Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ghobad Behzadi pour | -- | 1177 | 2023-06-09 11:40:20 | | | |
| 2 | Dean Liu | -23 word(s) | 1154 | 2023-06-12 02:56:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pour, G.B.; Ashourifar, H.; Aval, L.F.; Solaymani, S. CNTs-Supercapacitors. Encyclopedia. Available online: https://encyclopedia.pub/entry/45387 (accessed on 12 March 2026).
Pour GB, Ashourifar H, Aval LF, Solaymani S. CNTs-Supercapacitors. Encyclopedia. Available at: https://encyclopedia.pub/entry/45387. Accessed March 12, 2026.
Pour, Ghobad Behzadi, Hassan Ashourifar, Leila Fekri Aval, Shahram Solaymani. "CNTs-Supercapacitors" Encyclopedia, https://encyclopedia.pub/entry/45387 (accessed March 12, 2026).
Pour, G.B., Ashourifar, H., Aval, L.F., & Solaymani, S. (2023, June 09). CNTs-Supercapacitors. In Encyclopedia. https://encyclopedia.pub/entry/45387
Pour, Ghobad Behzadi, et al. "CNTs-Supercapacitors." Encyclopedia. Web. 09 June, 2023.
Copy Citation
Carbon nanotubes (CNTs), due to mechanical, electrical, and surface area properties and their ability to adapt to different nanocomposite structures, are very substantial in supercapacitor electrodes.
supercapacitor
CNTs
PANI
specific capacitance
1. Introduction
Supercapacitors are capable energy storage systems, as they offer fast charge/discharge, high cycle stability, high power density, and stable electrical properties. Supercapacitors in several industries are used for energy harvesting and high-power systems in electric vehicles. Recently, supercapacitors based on CNTs composite electrodes have received more attention due to the electrical and chemical properties of CNTs [1][2][3][4][5][6][7].
2. CNTs/Metal-Based Supercapacitors
Increasing energy demands are causing the growth of new materials for supercapacitors’ electrodes and electrolytes. Recently, CNT/metal nanocomposites were widely investigated for the development of supercapacitor electrode materials such as CNTs/HCNFs/MOF, CNTs/NiPMo12, CNT/BT/Zn-N, CNTs@NHP/Co@LDH-CoZnAl, CNTs/ZnCo2O4, CNTs/NiO/MnO2, CNTs/Ti3C2TX, CNTs/KCl, CNTs/V2CTx MXene, CNTs/NiC32N8H16, CNTs/MnS, CNTs/MnO2, CNTs/Ni–Va and CNTs/ZnWO4/Ni foam, CNTs/NiO/MnO2, CNTs/ZnO/C, CNTs/NiCo2S4@N, CNTs@Bi2S3/MoS2, CNTs/Cu2P2O7, and CNTs/MXene with a SC of 712 F/g, 815.6 F/g, 252 F/g, 869.6 F/g, 888 F/g, 23 F/g, 270.5 F/g, 334.4 F/g, 1842 F/g, 12 F/g, 8 F/g, 1298 F/g, 386 F/g, 1493 F/g, 4552 F/g, 1320 F/g, 650 F/g, 254 F/g, 1338 F/g, 465 F/g, and 401.4 F/g, respectively [8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27]. A comparison of SC, power, and energy densities of CNT/metal-based supercapacitors published in 2022 is mentioned in Table 1.
Table 1. Comparison of CNT/metal electrode-based supercapacitors.
