Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Emilia Trif | -- | 2365 | 2023-06-08 19:38:11 | | | |
| 2 | Conner Chen | Meta information modification | 2365 | 2023-06-12 05:17:14 | | | | |
| 3 | Conner Chen | -41 word(s) | 2324 | 2023-07-10 05:29:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Trif, E.; Cerbu, C.; Olah, D.; Zăblău, S.D.; Spînu, M.; Potârniche, A.V.; Pall, E.; Brudașcă, F. Medical Uses of FFC Based on Targeted Species. Encyclopedia. Available online: https://encyclopedia.pub/entry/45363 (accessed on 07 February 2026).
Trif E, Cerbu C, Olah D, Zăblău SD, Spînu M, Potârniche AV, et al. Medical Uses of FFC Based on Targeted Species. Encyclopedia. Available at: https://encyclopedia.pub/entry/45363. Accessed February 07, 2026.
Trif, Emilia, Constantin Cerbu, Diana Olah, Sergiu Dan Zăblău, Marina Spînu, Adrian Valentin Potârniche, Emoke Pall, Florinel Brudașcă. "Medical Uses of FFC Based on Targeted Species" Encyclopedia, https://encyclopedia.pub/entry/45363 (accessed February 07, 2026).
Trif, E., Cerbu, C., Olah, D., Zăblău, S.D., Spînu, M., Potârniche, A.V., Pall, E., & Brudașcă, F. (2023, June 08). Medical Uses of FFC Based on Targeted Species. In Encyclopedia. https://encyclopedia.pub/entry/45363
Trif, Emilia, et al. "Medical Uses of FFC Based on Targeted Species." Encyclopedia. Web. 08 June, 2023.
Copy Citation
Florfenicol is a bacteriostatic antibiotic that is primarily used in veterinary medicine to treat a range of diseases in farm and aquatic animals. This synthetic analog of thiamphenicol and chloramphenicol works by inhibiting ribosomal activity, thereby disrupting bacterial protein synthesis, and has been proven in its effectiveness against a variety of Gram-positive and Gram-negative bacterial groups. Additionally, florfenicol has been found to possess anti-inflammatory properties and reduce immune cell proliferation and cytokine production.
florfenicol
alternative drug delivery
antibiotic-loaded nanoparticles
1. Introduction
Florfenicol (d-(threo)-1-(methylsulphoylphenyl)2-dichloroacetamide-3-floro-1-propanol) (FFC) is a synthetic antibiotic [1] included in the class of amphenicols (along with chloramphenicol, thiamphenicol, and azidamfenicol), with placement made by their phenylpropanoid structure [2]. FFC is the only antibiotic from the abovementioned class that was exclusively designed for veterinary therapeutics since it complements the shortcomings of chloramfenicol: an antimicrobial that lost its approval because of its toxic secondary effects on humans through food products of animal origin [3]. It is an antibiotic with a bacteriostatic action, provided by its binding to the bacterial 50S ribosomal subunits [2] and inhibiting the peptidyl transferase, which is an enzyme indispensable for protein synthesis [4]. FFC (chemical structure illustrated in Figure 1) is a derivative analog of chloramphenicol and thiamphenicol that presents only an active D-threo stereoisomer with an antimicrobial effect [5].
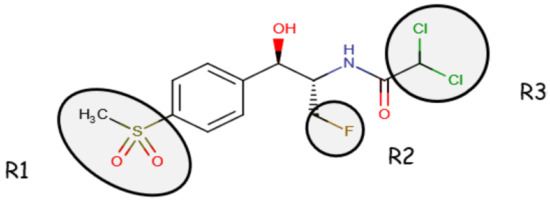
Figure 1. Chemical structure of FF (compared to Chloramphenicol, R2- hydroxyl group replaced by fluorine atom and R1-p-nitro group and the sulfomethyl group [2]) (Source for the modified figure: National Center for Biotechnology Information (2023). Pub Chem Compound Summary for CID 156406, Florfenicol amine).
