method of irradiation with lower toxicity.
3. Gynecologic Cancers
3.1. SLNB Is a Well-Documented Technique in Vulvar Cancers
Locally advanced vulvar carcinomas are usually treated conservatively thanks to chemoradiation, whereas early-stage treatment consists of radical resection with nodal assessment and can be followed by adjuvant radiotherapy. Lymph node staging is a major prognostic factor in vulvar cancers [25]. For FIGO IB to II and lateral lesions (≥2 cm from vulvar midline) with clinically/radiologically node negative tumors, SLNB is recommended, since nSLN is associated with low morbidity, groin recurrence and disease-specific mortality, while being more cost-effective than extensive lymphadenectomy [26].
In case of pSLN, the management of ipsilateral groin with lymphadenectomy and radiotherapy should be discussed [27]. The GROINSS-V-II trial studied 322 patients with pSLN to evaluate whether groin dissection could be replaced by inguinofemoral radiotherapy. Due to high groin recurrence, the protocol had to be amended to allow for patients with SLN > 2 mm (macrometastasis) to undergo lymphadenectomy as the standard of care, but patients with SLN ≤ 2 mm (micrometastasis) could continue to receive radiotherapy. The 2-year groin recurrence rate was low for patients with micrometastasis (1.6%), but high for patients with macrometastasis when treated by radiotherapy (22%) compared to those treated by lymphadenectomy (6.9%). Ipsilateral inguinofemoral irradiation appears to be a low-morbidity option for patients with micrometastasis but should not be the first intention in case of macrometastasis [28].
How to manage contralateral groin remains unclear. Two retrospective monocentric studies suggested not treating contralateral groin, since they found very low rates of contralateral involvement: 0% (0/28) patients and 5.3% (1/19) patients, respectively [29][30]. However, a recent study reported a higher rate of contralateral involvement, at 22.2% (4/18) of patients, after an initial diagnosis of unilateral metastasis, supporting current guidelines in favor of contralateral prophylactic treatment by either lymphadenectomy or radiotherapy [31].
For larger tumors (greater than 4 cm), the negative predictive value deteriorates, so there is no strong evidence to recommend using the SLN technique [30].
3.2. SLNB Is Not the Standard Reference for Node Staging in Cervical Cancers at Present
Lymph node status leads the indication for radiotherapy in cervical cancers. The treatment is exclusively chemoradiation if metastatic lymph nodes are detected before radical surgery, or adjuvant chemoradiation if detected after resection. SLNB is currently employed in addition to pelvic node dissection but not alone, despite some interesting performances [32]. Indeed, questions have been raised about the ability to detect micrometastasis, reliability in intraoperative detection and the limited evidence obtained from prospective studies [33]. The SENTIX trial evaluated intraoperative SLN frozen section and SLNB without pelvic node dissection in 395 patients: SLN pathological examinations achieved high detection for node staging, but the intraoperative SLN frozen section failed to detect about 50% of pathological nodes [34]. Ongoing SENTICOL III and PHENIX trials are enrolling patients with early-stage cervical cancer. The SENTICOL III trial follows the SENTICOL II trial, which showed the decreased morbidity of SLNB alone [35] and randomizes patients between SLNB alone (experimental arm) and SLNB plus pelvic node dissection (reference arm). In the PHENIX trial, all patients undergo SLNB and are allocated into either the PHENIX-I (if nSLN) or PHENIX-II (if pSLN) cohorts. Patients in each cohort are randomized after the SLNB between observation (experimental arm) and pelvic node dissection (reference arm). The primary outcome of these two trials is disease-free survival to demonstrate non-inferiority, and results are expected in 2026 [36][37].
For more advanced cervical cancers, higher than FIGO 2018 stage Ib3, the involvement of para-aortic nodes needs to be assessed to guide irradiation volumes. This assessment is based on FDG PET-CT and para-aortic lymphadenectomy. The role played by SLNB is little documented and thus cannot be recommended [38][39].
4. Urologic Cancers
4.1. Penile Cancers Represent a Leading Indication of SLNB
In penile cancers, SLNB is a highly recommended procedure for the management of clinically node-negative patients based on the European Association of Urology guidelines [40]. Systematic reviews have confirmed the relevance of SLNB in this cancer, which has a very stereotyped echelon-based pattern of lymphatic drainage [41][42]. SLNs are detected during surgery with a high sensitivity and specificity of about 77% and 100%, respectively [43], especially when using blue dye and radiotracer in combination [44]. Performances appear even better when acquiring 3D-imaging in SPECT/CT before surgical detection, to increase the detection rate [45] and decrease the rate of false-positive nodes [46].
