Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Makoto Nakashima | -- | 1385 | 2023-06-09 02:36:33 | | | |
| 2 | Conner Chen | Meta information modification | 1385 | 2023-06-12 05:18:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nakashima, M.; Uchimaru, K. The Functions of CD30. Encyclopedia. Available online: https://encyclopedia.pub/entry/45368 (accessed on 01 March 2026).
Nakashima M, Uchimaru K. The Functions of CD30. Encyclopedia. Available at: https://encyclopedia.pub/entry/45368. Accessed March 01, 2026.
Nakashima, Makoto, Kaoru Uchimaru. "The Functions of CD30" Encyclopedia, https://encyclopedia.pub/entry/45368 (accessed March 01, 2026).
Nakashima, M., & Uchimaru, K. (2023, June 09). The Functions of CD30. In Encyclopedia. https://encyclopedia.pub/entry/45368
Nakashima, Makoto and Kaoru Uchimaru. "The Functions of CD30." Encyclopedia. Web. 09 June, 2023.
Copy Citation
Previous studies have identified the functional roles of CD30, a member of the tumor necrosis factor receptor superfamily, in CD30-expressing malignant lymphomas, including Hodgkin lymphoma (HL), anaplastic large cell lymphoma (ALCL), and a portion of peripheral T-cell lymphoma (PTCL), adult T-cell leukemia/lymphoma (ATL), and diffuse large B-cell lymphoma (DLBCL).
TNFRSF
TNFSF
CD30 (TNFRSF8)
Trogocytosis
1. Introduction
Members of the tumor necrosis factor receptor superfamily (TNFRSF) and TNF superfamily (TNFSF) play crucial roles in both innate and adaptive immunity. It has been suggested that the divergence of the TNFRSF and TNFSF families paralleled the emergence of the adaptive immune system, at least through en bloc duplication [1]. As the cloning of the first prototypic members TNF itself and lymphotoxin α, 19 additional TNFSF members and 29 cognate receptors have been identified [2]. In addition to their roles in many important biological processes (development, organogenesis, immunity, cell death, and survival), TNFRSF and TNFSF members are implicated in various acquired or genetic human diseases, ranging from septic shock to autoimmune disorders, allograft rejection, and cancer.
The group of TNFRSF2, 4, 8, 9, 12, 14, and 18 is located on chromosome 1p36.2–36.3, most of which have cognate ligands in TNFSF subfamilies. The biological functions of the group involve T-cell activation, T-cell homeostasis, and T-cell survival [3]. These observations support a co-evolution perspective of TNFRSF and TNFSF families in the immune system. Malignant lymphoma cells express, depending on their immunophenotype, several TNF receptor and ligand superfamily members. B-cell NHLs frequently express CD27/CD27L (TNFRSF7/TNFSF7), CD30 or CD30L (TNFRSF8 or TNFSF8), CD40 (TNFRSF5), and TNFRs/TNF positive (TNFRSF1 and 2/TNFSF2), but T-cell NHLs show expression of CD30, CD40L (TNFSF5), and TNFRs/TNF [4].
CD30, a member of TNFRSF, is a type I single transmembrane protein consisting of 595 amino acids, whose molecular weight is 105–120 kDa [5]. CD30 was initially found to be strongly expressed on Hodgkin and Reed–Sternberg (H-RS) cells of classical Hodgkin lymphoma (cHL) using an anti-Ki-1 antibody. Ki-1, the first anti-CD30 monoclonal antibody, was raised against the cHL patient-derived cell line L428 and observed to react uniquely with primary and cultured H-RS cells [6]. However, soon after, it was found that CD30 is also strongly expressed in a rare subtype of NHL, called anaplastic large cell lymphoma (ALCL) [7]. Later studies showed the expression of CD30 in other pathological conditions, such as viral infection. CD30 expression is often observed in cells infected with HTLV-1, human immunodeficiency virus type 1 (HIV-1), and Epstein–Barr virus (EBV). However, its expression is limited, and normal cells of lymphoid lineage express CD30 only in some activated lymphocytes; furthermore, their expression level is not as high as that of HL cells and ALCL cells. These observations suggest that CD30 is an activation-associated antigen [8][9]. Normal cells of non-lymphoid lineage express CD30 only in uterine decidual cells.