| Electrode Materials | CR (%)/Cycles | SC (F/g) | Energy Density (Wh/kg) |
Power Density (W/kg) |
Reference |
|---|---|---|---|---|---|
| CNTs/Ni | 83.2/5000 | 97 | 32.6 | 476.5 | [28] |
| CNTs/NiCo2O4/Ni/C | - | 1945.9 | 58.31 | 749.7 | [29] |
| CNTs/NiCoO2 | 92/5000 | 1587 | 41.8 | 412 | [30] |
| CNTs/NiS2/NiCo2S4 | 83/10,000 | 2905 | 58.1 | - | [31] |
| CNTs/NiFe2O4 | 89.16/5000 | 670 | 23.39 | 466.66 | [32] |
| CNTs/Co-S@carbon nanofibers | 96.9/10,000 | 416.5 | 10.3 | 320 | [33] |
| CNTs/Co3V2O8 | 95.26/3000 | 120.17 | 37.55 | - | [34] |
| CNTs/ZIF/MoS2 | -/10,000 | 262 | 52.4 | 3680 | [35] |
| CNTs/ZnCoS | 96/10,000 | 2957.6 | 68.8 | 700 | [36] |
| CNTs/MnO2 | 78.26/6000 | 253.86 | 32 | 413.70 | [37] |
| CNTs/Ti3C2/MnCo2S4 | 94.09/5000 | 823 | 49.5 | 350 | [38] |
| CNTs/Bi-Fe-P | 85.6/8000 | 532 | 81.5 | 890.2 | [39] |
| CNTs/cerium selenide nanopebbles | 84.1/4000 | 451.4 | 36.3–14.5 | 2800–5600 | [40] |
| CNTs/Nb2O5 | 96/10,000 | 192 | 5.4–2.7 | 98.7–24.671 | [41] |
| CNTs/Nitrogen–Boron–Carbon | 95.7/3000 | 432.31 | 11.67–7.74 | 300–1485 | [42] |
| CNTs/SnS2-BN | 101/- | 87 | 49 | - | [43] |
Metals that are organic due to chemical and physical properties such as porosity were proposed for supercapacitors’ electrode material. A comparison of supercapacitors based on CNT/Ni electrode structures is reported in Table 1 [28][29][30][31][32]. Sun et al. [28] synthesized the CNT/Ni composite electrode for the supercapacitor electrode and reported that the SC of the supercapacitor was 97 F/g and 83.2% retention after 5000 cycles. They reached 32.6 Wh/kg and 476.5 W/kg energy density and power density, respectively, for CNT/Ni supercapacitors. The hierarchical electrode materials are attractive for energy storage applications. The supercapacitor is based on the CNT/NiCo2O4/Ni/C electrode structure described in Ref. [29]. The paper mentioned that the SC of a supercapacitor with the hierarchical electrode material was 1945.9 F/g with an energy density of 58.31 Wh/kg at a power density of 749.7 W/kg. One of the disadvantages of supercapacitors is related to the low energy density that can be improved by pseudocapacitive materials from redox reactions. A supercapacitor electrode based on the CNT/NiCoO2 mesoporous structure presented the SC of 1587 F/g and 92% CR after 5000 cycles, with an energy density of 41.8 Wh/kg at a power density of 412 W/kg [30]. Geioushy et al. [31] introduced the CNT/NiS2/NiCo2S4 nanostructure as electrode material for energy storage applications. They achieved the SC of 1587 F/g and 83% CR after 10,000 cycles. The composition of the binary metal oxide with CNTs is considered for increased energy storage in recent reports. The supercapacitor with the CNT/NiFe2O4 electrode structure demonstrated energy and power densities of 23.39 Wh/kg and 466.66 W/kg, respectively [32]. Supercapacitors with long-cycle stability and flexible electrodes are in demand for portable electronic equipment. Yao et al. [33] investigated the flexible quasi-solid-state supercapacitor based on the CNT/Co-S/carbon nanofibers’ electrode and stated that the SC of the supercapacitor was 416.5 F/g and 96.9% CR after 10,000 cycles. Due to the tunable structure and stability properties of the binary metal, oxides were used for electrode materials’ construction. The supercapacitor with the gel polymer electrolyte and the CNT/Co3V2O8 electrode was proposed in Ref. [34]. The paper detailed that the SC of the supercapacitor was 120.17 F/g and 95.26% CR after 3000 cycles. Despite the capability of supercapacitors in energy storage, there is a big difference in energy densities between batteries and supercapacitors. Houpt et al. [35] introduced a hybrid framework with the