However, no studies investigated the use of SLNM and SLNB in radiation oncology, mainly because the benefits of nodal irradiation have not been demonstrated. In the absence of nodal involvement, prophylactic inguinal irradiation at 50 Gy showed no decrease in recurrences compared to surveillance [47]. If lymph nodes are involved, inguinal dissection is performed, and adjuvant radiotherapy might be offered in case of bad prognosis factors. The use of adjuvant radiotherapy is under debate since a systematic review showed no benefits, and thus a standard recommendation cannot be made [48].
4.2. SLNB Is Non-Mature in Bladder, Testicular, and Renal Cancers
SLNB has been described in bladder cancers for more than 20 years, but still presents non-negligible rates of false-negative lymph nodes, thus requiring further investigation, notably regarding radiotracers and detection techniques [49][50].
In testicular cancers, SLNB appears safe in prospective studies, but its value for guiding adjuvant treatment remains to be demonstrated [51][52].
In renal cancers, SLNM is not an easy technique to reproduce because it can be non-contributory in 30% of cases due to a lack of drainage of the radiotracer through lymphatic vessels [53]. Aside from these technical difficulties, its ability to detect and then treat lymph nodes is debated since it does not seem to change overall survival according to a recent meta-analysis [54].
5. Anal Cancer
5.1. SLNB Shows Better Performances Than FDG PET-CT for Detecting Metastases in Inguinal Nodes
The standard treatment of anal cancers is based on radiotherapy for T1 N0 tumors or concurrent radiochemotherapy (most often with 5FU and mitomycine C) for the others. Irradiation concerns the gross tumor, pelvic nodes, and inguinal nodes. Cancer staging currently relies on FDG PET-CT due to its high sensitivity. For instance, a study showed the perfect sensitivity of PET-CT, which did not miss any metastatic inguinal nodes, but reported a significant number of false-positive images, leading to a poor positive predictive value of only 43% [55]. Another study evaluated the SLNB of inguinal nodes and found this technique to be superior to FDG PET-CT, with fewer false-positive and false-negative patients [56]. In addition to better accuracy, a study revealed that pSLN was associated with oncological outcomes and a much better prognosis factor than positive inguinal uptake in FDG PET-CT. In fact, inguinal pSLN was significantly associated with a decrease in disease-free (21 vs. 56 months; p = 0.046) and overall (28 vs. 59 months; p = 0.028) survival [57]. Inguinal SLNB should be used more [58], as several literature reviews have reported good reproducibility and performance and acceptable rates of complications, but its deployment is limited by a lack of trials with a large population, notably because anal cancer is a rather rare cancer [59][60].
5.2. SLNB Could Spare Groin Irradiation and Its Toxicities
The selection of patients for groin irradiation currently depends on tumor size: T1 tumors are not a systematic indication, while groin irradiation is generally indicated for T2 or higher tumors. These rules present two problems: first, some T1 tumors may have occult inguinal metastasis whereas some T2 tumors may not, and second, groin irradiation can be poorly tolerated. The idea of adjusting radiation fields based on SLNB is not new in anal cancers [61]. A pilot study tested the feasibility of performing inguinal SLNB on patients with T1 or T2 anal tumors and irradiating the groin only in cases of pSLN [62]. The results of SLNB changed management in half (10/20) of their patients: 4 patients with a T1 tumor and pSLN received groin irradiation that was not initially indicated, and 6 patients with a T2 tumor and nSLN avoided groin irradiation that was initially indicated. Nevertheless, treatment de-escalation requires caution because a prospective study agreed with the feasibility of SLNB but also reported the cases of 2 out of 14 patients with nSLN who were spared groin irradiation, and who then developed inguinal metastasis at one year and two years, respectively [63]. Another study combined the use of FDG PET-CT and SLNB for inguinal staging, and patients presenting no sign of inguinal involvement in both exams avoided groin irradiation, and then presented significantly less inguinal dermatitis, especially severe dermatitis (grades 1–2: 12% vs. 50% and grades 3–4: 0% vs. 17%; p < 0.05) [64]. A retrospective study confirmed the difference between patients with nSLN and pSLN in terms of prognosis for disease-free and overall survival, and showed that it seemed safe not to target inguinal nodes in cases of nSLN, as none of their patients presented inguinal recurrence after a mean follow-up of 43 months [65].