CD30 is strongly expressed in some malignant lymphomas, and its overexpression characterizes cHL and ALCL. Antibody-drug conjugates (ADC) targeting CD30, such as brentuximab vedotin (BV), have shown striking clinical efficiency in cHL and ALCL. CD30 also overexpresses in a portion of peripheral T-cell lymphoma (PTCL), adult T-cell leukemia/lymphoma (ATL), NK lymphoma, and diffuse large B-cell lymphoma (DLBCL), in which clinical studies of BV therapy have been examined.
ATL is a poor prognosis T-cell malignancy that is caused by human T-cell leukemia virus type I (HTLV-1) infection. ATL develops after about 50 years of clinical latency in 2–5% of HTLV-1 carriers [10]; it is estimated that 5–10 million carriers exit worldwide [11]. ATL is endemic in several regions of the world, in particular south-western Japan, the Caribbean basin, and parts of central Africa. Transformation of HTLV-1–infected T cells in vivo is a multistage process, which reflects the status of HTLV-1 infection, i.e., asymptomatic state, smoldering, chronic, lymphoma, or acute type of ATL [12]. The median survival times for each ATL type in the Japanese nationwide, multicenter, hospital-based study are 1815, 778, 305, and 252 days, respectively [13]. The emergence of malignant cells with polylobulated nuclei, (the typical appearance is termed “flower cells”) characterizes ATL. ATL cells show monoclonal integration of HTLV-1 and mainly express CD4, CADM1, CCR4, CD25, and CD45RO, but usually lack CD7 and frequently downregulate CD3 expression. ATL cells acquire abnormalities in the genome, epigenetics, gene expression, and signal transduction.
2. The Functions of CD30
2.1. CD30L: TNFSF8
The ligand for CD30 (CD30L), also known as CD153, a member of the tumor necrosis factor (TNF) superfamily, is a type II single transmembrane protein consisting of 234 amino acids, whose molecular weight is 26–40 kDa. CD30L was identified and cloned by Smith CA et al. using a soluble fusion protein consisting of the extracellular domain of human CD30 linked to the hinge, CH2, and CH3 domains of human immunoglobulin G1 heavy chain [14]. CD30L is expressed relatively broadly, including in granulocytes, macrophages, mast cells, and activated lymphocytes. In terms of pathological conditions, this protein is expressed in a subset of myeloid and lymphoid leukemias and in Burkitt lymphoma [9][15].
2.2. CD30 Signal Transduction
Stimulation of CD30-expressing cells by CD30L elicits various cellular signaling responses, including proliferation, survival, cytokine secretion, and cell death, depending on the cell type and the cellular differentiation state. These signals are triggered after CD30 binds to the trimeric CD30L expressed on the surface of surrounding cells. The ligation of CD30L to CD30 triggers the recruitment of intracellular adaptor proteins, such as TNFR-associated factor (TRAF) proteins, to the TRAF binding domain of the cytoplasmic tail of trimerized CD30, resulting in further modifications to downstream signaling molecules [9]. TRAF family members are critical signal transducers that relay signals between stimulus-sensing surface receptors and transcription regulators, ultimately altering gene expression [16]. Members of the TRAF family of proteins (TRAF1–6) have been initially identified as modulators of signaling cascades downstream of TNFRSF members through their adaptor function and/or E3 ubiquitin ligase activity [17]. TRAF1, 2, 3, and 5 bind to the cytoplasmic tail of CD30, and TRAFs induce the activation of the transcription factors of the NF-κB family (Figure 1A). NF-κB can be activated via two major pathways: the canonical and non-canonical signaling pathways. CD30 signaling induces the activation of both of these pathways [18]. The canonical NF-κB pathway is regulated by TAK1 kinase activation, which induces the ubiquitination and proteasomal degradation of IκB family members, resulting in the release and nuclear translocation of NF-κB1/p50–RelA/p65 and NF-κB1/p50–c-Rel dimers. On the other hand, the non-canonical NF-κB pathway depends on NF-κB-inducing kinase (NIK) activation. NIK can phosphorylate and activate IKKα, which, in turn, promotes p100 processing to generate NF-κB2/p52 and allows its nuclear translocation with RelB. It has been reported that the overexpression of CD30 induces CD30 signaling independent of CD30L [19].