FFC is documented as effective against a large group of pathogenic bacteria, both aerobic and anaerobic, including Gram-positive and Gram-negative [6], and also against certain types of ryckettsia and chlamydia [7]. Its effects have lead to the development of medical applications for livestock, which are to be detailed further. Additionally, FFC has been demonstrated to possess an anti-inflammatory effect by inhibiting the NK-kB pathway in vitro [8]. Even though FFC complements the shortcomings of the antimicrobial substance from which it originates (one of the most important is that is not incriminated to the same extent as the occurrence of aplastic anemia [9]), it still presents certain limitations that could eventually led to the necessity of finding a replacement. Although florfenicol can be classified as a relatively new antibiotic (first reported use in 1990 in Japan) [7], its widespread use for both metaphylactic and prophylactic purposes [10] and as a growth promoter in aquaculture [11] has led to the premature development of resistance genes. This phenomenon affects both veterinary and human medicine, as bacteria with florfenicol-resistant genes have been identified among the human population, despite the fact that it is exclusively used in veterinary medicine [12]. This underscores once again the importance of rationally using antimicrobial substances in veterinary medicine [13], as this consequence affects all biomedical fields. The improper use of this antibiotic, such as underdosing, overdosing, or unjustified administration, may lead to an inability to obtain an appropriate therapeutic response in the future. Not only does the development of resistance over the time limit the usefulness of florfenicol in therapeutic applications of veterinary medicine, but its low solubility in water also presents a challenge [14]. This property makes it difficult to distribute this antibiotic in a stable and efficient form for oral administration, as it requires a prior process of solubilization in organic solvents [15]. In addition, florfenicol has been identified as having certain immunosuppressive potential through a significant reduction in immune cell proliferation: an effect that can have serious clinical and economic consequences [8]. For example, it can lead to a decreased humoral immune response in livestock (animals following vaccination), as well as a dose-dependent harmful effect on the reproductive system, especially in birds [16]. Despite all these considerations, florfenicol is still considered an effective antibiotic and is recommended for use in veterinary medicine. However, there is a need to improve its pharmacokinetics, pharmacodynamics, and routes of administration in order to increase its efficacy. Therefore, various biomedical techniques have been developed to address these issues for florfenicol as well as other antibiotics [17][18], through the application of nanotechnology, in order to obtain nanoscale applications for FFC. According to the Encyclopedia of Pharmaceutical Technology, nanostructures can be defined as solid colloidal particles that range in size from 1 to 1000 nm and serve as drug carriers, containing an active ingredient in a dissolved, entrapped, or encapsulated form, to which the active ingredient can be adsorbed or attached [19]. Novel forms of administering FFC, such as nanoemulsions [14] and polymeric nanoparticles made of natural polymers such as chitosan [20] or synthetic polymers such as PLGA [9], show great potential for enhancing the efficacy of this antibiotic due to their unique properties such as controlled release, stability, and targeting ability. For instance, polymeric nanoparticles based on PLGA are able to offer a sustained release of FFC and provide targeted delivery to specific cells or tissues, while nanoemulsions can improve the solubility and bioavailability of FFC. However, these novel delivery systems also present certain limitations and drawbacks, such as potential toxicity or challenges in achieving an optimal particle size and stability. These factors must be carefully considered and addressed in order to fully exploit the potential of nanotechnology for the improvement of the therapeutic use of FFC.
2. Classification of Medical Uses of FFC Based on Targeted Species
2.1. FFC Use in Companion Animals
In dogs and cats, florfenicol is frequently used for the treatment of dermatological conditions of bacterial etiology, such as external otitis (a pathology not to be neglected since it represents the third most common diagnosis in companion animals [21]). Randomized clinical trials confirmed the efficacy of the topical application of florfenicol combined with terbinafine (through the available commercial form- topical gel) in this pathology [22][23]. Research articles, both from 2008 [24] and 2015 [1], conclude that florfenicol could represent a useful therapeutical option for other bacterial infections in dogs, but this clinical approach should be made carefully since there are no available studies regarding FF efficacy and its possible toxicity [1][25].
2.2. FFC Use in Rabbits
FFC is known to be effective against various digestive and respiratory tract infections [26], including blocking the growth of Streptococcus agalactiae, a pathogen that causes severe and antibiotic-resistant infectious diseases in domestic rabbits [27][28]. Additionally, since the simultaneous use of coccidiostats and antibiotics is a common practice in rabbit care, both for preventing and treating parasitic and infectious diseases, FFC can be associated with three different coccidiostats, but in this documented case, its elimination was reported as strongly accelerated [29].
2.3. FFC Use in Ruminants
FFC is mainly used for large ruminants in the treatment of bovine respiratory diseases caused by etiological agents such as Pasteurella multocida, Mannheimia haemolytica, or Histophilus somni [6], which are included in the undifferentiated fever syndrome of the bovine: a syndrome that is frequently associated with other pathologies such as BVD (bovine viral diarrhea) [30]. Even though there are other therapeutical alternatives for this syndrome, FFC was presented as the most preferable option for treatment when comparing the advantages versus costs [31]. Regarding its pharmacokinetics, previous data state that FFC presents good bioavailability when administered parenterally and orally (except when given as a milk replacer). This concludes that a treatment protocol that includes one or two administrations per day (depending on the MIC of the involved pathological agent) could be very effective [32].