In conclusion, disease staging in anal cancers is currently based on FDG PET-CT, which has shown good performances for pelvic nodes or visceral metastasis. However, FDG PET-CT has its limits for inguinal status, with a high rate of false positives. Inguinal SLNB can be seen as a more reliable alternative to inguinal staging, as well as to “LFGRT” where radiation fields are tailored to each patient. As anal cancers are uncommon, data on oncologic outcomes are lacking and comparative trials are needed.
References
- Leong, S.P.; Pissas, A.; Scarato, M.; Gallon, F.; Pissas, M.H.; Amore, M.; Wu, M.; Faries, M.B.; Lund, A.W. The lymphatic system and sentinel lymph nodes: Conduit for cancer metastasis. Clin. Exp. Metastasis 2022, 39, 139–157.
- Jakobsen, J.K. Sentinel node biopsy in uro-oncology: A history of the development of a promising concept. Urol. Oncol. 2015, 33, 486–493.
- Hamdy, O.; Farouk, O.; El-Badrawy, A.; Denewer, A.; Setit, A. Sentinel lymph node biopsy in breast cancer guided by CT lymphography; History, evolution and current applications. Breast Dis. 2021, 40, 219–225.
- Nieweg, O.E.; Uren, R.F.; Thompson, J.F. The history of sentinel lymph node biopsy. Cancer J. 2015, 21, 3–6.
- Moncayo, V.M.; Alazraki, A.L.; Alazraki, N.P.; Aarsvold, J.N. Sentinel Lymph Node Biopsy Procedures. Semin. Nucl. Med. 2017, 47, 595–617.
- Krag, D.N.; Anderson, S.J.; Julian, T.B.; Brown, A.M.; Harlow, S.P.; Costantino, J.P.; Ashikaga, T.; Weaver, D.L.; Mamounas, E.P.; Jalovec, L.M.; et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010, 11, 927–933.
- Noguchi, M.; Inokuchi, M.; Noguchi, M.; Morioka, E.; Ohno, Y.; Kurita, T. Axillary surgery for breast cancer: Past, present, and future. Breast Cancer 2021, 28, 9–15.
- Galimberti, V.; Cole, B.F.; Viale, G.; Veronesi, P.; Vicini, E.; Intra, M.; Mazzarol, G.; Massarut, S.; Zgajnar, J.; Taffurelli, M.; et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1385–1393.
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs No Axillary Dissection on 10-Year Overall Survival Among Women With Invasive Breast Cancer and Sentinel Node Metastasis: The ACOSOG Z0011 (Alliance) Randomized Clinical Trial. JAMA 2017, 318, 918–926.
- Donker, M.; van Tienhoven, G.; Straver, M.E.; Meijnen, P.; van de Velde, C.J.H.; Mansel, R.E.; Cataliotti, L.; Westenberg, A.H.; Klinkenbijl, J.H.G.; Orzalesi, L.; et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014, 15, 1303–1310.
- Sávolt, Á.; Péley, G.; Polgár, C.; Udvarhelyi, N.; Rubovszky, G.; Kovács, E.; Győrffy, B.; Kásler, M.; Mátrai, Z. Eight-year follow up result of the OTOASOR trial: The Optimal Treatment of the Axilla–Surgery Or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: A randomized, single centre, phase III, non-inferiority trial. Eur. J. Surg. Oncol. 2017, 43, 672–679.
- Ortega Expósito, C.; Falo, C.; Pernas, S.; Pérez Carton, S.; Gil Gil, M.; Ortega, R.; Pérez Montero, H.; Stradella, A.; Martinez, E.; Laplana, M.; et al. The effect of omitting axillary dissection and the impact of radiotherapy on patients with breast cancer sentinel node macrometastases: A cohort study following the ACOSOG Z0011 and AMAROS trials. Breast Cancer Res. Treat. 2021, 189, 111–120.
- Castelo, M.; Hu, S.Y.; Dossa, F.; Acuna, S.A.; Scheer, A.S. Comparing Observation, Axillary Radiotherapy, and Completion Axillary Lymph Node Dissection for Management of Axilla in Breast Cancer in Patients with Positive Sentinel Nodes: A Systematic Review. Ann. Surg. Oncol. 2020, 27, 2664–2676.