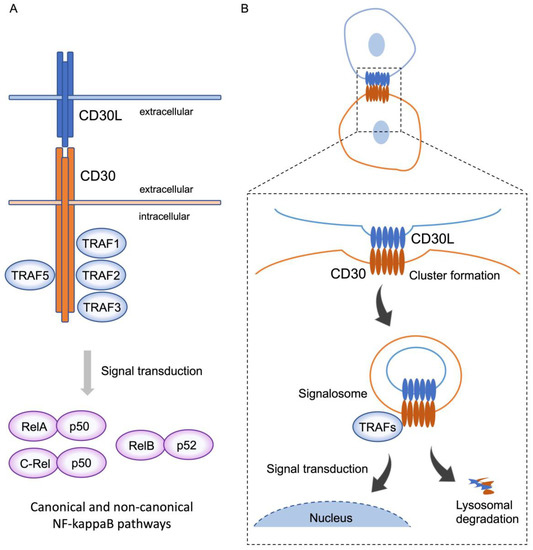
Figure 1. CD30 signaling via trogocytosis. (A) CD30 binds to trimerized CD30L, and, subsequently, recruits TRAF1, 2, 3, and 5 to the intracellular domain of CD30 molecules. Activation of TRAFs triggers downstream signal transduction and induces nuclear translocation of RelA-p50 and C-rel-p50, and RelB-p52, known as the canonical and non-canonical pathways, respectively. This signal transduction elicits various cellular signaling responses, including proliferation, survival, cytokine secretion, and cell death, depending on the cell type and the cellular differentiation state. (B) On the contact surface of CD30L and CD30-expressing cells, CD30L and CD30 form huge clusters, with CD30 extracting CD30L from the adjoining cell, along with part of their plasma membrane, triggering internalization of the clustered complex. These complexes simultaneously generate signalosomes, resulting in intracellular signaling, and are, subsequently, degraded in lysosomes. This phenomenon represents trogocytosis-mediated signal transduction.
2.3. CD30 Signal Transduction via Trogocytosis
Trogocytosis is a biological process whereby a cell (receiving cell) nibbles membrane fragments from another cell (donor cell), leading to the transfer of cell surface molecules along with membrane fragments. This phenomenon was first observed by Cone et al. over 50 years ago [20]. They noted the presence of allogeneic MHC class II (MHCII) molecules on adoptively transferred T cells. As this seminal observation, numerous researchers have shown that the T cell receptor (TCR) rapidly acquires MHC molecules from antigen-presenting cells (APCs) via the immunological synapse formed at the cell–cell contact area [21][22]. Several recent studies reported that trogocytosis of MHC class I (MHCI) and MHCII occurs not only between T cells and APCs, but also between various cell types, including APCs–natural killer (NK) cells; tumor cells–T; or NK cells; etc. [23][24], suggesting that the type of cell receiving such MHC may impact antigen-specific T cell activation.
References
- Collette, Y.; Gilles, A.; Pontarotti, P.; Olive, D. A co-evolution perspective of the TNFSF and TNFRSF families in the immune system. Trends Immunol. 2003, 24, 387–394.
- Vanamee, É.S.; Faustman, D.L. Structural principles of tumor necrosis factor superfamily signaling. Sci. Signal. 2018, 11.
- Ware, C.F. The TNF Superfamily-2008. Cytokine Growth Factor. Rev. 2008, 19, 183–186.
- Gruss, H.J.; Dower, S.K. Tumor necrosis factor ligand superfamily: Involvement in the pathology of malignant lymphomas. Blood 1995, 85, 3378–3404.
- Dürkop, H.; Latza, U.; Hummel, M.; Eitelbach, F.; Seed, B.; Stein, H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin’s disease. Cell 1992, 68, 421–427.
- Schwab, U.; Stein, H.; Gerdes, J.; Lemke, H.; Kirchner, H.; Schaadt, M.; Diehl, V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin’s disease and a subset of normal lymphoid cells. Nature 1982, 299, 65–67.