In cattle, florfenicol products are also intended for therapeutic use in acute interdigital necrobacillosis, presenting good antimicrobial activity against Fusobacterium necrophorum and B. melaninogenicus [33], but also in the case of keratoconjunctivitis produced by Moraxella bovis [6]. Despite the fact that neither florfenicol nor chloramfenicol was approved for digestive tract infections produced by bacteria from the Escherichia genus, resistance genes were found in clinical isolates from cattle with diarrheal syndrome [34], highlighting the rapid emergence of bacterial resistance against the amphenicols antimicrobial group. To consolidate this hypothesis, as a result of a study performed on a commercial farm, it was concluded that even though the administration of florfenicol in dairy calves did not significantly affect the soil microbiome, by direct contact with fecal matter, grazing, and other farming activities, it led to an overall rise in the antibiotic resistance genes present at this level (resistome). Many of these genes have the potential to be transferred, increasing the risk of the soil-animal and later on animal–human transmissions in antibiotic-resistant genes [35].
Although FFC is a broad-spectrum antibiotic approved by the Food and Drug Administration (FDA) for use in cattle, swine, and fish and by the European Medicines Agency (EMA) for use in cattle, sheep, and fish, maximum residue limits have been extrapolated in all species that produce food for human consumption, including goats, due to the limited number of products available for therapeutic approaches in this species [36]. Although, by extrapolation, florfenicol is approved by the FDA and EMA for use both in large and small ruminants, neither agency has approved its use in lactating animals, even though there are no tolerance values allowed for milk, and its use in the mentioned situations was conducted outside of the manufacturer recommendations made (extra-label/off-label) [36][37][38].
Based on the clinical score and complete remission, as well as the laboratory results obtained from performing antibiograms, FFC can be a good choice when treating respiratory infections in small ruminants caused by Mannheimia haemolytica [39]. Additionally, a study was identified that tested the efficacy of florfenicol in its therapeutic approach to caseous lymphadenitis in sheep and goats caused by Corynebacterium pseudotuberculosis. A comparison of the control groups and those who received florfenicol therapy showed an improvement in clinical scores, suggesting the effective treatment and maintenance of remission in caseous abscesses [39]. Considering these findings, as well as the clinical and bacteriological results, FFC may be effective in combating this pathology, but more studies are needed in order to confirm this [39]. Given the intracellular localization of the bacteria responsible for caseous lymphadenitis and the formation of biofilm in natural infections [40], which reduces the effectiveness of drugs, the successful use of florfenicol in alleviating this pathology and the clinical recovery represents aspects that can counteract the costs involved, make this antimicrobial beneficial for disease management at the herd level [39].
2.4. FFC Use in Equine
Even though it complements the limitations of chloramfenicol (estimated half-life of 1 h and consecutively the need for multiple dose administration for 24 h in order to achieve an optimal plasma concentration) [41], FFC is not the first option when needing an antimicrobial agent in equine species. Adverse reactions such as modifications in stool consistency and modified smells (signs that can indicate incipient enterocolitis) [42] and also modified biochemical parameters (increased bilirubin levels in plasma) have been documented. Additional safety tests were considered necessary concerning the use of florfenicol in equidae, but since the study was published in 1996 [41], no additional data were identified.
2.5. FFC Use in Swine
In pigs, FFC is used for the treatment of bacterial respiratory diseases caused by agents such as Actinobacillus pleuropneumoniae, Pasteurella multocida, Bordetella bronchiseptica, and Salmonella choleraesuis [6][43]. Although it has high efficacy against these pathogens, FF should be used with caution in this species as it can cause changes in the intestinal microbiome, promoting the emergence of resistance genes (plasmid-mediated resistance or cross-resistance) [44]. Additionally, the synergistic effect of florfenicol and macrolide antimicrobials (such as tilmicosin) has been identified for the treatment of respiratory diseases in pigs [45]. A recent study presented florfenicol to be a reliable option for the treatment of arthritis in pigs caused by Streptococcus suis, but with dose adjustment dependent on the minimum inhibitory concentration for the specific pathogen determined beforehand (the study showed favorable results with an initial dose of 30 mg/kg body weight), followed by maintenance doses of 15 mg/kg [46].