- Whelan, T.J.; Olivotto, I.A.; Parulekar, W.R.; Ackerman, I.; Chua, B.H.; Nabid, A.; Vallis, K.A.; White, J.R.; Rousseau, P.; Fortin, A.; et al. Regional Nodal Irradiation in Early-Stage Breast Cancer. N. Engl. J. Med. 2015, 373, 307–316.
- Poortmans, P.M.; Collette, S.; Kirkove, C.; Van Limbergen, E.; Budach, V.; Struikmans, H.; Collette, L.; Fourquet, A.; Maingon, P.; Valli, M.; et al. Internal Mammary and Medial Supraclavicular Irradiation in Breast Cancer. N. Engl. J. Med. 2015, 373, 317–327.
- Kim, Y.B.; Byun, H.K.; Kim, D.Y.; Ahn, S.J.; Lee, H.S.; Park, W.; Kim, S.S.; Kim, J.H.; Lee, K.C.; Lee, I.J.; et al. Effect of Elective Internal Mammary Node Irradiation on Disease-Free Survival in Women with Node-Positive Breast Cancer: A Randomized Phase 3 Clinical Trial. JAMA Oncol. 2022, 8, 96–105.
- Hindié, E.; Groheux, D.; Brenot-Rossi, I.; Rubello, D.; Moretti, J.L.; Espié, M. The sentinel node procedure in breast cancer: Nuclear medicine as the starting point. J. Nucl. Med. 2011, 52, 405–414.
- Nikolaevich, N.S.; Vasilevich, K.S. Why do we need irradiation of internal mammary lymph nodes in patients with breast cancer: Analysis of lymph flow and radiotherapy studies. Rep. Pract. Oncol. Radiother. 2017, 22, 37–41.
- Cheville, A.L.; Brinkmann, D.H.; Ward, S.B.; Durski, J.; Laack, N.N.; Yan, E.; Schomberg, P.J.; Garces, Y.I.; Suman, V.J.; Petersen, I.A. The addition of SPECT/CT lymphoscintigraphy to breast cancer radiation planning spares lymph nodes critical for arm drainage. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 971–977.
- Wang, W.; Ward, R.; Jia, D.; Ashworth, S.; Estoesta, E.; Moodie, T.; Ahern, V.; Stuart, K.; Ngui, N.; French, J.; et al. Location of arm draining lymph node in relation to breast cancer radiotherapy field and target volume. Radiother. Oncol. 2019, 133, 193–197.
- Waldstein, C.; Moodie, T.; Ashworth, S.; Ahern, V.; Stuart, K.; Wang, W. Feasibility of arm-draining lymph node-sparing radiotherapy of breast cancer: A pilot planning study. J. Med. Imaging Radiat. Oncol. 2021, 65, 951–955.
- Pilewskie, M.; Morrow, M. Axillary Nodal Management Following Neoadjuvant Chemotherapy: A Review. JAMA Oncol. 2017, 3, 549–555.
- Boileau, J.F.; Poirier, B.; Basik, M.; Holloway, C.M.B.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J. Clin. Oncol. 2015, 33, 258–264.
- Currey, A.; Patten, C.R.; Bergom, C.; Wilson, J.F.; Kong, A.L. Management of the axilla after neo-adjuvant chemotherapy for breast cancer: Sentinel node biopsy and radiotherapy considerations. Breast J. 2018, 24, 902–910.
- Brincat, M.R.; Muscat Baron, Y. Sentinel Lymph Node Biopsy in the Management of Vulvar Carcinoma: An Evidence-Based Insight. Int. J. Gynecol. Cancer 2017, 27, 1769–1773.
- Collarino, A.; Fuoco, V.; Garganese, G.; Pereira Arias-Bouda, L.M.; Perotti, G.; Manca, G.; Vidal-Sicart, S.; Giammarile, F.; de Geus-Oei, L.F.; Scambia, G.; et al. Lymphoscintigraphy and sentinel lymph node biopsy in vulvar carcinoma: Update from a European expert panel. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1261–1274.
- Weinberg, D.; Gomez-Martinez, R.A. Vulvar Cancer. Obstet. Gynecol. Clin. N. Am. 2019, 46, 125–135.