CNT/ZIF/MoS2 electrode material. They stated that the power density was 3682 W/kg for the supercapacitor with a SC of 262 F/g. A supercapacitor based on the metal–organic CNT/ZnCoS electrode demonstrated the SC of 2957.6 F/g with a 96% CR after 10,000 cycles [36]. Metal–oxygen material such as CNTs/MnO2 was used for the supercapacitor electrode and achieved the SC of 253.86 F/g with 78.26% CR after 6000 cycles, with energy and power densities of 32 Wh/kg and 413.7 W/kg, respectively [37]. The improvement of supercapacitors’ performance is related to the electrode materials and structural design. The CNT/Ti3C2/MnCo2S4 composite electrode with positively charged CNTs and negatively charged Ti3C2 was investigated in Ref. [38]. The paper mentioned that the symmetric supercapacitor demonstrated the SC of 823 F/g and energy and power densities of 49.5 Wh/kg and 350 W/kg respectively. Pseudocapacitive materials due to their good redox processes and low cost are proper for supercapacitor fabrication. A supercapacitor based on the CNT/Bi-Fe-P electrode exhibited an energy density and power densities of 81.5 Wh/kg and 890.2 W/kg, respectively, with 85.6% CR after 8000 cycles [39]. Problems in flexible energy storage devices are related to mechanical properties, cyclic stability, and small capacitance. A flexible symmetric supercapacitor based on the CNT/cerium selenide nanopebbles’ electrode and poly (vinyl alcohol) (PVA)-LiClO4 gel electrolyte was proposed in Ref. [40]. The study achieved energy and power densities in a range of 36.3–14.5 Wh/kg and 2800–5600 W/kg, respectively. There is sodium due to an abundance in the Earth’s crust, leading to low-cost energy storage and renewable energies. In combination with niobium pentoxide and CNTs, sodium ions can make intercalation into niobium pentoxide and electrostatic adsorption into CNTs.
Real et al. [41] investigated a flexible, sodium-ion pseudocapacitor with the CNT/Nb2O5 electrode and demonstrated a SC of 192 F/g and 96% CR after 10,000 cycles. A supercapacitor based on the CNT/Nitrogen–Boron–Carbon electrode reached energy density and power density in a range between 11.67–7.74 Wh/kg and 300–1485 W/kg, respectively [42]. Due to large-volume oscillations, the low inherent conductivity and stability of the bare SnS2 are not expected for supercapacitor devices. A supercapacitor with CNT/SnS2-BN electrode material stated the SC of 87 F/g [43]. As can be observed from Figure 1 for CNT/metal-based supercapacitors, the energy density is higher, and for CNT/graphene-based supercapacitors, the power density is highlighted. The CNT/metal-based supercapacitors achieved an energy density of about 80 Wh/kg, and the CNT/graphene-based supercapacitor reached a power density of 9000 W/kg.
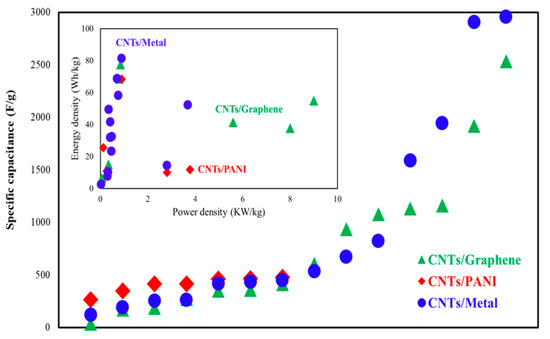
Figure 1. Comparison of specific capacitance, power density, and energy density for supercapacitors based on the CNTs/metal, CNTs/graphene, and CNTS/PANI.
References
- Cevik, E.; Asiri, S.M.M.; Qahtan, T.F.; Bozkurt, A. Fabrication of high mechanical stability electrodes and bio-electrolytes for high-performance supercapacitor application. J. Alloys Compd. 2022, 913, 165230.
- Jalalah, M.; Rudra, S.; Aljafari, B.; Irfan, M.; Almasabi, S.S.; Alsuwian, T.; Khazi, M.I.; Nayak, A.K.; Harraz, F.A. Sustainable synthesis of heteroatom-doped porous carbon skeleton from Acacia auriculiformis bark for high-performance symmetric supercapacitor device. Electrochim. Acta 2022, 414, 140205.
- Keum, K.; Park, D.; Park, M.; Lee, Y.; Lee, H.; Jeong, H.; Kim, J.W.; Kim, D.-W.; Ha, J.S. All vanadium-based Li-ion hybrid supercapacitor with enhanced electrochemical performance via prelithiation. J. Alloys Compd. 2022, 914, 165288.