2.6. FFC Use in Aviary Medicine
FFC is an antibacterial medication that is used quite frequently in bird pathology, but due to its low solubility in water, administering it in drinking water can lead to a considerable variation in the concentration of the active substance among treated individuals [15]. However, administering an individual injectable preparation can have a negative effect on carcass quality. In addition, the porto-renal system in birds can reduce the bioavailability of this antimicrobial when administered in the caudofemoral region of the body [15]. Moreover, it has been used over time for prophylactic purposes in order to prevent the gastrointestinal and respiratory infections caused by microorganisms that are sensitive to florfenicol, such as Ornithobacterium rhinotracheale, Mannheimia haemolytica, Salmonella typhi, Pasteurella multocida, Enterobacter cloacae, Escherichia coli, Haemophilus somnus, Klebsiella pneumoniae, Shigella dysenteriae, and Staphylococcus aureus [47][48][49][50]. Following the existence of a suspicion, pharmacovigilance studies were carried out consisting of a five-day treatment at a dose of 10 mg/kg (both males and females treated metaphylactically) to manage Escherichia coli infection [16]. The test revealed a severe decrease in egg hatching, resulting in embryonic death, as a result of using the antimicrobial substance outside the manufactures’ recommendations. It was thus recommended to limit its use in breeding flocks, except in special situations where the value of the breeding flock indicated that their clinical condition was more important than a decrease in egg fertility.
2.7. FFC Use in Aquaculture
Data from previous pharmacokinetic and in vitro sensitivity studies have shown florfenicol to be a good alternative to the most commonly used antimicrobial agents when treating bacterial infections in fish [51][52][53] since its first approval for use in aquaculture in the United States in 2000 [54]. Florfenicol can be used specifically to combat bacterial pathologies in rainbow trout caused by Flavobacterium psychrophilum [55]. Florfenicol has proven effective in treating furunculosis: a severe disease in salmonids caused by Aeromonas salmonicida [56]. Additionally, studies have demonstrated its superiority over thiamphenicol, chloramphenicol, and oxytetracycline in experimental infections with Pasteurella piscicida in carp, Edwardsiella tarda in Japanese perch, and Vibrio anguillarum in certain species of carp [53].
The effectiveness of florfenicol when treating sea bass pathologies caused by bacterial agents such as Flavobacterium columnare has subsequently been determined, significantly reducing mortality without producing the macroscopic lesions associated with florfenicol treatment [52].
2.8. Available Formulations for Clinical Use
The following table provides an overview of florfenicol use in veterinary medicine, classified by targeted species and the availability of commercial products, including their indications. It is important to note that the availability of commercial products may vary over time and across regions.
References
- Birdane, Y.O.; Birdane, F.M. Pharmacokinetics of Florfenicol Following Intravenous and Intramuscular Administration in Dogs. Vet. Med. 2015, 60, 323–329.
- Wei, C.F.; Shien, J.H.; Chang, S.K.; Chou, C.C. Florfenicol as a Modulator Enhancing Antimicrobial Activity: Example Using Combination with Thiamphenicol against Pasteurella multocida. Front. Microbiol. 2016, 7, 389.
- Picco, E.J.; Díaz, D.C.; Valtorta, S.E.; Boggio, J.C. Chronotoxicology of Florfenicol. Chronobiol. Int. 2001, 18, 567–572.
- Hayes, J.M.; Eichman, J.; Katz, T.; Gilewicz, R. Stability of Florfenicol in Drinking Water. J. AOAC Int. 2003, 86, 22–29.
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular Basis of Bacterial Resistance to Chloramphenicol and Florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542.
- Papich, M.G. Florfenicol. In Saunders Handbook of Veterinary Drugs; WB Saunders: Philadelphia, PA, USA, 2016; pp. 327–329.
- Li, P.; Zhu, T.; Zhou, D.; Lu, W.; Liu, H. Analysis of Resistance to Florfenicol and the Related Mechanism of Dissemination in Different Animal-Derived Bacteria. Front. Cell. Infect. Microbiol. 2020, 10, 369.
- Shuang, G.; Yu, S.; Weixiao, G.; Dacheng, W.; Zhichao, Z.; Jing, L.; Xuming, D. Immunosuppressive Activity of Florfenicol on the Immune Responses in Mice. Immunol. Investig. 2011, 40, 356–366.
- Karp, F.; Busatto, C.; Turino, L.; Luna, J.; Estenoz, D. PLGA Nano- and Microparticles for the Controlled Release of Florfenicol: Experimental and Theoretical Study. J. Appl. Polym. Sci. 2019, 136, 47248.
- González-Martín, J.V.; Elvira, L.; Cerviño López, M.; Pérez Villalobos, N.; Calvo López-Guerrero, E.; Astiz, S. Reducing Antibiotic Use: Selective Metaphylaxis with Florfenicol in Commercial Feedlots. Livest. Sci. 2011, 141, 173–181.