- Stein, H.; Mason, D.Y.; Gerdes, J.; O’Connor, N.; Wainscoat, J.; Pallesen, G.; Gatter, K.; Falini, B.; Delsol, G.; Lemke, H.; et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: Evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985, 66, 848–858.
- Croager, E.J.; Muir, T.M.; Abraham, L.J. Analysis of the human and mouse promoter region of the non-Hodgkin’s lymphoma-associated CD30 gene. J. Interferon Cytokine Res. 1998, 18, 915–920.
- Horie, R.; Watanabe, T. CD30: Expression and function in health and disease. Semin. Immunol. 1998, 10, 457–470.
- Iwanaga, M.; Watanabe, T.; Yamaguchi, K. Adult T-cell leukemia: A review of epidemiological evidence. Front. Microbiol. 2012, 3, 322.
- Gessain, A.; Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front. Microbiol. 2012, 3, 388.
- Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br. J. Haematol. 1991, 79, 428–437.
- Imaizumi, Y.; Iwanaga, M.; Nosaka, K.; Ishitsuka, K.; Ishizawa, K.; Ito, S.; Amano, M.; Ishida, T.; Uike, N.; Utsunomiya, A.; et al. Prognosis of patients with adult T-cell leukemia/lymphoma in Japan: A nationwide hospital-based study. Cancer Sci. 2020, 111, 4567–4580.
- Smith, C.A.; Gruss, H.J.; Davis, T.; Anderson, D.; Farrah, T.; Baker, E.; Sutherland, G.R.; Brannan, C.I.; Copeland, N.G.; Jenkins, N.A.; et al. CD30 antigen, a marker for Hodgkin’s lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell 1993, 73, 1349–1360.
- Croager, E.J.; Abraham, L.J. Characterisation of the human CD30 ligand gene structure. Biochim. Biophys. Acta 1997, 1353, 231–235.
- Pedros, C.; Altman, A.; Kong, K.F. Role of TRAFs in Signaling Pathways Controlling T Follicular Helper Cell Differentiation and T Cell-Dependent Antibody Responses. Front. Immunol. 2018, 9, 2412.
- Xie, P. TRAF molecules in cell signaling and in human diseases. J. Mol. Signal. 2013, 8, 7.
- Wright, C.W.; Rumble, J.M.; Duckett, C.S. CD30 activates both the canonical and alternative NF-kappaB pathways in anaplastic large cell lymphoma cells. J. Biol. Chem. 2007, 282, 10252–10262.
- Horie, R.; Watanabe, T.; Morishita, Y.; Ito, K.; Ishida, T.; Kanegae, Y.; Saito, I.; Higashihara, M.; Mori, S.; Kadin, M.E.; et al. Ligand-independent signaling by overexpressed CD30 drives NF-kappaB activation in Hodgkin-Reed-Sternberg cells. Oncogene 2002, 21, 2493–2503.
- Cone, R.E.; Sprent, J.; Marchalonis, J.J. Antigen-binding specificity of isolated cell-surface immunoglobulin from thymus cells activated to histocompatibility antigens. Proc. Natl. Acad. Sci. USA 1972, 69, 2556–2560.
- Huang, J.F.; Yang, Y.; Sepulveda, H.; Shi, W.; Hwang, I.; Peterson, P.A.; Jackson, M.R.; Sprent, J.; Cai, Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science 1999, 286, 952–954.
- Hwang, I.; Huang, J.F.; Kishimoto, H.; Brunmark, A.; Peterson, P.A.; Jackson, M.R.; Surh, C.D.; Cai, Z.; Sprent, J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J. Exp. Med. 2000, 191, 1137–1148.
- Roda-Navarro, P.; Reyburn, H.T. Intercellular protein transfer at the NK cell immune synapse: Mechanisms and physiological significance. FASEB J. 2007, 21, 1636–1646.
- Dhainaut, M.; Moser, M. Regulation of immune reactivity by intercellular transfer. Front. Immunol. 2014, 5, 112.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
644
Revisions:
2 times
(View History)
Update Date:
12 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