- Oonk, M.H.M.; Slomovitz, B.; Baldwin, P.J.W.; van Doorn, H.C.; van der Velden, J.; de Hullu, J.A.; Gaarenstroom, K.N.; Slangen, B.F.M.; Vergote, I.; Brännström, M.; et al. Radiotherapy Versus Inguinofemoral Lymphadenectomy as Treatment for Vulvar Cancer Patients With Micrometastases in the Sentinel Node: Results of, GROINSS-V II. J. Clin. Oncol. 2021, 39, 3623–3632.
- Woelber, L.; Eulenburg, C.; Grimm, D.; Trillsch, F.; Bohlmann, I.; Burandt, E.; Dieckmann, J.; Klutmann, S.; Schmalfeldt, B.; Mahner, S.; et al. The Risk of Contralateral Non-sentinel Metastasis in Patients with Primary Vulvar Cancer and Unilaterally Positive Sentinel Node. Ann. Surg. Oncol. 2016, 23, 2508–2514.
- Nica, A.; Covens, A.; Vicus, D.; Kupets, R.; Osborne, R.; Cesari, M.; Gien, L.T. Sentinel lymph nodes in vulvar cancer: Management dilemmas in patients with positive nodes and larger tumors. Gynecol. Oncol. 2019, 152, 94–100.
- Winarno, A.S.; Mondal, A.; Martignoni, F.C.; Fehm, T.N.; Hampl, M. The potential risk of contralateral non-sentinel groin node metastasis in women with early primary vulvar cancer following unilateral sentinel node metastasis: A single center evaluation in University Hospital of Düsseldorf. BMC Womens Health 2021, 21, 23.
- Balaya, V.; Guani, B.; Bonsang-Kitzis, H.; Deloménie, M.; Ngô, C.; Montero Macias, R.; Koual, M.; Nguyen-Xuan, H.T.; Bats, A.S.; Mathevet, P.; et al. Sentinel lymph node biopsy in early-stage cervical cancer: Current state of art. Bull. Cancer 2020, 107, 696–706.
- Cibula, D.; McCluggage, W.G. Sentinel lymph node (SLN) concept in cervical cancer: Current limitations and unanswered questions. Gynecol. Oncol. 2019, 152, 202–207.
- Cibula, D.; Kocian, R.; Plaikner, A.; Jarkovsky, J.; Klat, J.; Zapardiel, I.; Pilka, R.; Torne, A.; Sehnal, B.; Ostojich, M.; et al. Sentinel lymph node mapping and intraoperative assessment in a prospective, international, multicentre, observational trial of patients with cervical cancer: The SENTIX trial. Eur. J. Cancer 2020, 137, 69–80.
- Mathevet, P.; Lécuru, F.; Uzan, C.; Boutitie, F.; Magaud, L.; Guyon, F.; Querleu, D.; Fourchotte, V.; Baron, M.; Bats, A.S.; et al. Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: Results of a multicentre randomised trial (SENTICOL-2). Eur. J. Cancer 2021, 148, 307–315.
- Lecuru, F.R.; McCormack, M.; Hillemanns, P.; Anota, A.; Leitao, M.; Mathevet, P.; Zweemer, R.; Fujiwara, K.; Zanagnolo, V.; Zahl Eriksson, A.G.; et al. SENTICOL III: An international validation study of sentinel node biopsy in early cervical cancer. A GINECO, ENGOT, GCIG and multicenter study. Int. J. Gynecol. Cancer 2019, 29, 829–834.
- Tu, H.; Huang, H.; Xian, B.; Li, J.; Wang, P.; Zhao, W.; Chen, X.; Xie, X.; Wang, C.; Kong, B.; et al. Sentinel lymph node biopsy versus pelvic lymphadenectomy in early-stage cervical cancer: A multi-center randomized trial (PHENIX/CSEM 010). Int. J. Gynecol. Cancer 2020, 30, 1829–1833.
- Lavoué, V.; Bats, A.S.; Rouzier, R.; Coutant, C.; Barranger, E.; Daraï, E. Sentinel lymph node procedure followed by laparoscopic pelvic and paraaortic lymphadenectomy in women with IB2-II cervical cancer. Ann. Surg. Oncol. 2007, 14, 2654–2661.
- Chéreau, E.; Feron, J.G.; Ballester, M.; Coutant, C.; Bezu, C.; Rouzier, R.; Touboul, E.; Daraï, E. Contribution of pelvic and para-aortic lymphadenectomy with sentinel node biopsy in patients with IB2-IIB cervical cancer. Br. J. Cancer 2012, 106, 39–44.