- Mandal, M.; Subudhi, S.; Nayak, A.K.; Alam, I.; Subramanyam, B.V.R.S.; Maheswari, R.P.; Patra, S.; Mahanandia, P. In-situ synthesis of mixed-phase carbon material using simple pyrolysis method for high-performance supercapacitor. Diam. Relat. Mater. 2022, 127, 109209.
- Ahmad, H.; Khan, R.A.; Koo, B.H.; Alsalme, A. Systematic study of physicochemical and electrochemical properties of carbon nanomaterials. RSC Adv. 2022, 12, 15593–15600.
- Ye, T.; Wu, H.; Shao, Y.; Ye, Z.; Li, G.; Wang, J.; Chen, K. A Study on the Effect of Graphene/Carbon Nanotubes on the Enhanced Capacitance of IrO2-ZnO-G(CNT)/TiElectrodes. Energy Fuels 2022, 36, 3259–3271.
- Rehman, Z.U.; Raza, M.A.; Chishti, U.N.; Hussnain, A.; Maqsood, M.F.; Iqbal, M.Z.; Iqbal, M.J.; Latif, U. Role of Carbon Nanomaterials on Enhancing the Supercapacitive Performance of Manganese Oxide-Based Composite Electrodes. Arab. J. Sci. Eng. 2022, 2022, 1–16.
- Kim, T.; Subedi, S.; Dahal, B.; Chhetri, K.; Mukhiya, T.; Muthurasu, A.; Gautam, J.; Lohani, P.C.; Acharya, D.; Pathak, I.; et al. Homogeneous Elongation of N-Doped CNTs over Nano-Fibrillated Hollow-Carbon-Nanofiber: Mass and Charge Balance in Asymmetric Supercapacitors Is No Longer Problematic. Adv. Sci. 2022, 9, 2200650.
- Zhuo, J.-L.; Wang, Y.-L.; Wang, Y.-G.; Xu, M.-Q.; Sha, J.-Q. Surfactant-assisted fabrication and supercapacitor performances of a 12-phosphomolybdate-pillared metal-organic framework containing a helix and its SWNT nanocomposites. CrystEngComm 2022, 24, 579–586.
- Shi, L.; Yang, W.; Zha, X.; Zeng, Q.; Tu, D.; Li, Y.; Yang, Y.; Xu, J.; Chen, F. Metal-organic frameworks-derived porous carbon nanotube for high performance supercapacitor electrode materials. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129862.
- Habibi, R.; Mehrpooya, M.; Ganjali, M. Synthesis of ternary CoZnAl layered double hydroxide and Co-embedded N-doped carbon nanotube hollow polyhedron nanocomposite as a bifunctional material for ORR electrocatalyst and supercapacitor electrode. J. Energy Storage 2022, 54, 105377.
- Isacfranklin, M.; Daphine, S.; Yuvakkumar, R.; Kungumadevi, L.; Ravi, G.; Al-Sehemi, A.G.; Velauthapillai, D. ZnCo2O4/CNT composite for efficient supercapacitor electrodes. Ceram. Int. 2022, 48, 24745–24750.
- Peçenek, H.; Dokan, F.K.; Onses, M.S.; Yılmaz, E.; Sahmetlioglu, E. Highly compressible binder-free sponge supercapacitor electrode based on flower-like NiO/MnO2/CNT. J. Alloys Compd. 2022, 913, 165053.
- Liu, X.; Zhou, R.; Yang, D.; Lu, S.; Wang, J.; Liu, C.; Wang, S. A Supercapacitor Electrode Synthesis Strategy: Proton Acid-Treated Ti3C2Tx Film with Single-walled Carbon Nanotubes as a Reinforcement. ChemistrySelect 2022, 7, e202200690.
- Lv, S.; Ma, L.; Shen, X.; Tong, H. Potassium chloride-catalyzed growth of porous carbon nanotubes for high-performance supercapacitors. J. Alloys Compd. 2022, 906, 164242.
- Zahra, S.A.; Anasori, B.; Iqbal, M.Z.; Ravaux, F.; Al Tarawneh, M.; Rizwan, S. Enhanced electrochemical performance of vanadium carbide MXene composites for supercapacitors. APL Mater. 2022, 10, 060901.
- Gyulasaryan, H.T.; Azizbekyan, G.G.; Sisakyan, N.S.; Chilingaryan, G.N.; Grapov, D.V.; Kukuts, Y.M.; Sharoyan, E.G.; Manukyan, A.S. Electrode Material for Supercapacitors Based on Products of Solid Phase Pyrolysis of Metal-Phthalocyanines. J. Contemp. Phys. 2022, 57, 76–80.
- Tamilselvan, A.; Kundu, M. Ex-situ synthesis of MnS nanoparticles imbedded with carbon nanotubes as a high-performance electrode material for supercapacitors. Mater. Today Proc. 2022, 68, 146–151.
- Teng, S.; Shi, S.; Wang, G.; Xiang, Y.; Wan, G. Ozone-activated CNTs to induce uniform coating of MnO2 as high-performance supercapacitor electrodes. Fuller. Nanotub. Carbon Nanostructures 2022, 30, 1163–1169.
- Tu, Q.; Zhang, J.; Cai, S.; Zhang, K.; Zhan, H.; Huang, S.; Chen, L.; Sun, X. One-Step Preparation of Hierarchical Composite for Advanced Asymmetrical Supercapacitor. Adv. Eng. Mater. 2022, 24, 2101174.
- Tourchi Moghadam, M.T.; Seifi, M.; Jamali, F.; Azizi, S.; Askari, M.B. ZnWO4-CNT as a superior electrode material for ultra-high capacitance supercapacitor. Surf. Interfaces 2022, 32, 102134.
- Peçenek, H.; Dokan, F.K.; Onses, M.S.; Yılmaz, E.; Sahmetlioglu, E. Outstanding supercapacitor performance with intertwined flower-like NiO/MnO2/CNT electrodes. Mater. Res. Bull. 2022, 149, 111745.
- Otun, K.O.; Xaba, M.S.; Zong, S.; Liu, X.; Hildebrandt, D.; El-Bahy, S.M.; Alotaibi, M.T.; El-Bahy, Z.M. ZIF-8-derived ZnO/C decorated hydroxyl-functionalized multi-walled carbon nanotubes as a new compositeelectrode for supercapacitor application. Colloids Interface Sci. Commun. 2022, 47, 100589.
- Ramesh, S.; Karuppasamy, K.; Vikraman, D.; Yadav, H.M.; Kim, H.-S.; Sivasamy, A.; Kim, H.S. Fabrication of NiCo2S4 accumulated on metal organic framework nanostructured with multiwalled carbon nanotubes composite material for supercapacitor application. Ceram. Int. 2022, 48, 29102–29110.
- Sakthivel, P.; Anandha babu, G.; Karuppiah, M.; Asaithambi, S.; Balaji, V.; Pandian, M.S.; Ramasamy, P.; Mohammed, M.K.A.; Navaneethan, N.; Ravi, G. Electrochemical energy storage applications of carbon nanotube supported heterogeneous metal sulfide electrodes. Ceram. Int. 2022, 48, 6157–6165.
- Agarwal, A.; Majumder, S.; Sankapal, B.R. Multi-walled carbon nanotubes supported copper phosphate microflowers for flexible solid-state supercapacitor. Int. J. Energy Res. 2022, 46, 6177–6196.
- Li, K.; Zhang, P.; Soomro, R.A.; Xu, B. Alkali-Induced Porous MXene/Carbon Nanotube-Based Film Electrodes for Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 4180–4186.
- Sun, S.; Wang, Y.; Chen, L.; Chu, M.; Dong, Y.; Liu, D.; Liu, P.; Qu, D.; Duan, J.; Li, X. MOF(Ni)/CNT composites with layer structure for high capacitive performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128802.
- Yang, R.; Bai, X.; Guo, X.; Song, K.; Jia, L.; Chen, X.; Wang, J. Hierarchical NiCo2O4 nanostructured arrays decorated over the porous Ni/C as battery-type electrodes for supercapacitors. Appl. Surf. Sci. 2022, 586, 152574.
- Wang, B.Q.; Gong, S.H.; Sun, Q.S.; Liu, F.; Wang, X.C.; Cheng, J.P. Carbon nanotubes refined mesoporous NiCoO2 nanoparticles for high−performance supercapacitors. Electrochim. Acta 2022, 402, 139575.