- Djajadiningrat, R.S.; Graafland, N.M.; van Werkhoven, E.; Meinhardt, W.; Bex, A.; van der Poel, H.G.; van Boven, H.H.; Valdés Olmos, R.A.; Horenblas, S. Contemporary management of regional nodes in penile cancer-improvement of survival? J. Urol. 2014, 191, 68–73.
- Brouwer, O.R.; van der Poel, H.G.; Bevers, R.F.; van Gennep, E.J.; Horenblas, S. Beyond penile cancer, is there a role for sentinel node biopsy in urological malignancies? Clin. Transl. Imaging 2016, 4, 395–410.
- Mehralivand, S.; van der Poel, H.; Winter, A.; Choyke, P.L.; Pinto, P.A.; Turkbey, B. Sentinel lymph node imaging in urologic oncology. Transl. Androl. Urol. 2018, 7, 887–902.
- Neto, A.S.; Tobias-Machado, M.; Ficarra, V.; Wroclawski, M.L.; Amarante, R.D.M.; Pompeo, A.C.L.; Del Giglio, A. Dynamic sentinel node biopsy for inguinal lymph node staging in patients with penile cancer: A systematic review and cumulative analysis of the literature. Ann. Surg. Oncol. 2011, 18, 2026–2034.
- Sadeghi, R.; Gholami, H.; Zakavi, S.R.; Kakhki, V.R.D.; Tabasi, K.T.; Horenblas, S. Accuracy of sentinel lymph node biopsy for inguinal lymph node staging of penile squamous cell carcinoma: Systematic review and meta-analysis of the literature. J. Urol. 2012, 187, 25–31.
- Saad, Z.Z.; Omorphos, S.; Michopoulou, S.; Gacinovic, S.; Malone, P.; Nigam, R.; Muneer, A.; Bomanji, J. Investigating the role of SPECT/CT in dynamic sentinel lymph node biopsy for penile cancers. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1176–1184.
- Naumann, C.M.; Colberg, C.; Jüptner, M.; Marx, M.; Zhao, Y.; Jiang, P.; Hamann, M.F.; Jünemann, K.P.; Zuhayra, M.; Lützen, U. Evaluation of the diagnostic value of preoperative sentinel lymph node (SLN) imaging in penile carcinoma patients without palpable inguinal lymph nodes via single photon emission computed tomography/computed tomography (SPECT/CT) as compared to planar scintigraphy. Urol. Oncol. 2018, 36, 92.e17–92.e24.
- Leone, A.; Diorio, G.J.; Pettaway, C.; Master, V.; Spiess, P.E. Contemporary management of patients with penile cancer and lymph node metastasis. Nat. Rev. Urol. 2017, 14, 335–347.
- Robinson, R.; Marconi, L.; MacPepple, E.; Hakenberg, O.W.; Watkin, N.; Yuan, Y.; Lam, T.; MacLennan, S.; Adewuyi, T.E.; Coscione, A.; et al. Risks and Benefits of Adjuvant Radiotherapy After Inguinal Lymphadenectomy in Node-positive Penile Cancer: A Systematic Review by the European Association of Urology Penile Cancer Guidelines Panel. Eur. Urol. 2018, 74, 76–83.
- Kiss, B.; Thoeny, H.C.; Studer, U.E. Current Status of Lymph Node Imaging in Bladder and Prostate Cancer. Urology 2016, 96, 1–7.
- Zarifmahmoudi, L.; Ghorbani, H.; Sadri, K.; Tavakkoli, M.; Keshvari, M.; Salehi, M.; Sadeghi, R. Sentinel Node Biopsy in Urothelial Carcinoma of the Bladder: Systematic Review and Meta-Analysis. Urol. Int. 2019, 103, 373–382.
- Blok, J.M.; Kerst, J.M.; Vegt, E.; Brouwer, O.R.; Meijer, R.P.; Bosch, J.L.H.R.; Bex, A.; van der Poel, H.G.; Horenblas, S. Sentinel node biopsy in clinical stage I testicular cancer enables early detection of occult metastatic disease. BJU Int. 2019, 124, 424–430.
- Zarifmahmoudi, L.; Ghorbani, H.; Sadeghi, R.; Sadri, K.; Soltani, S.; Aghaee, A. Sentinel lymph node mapping in post chemotherapy nonseminoma testicular cancer patients undergoing retroperitoneal lymph node dissection: A series of nine cases. Asia Ocean J. Nucl. Med. Biol. 2022, 10, 36–42.