- Geioushy, R.A.; Attia, S.Y.; Mohamed, S.G.; Li, H.; Fouad, O.A. High-performance electrode materials for supercapacitor applications using Ni-catalyzed carbon nanostructures derived from biomass waste materials. J. Energy Storage 2022, 48, 104034.
- Sivakumar, M.; Muthukutty, B.; Panomsuwan, G.; Veeramani, V.; Jiang, Z.; Maiyalagan, T. Facile synthesis of NiFe2O4 nanoparticle with carbon nanotube composite electrodes for high-performance asymmetric supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129188.
- Yao, M.; Guo, C.; Zhang, Y.; Zhao, X.; Wang, Y. In situ encapsulation of metal sulfide into hierarchical nanostructured electrospun nanofibers as self-supported electrodes for flexible quasi-solid-state supercapacitors. J. Mater. Chem. C 2022, 10, 542–548.
- Fahimi, Z.; Moradlou, O. High-performance solid-state asymmetric supercapacitor based on Co3V2O8/carbon nanotube nanocomposite and gel polymer electrolyte. J. Energy Storage 2022, 50, 104697.
- Houpt, D.; Ji, J.; Yang, D.; Choi, J.H. High-Performance Supercapacitor Electrodes Based on Composites of MoS2 Nanosheets, Carbon Nanotubes, andZIF-8 Metal–Organic Framework Nanoparticles. ACS Appl. Nano Mater. 2022, 5, 1491–1499.
- Chen, H.-C.; Hou, L.-Y.; He, C.; Laing, P.-J.; Huang, C.-Y.; Kuo, W.-S. Metal-Organic Framework-Assisted Synthesis of Three-Dimensional ZnCoS Effloresced Paper for High-Performance Flexible All-Solid-State Battery-Type Supercapacitors with Ultrahigh Specific Capacitance. ACS Appl. Energy Mater. 2022, 5, 8262–8272.
- Li, Z.; Xiao, D.; Xu, C.; Li, Z.; Bi, S.; Xu, H.; Dou, H.; Zhang, X. MnO2/carbon nanotube free-standing electrode recycled from spent manganese-oxygen battery as high-performance supercapacitor material. J. Mater. Sci. 2022, 57, 8818–8827.
- Dang, A.; Sun, Y.; Fang, C.; Li, T.; Liu, X.; Xia, Y.; Ye, F.; Zada, A.; Khan, M. Rational design of Ti3C2/carbon nanotubes/MnCo2S4 electrodes for symmetric supercapacitors with high energy storage. Appl. Surf. Sci. 2022, 581, 152432.
- Khalafallah, D.; Zhi, M.; Hong, Z. Bi-Fe chalcogenides anchored carbon matrix and structured core–shell nanoarchitectures with appealing performances for supercapacitors. J. Colloid Interface Sci. 2022, 606, 1352–1363.
- Pandit, B.; Sankapal, B.R. Cerium Selenide Nanopebble/Multiwalled Carbon Nanotube Composite Electrodes for Solid-State Symmetric Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 3007–3017.
- Real, C.G.; Thaines, E.H.N.S.; Pocrifka, L.A.; Freitas, R.G.; Singh, G.; Zanin, H. Freestanding niobium pentoxide-decorated multiwalled carbon nanotube electrode: Charge storage mechanism insodium-ion pseudocapacitor and battery. J. Energy Storage 2022, 52, 104793.
- Ren, X.; Yuan, Z.; Ma, Y.; Zhang, C.; Qin, C.; Jiang, X. Nitrogen-/Boron-Doped Carbon from Poplar Powder and Carbon Nanotube Composite as Electrode Material for Supercapacitors. Energy Fuels 2022, 36, 2841–2850.
- Maity, C.K.; Sahoo, S.; Verma, K.; Nayak, G.C. SnS2@Conducting Energy Level-Induced Functionalized Boron Nitride for an Asymmetric Supercapacitor. Energy Fuels 2022, 36, 2248–2259.
More
Information
Subjects:
Energy & Fuels
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
866
Revisions:
2 times
(View History)
Update Date:
12 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