- Bex, A.; Vermeeren, L.; Meinhardt, W.; Prevoo, W.; Horenblas, S.; Valdés Olmos, R.A. Intraoperative sentinel node identification and sampling in clinically node-negative renal cell carcinoma: Initial experience in 20 patients. World J. Urol. 2011, 29, 793–799.
- Shi, X.; Feng, D.; Li, D.; Zhang, F.; Wei, W. The Role of Lymph Node Dissection for Non-Metastatic Renal Cell Carcinoma: An Updated Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 790381.
- Mistrangelo, M.; Pelosi, E.; Bellò, M.; Castellano, I.; Cassoni, P.; Ricardi, U.; Munoz, F.; Racca, P.; Contu, V.; Beltramo, G.; et al. Comparison of positron emission tomography scanning and sentinel node biopsy in the detection of inguinal node metastases in patients with anal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 73–78.
- Mistrangelo, M.; Pelosi, E.; Bellò, M.; Ricardi, U.; Milanesi, E.; Cassoni, P.; Baccega, M.; Filippini, C.; Racca, P.; Lesca, A.; et al. Role of positron emission tomography-computed tomography in the management of anal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 66–72.
- De Nardi, P.; Guarneri, G.; Canevari, C.; Tamburini, A.; Slim, N.; Passoni, P.; Rosati, R. Prognostic value of fluorodeoxyglucose positron emission tomography/computed tomography and inguinal sentinel lymph node biopsy in patients with anal cancer. Colorectal Dis. 2019, 21, 1017–1024.
- Mistrangelo, D.M.; Bellò, M.; Cassoni, P.; Milanesi, E.; Racca, P.; Munoz, F.; Fora, G.; Rondi, N.; Gilbo, N.; Senetta, R.; et al. Value of staging squamous cell carcinoma of the anal margin and canal using the sentinel lymph node procedure: An update of the series and a review of the literature. Br. J. Cancer 2013, 108, 527–532.
- Noorani, A.; Rabey, N.; Durrani, A.; Walsh, S.R.; Davies, R.J. Systematic review of sentinel lymph node biopsy in anal squamous cell carcinoma. Int. J. Surg. 2013, 11, 762–766.
- Tehranian, S.; Treglia, G.; Krag, D.N.; Dabbagh Kakhki, V.R.; Zakavi, S.R.; Sadeghi, R.; Keshtgar, M. Sentinel node mapping in anal canal cancer: Systematic review and meta-analysis. J. Gastrointestin Liver Dis. 2013, 22, 321–328.
- De Nardi, P.; Carvello, M.; Staudacher, C. New approach to anal cancer: Individualized therapy based on sentinel lymph node biopsy. World J. Gastroenterol. 2012, 18, 6349–6356.
- Gretschel, S.; Warnick, P.; Bembenek, A.; Dresel, S.; Koswig, S.; String, A.; Hünerbein, M.; Schlag, P.M. Lymphatic mapping and sentinel lymph node biopsy in epidermoid carcinoma of the anal canal. Eur. J. Surg. Oncol. 2008, 34, 890–894.
- de Jong, J.S.; Beukema, J.C.; van Dam, G.M.; Slart, R.; Lemstra, C.; Wiggers, T. Limited value of staging squamous cell carcinoma of the anal margin and canal using the sentinel lymph node procedure: A prospective study with long-term follow-up. Ann. Surg. Oncol. 2010, 17, 2656–2662.
- Slim, N.; Passoni, P.; Incerti, E.; Tummineri, R.; Gumina, C.; Cattaneo, G.M.; De Nardi, P.; Canevari, C.; Fiorino, C.; Ronzoni, M.; et al. Impact of sentinel lymph-node biopsy and FDG-PET in staging and radiation treatment of anal cancer patients. Sci. Rep. 2020, 10, 14613.
- De Nardi, P.; Mistrangelo, M.; Burtulo, G.; Passoni, P.; Slim, N.; Ronzoni, M.; Canevari, C.; Parolini, D.; Massimino, L.; Franco, P.; et al. Tailoring the radiotherapy approach in patients with anal squamous cell carcinoma based on inguinal sentinel lymph node biopsy. J. Surg. Oncol. 2021, 123, 315–321.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
617
Revisions:
2 times
(View History)
Update Date:
13 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