- Reda, R.M.; Ibrahim, R.E.; Ahmed, E.N.G.; El-Bouhy, Z.M. Effect of Oxytetracycline and Florfenicol as Growth Promoters on the Health Status of Cultured Oreochromis Niloticus. Egypt J. Aquat. Res. 2013, 39, 241–248.
- Fernández-Alarcón, C.; Singer, R.S.; Johnson, T.J. Comparative Genomics of Multidrug Resistance-Encoding IncA/C Plasmids from Commensal and Pathogenic Escherichia Coli from Multiple Animal Sources. PLoS ONE 2011, 6, e23415.
- Páll, E.; Niculae, M.; Brudașcă, G.F.; Ravilov, R.K.; Șandru, C.D.; Cerbu, C.; Olah, D.; Zăblău, S.; Potârniche, A.V.; Spinu, M.; et al. Assessment and Antibiotic Resistance Profiling in Vibrio Species Isolated from Wild Birds Captured in Danube Delta Biosphere Reserve, Romania. Antibiotics 2021, 10, 333.
- Zhang, Q.; Tang, S.S.; Qian, M.Y.; Wei, L.; Zhou, D.; Zhang, Z.J.; He, J.K.; Zhang, Q.J.; Zhu, P.; Xiao, X.L. Nanoemulsion Formulation of Florfenicol Improves Bioavailability in Pigs. J. Vet. Pharmacol. Ther. 2016, 39, 84–89.
- Bello, A.; Poźniak, B.; Smutkiewicz, A.; Świtała, M. The Influence of the Site of Drug Administration on Florfenicol Pharmacokinetics in Turkeys. Poult. Sci. 2022, 101, 536.
- AL-Shahrani, S.; Naidoo, V. Florfenicol Induces Early Embryonic Death in Eggs Collected from Treated Hens. BMC Vet. Res. 2015, 11, 213.
- Cerbu, C.; Kah, M.; White, J.C.; Astete, C.E.; Sabliov, C.M. Fate of Biodegradable Engineered Nanoparticles Used in Veterinary Medicine as Delivery Systems from a One Health Perspective. Molecules 2021, 26, 523.
- Paudel, S.; Cerbu, C.; Astete, C.E.; Louie, S.M.; Sabliov, C.; Rodrigues, D.F. Enrofloxacin-Impregnated PLGA Nanocarriers for Efficient Therapeutics and Diminished Generation of Reactive Oxygen Species. ACS Appl. Nano Mater. 2019, 2, 5035–5043.
- Devadasu, V.R.; Bhardwaj, V.; Kumar, M.N.V.R. Can Controversial Nanotechnology Promise Drug Delivery? Chem. Rev. 2013, 113, 1686–1735.
- Carmona, E.R.; Plaza, T.; Recio-Sánchez, G.; Parodi, J. Generation of a Protocol for the Synthesis of Chitosan Nanoparticles Loaded with Florfenicol through the Ionic Gelation Method. Rev. De Investig. Vet. Del Peru 2018, 29, 1195–1202.
- August, J.R. Otitis Externa. A Disease of Multifactorial Etiology. Vet. Clin. N. Am. Small Anim. Pract. 1988, 18, 731–742.
- King, S.B.; Doucette, K.P.; Seewald, W.; Forster, S.L. A Randomized, Controlled, Single-Blinded, Multicenter Evaluation of the Efficacy and Safety of a Once Weekly Two Dose Otic Gel Containing Florfenicol, Terbinafine and Betamethasone Administered for the Treatment of Canine Otitis Externa. BMC Vet. Res. 2018, 14, 307.
- Forster, S.L.; Real, T.; Doucette, K.P.; King, S.B. A Randomized Placebo-Controlled Trial of the Efficacy and Safety of a Terbinafine, Florfenicol and Betamethasone Topical Ear Formulation in Dogs for the Treatment of Bacterial and/or Fungal Otitis Externa. BMC Vet. Res. 2018, 14, 262.
- Park, B.K.; Lim, J.H.; Kim, M.S.; Hwang, Y.H.; Yun, H.I. Pharmacokinetics of Florfenicol and Its Metabolite, Florfenicol Amine, in Dogs. Res. Vet. Sci. 2008, 84, 85–89.
- Tameirao, E.R.; Soares, B.C.F.; Toma, H.S.; Wosiacki, S.R.; Ferrante, M. Eficacia de florfenicol para el tratamiento de pioderma por Staphylococcus intermedius en perros. Rev. Investig. Vet. Perú 2021, 32, e17678.
- Farag, V.M.; El-Shafei, R.A.; Elkenany, R.M.; Ali, H.S.; Eladl, A.H. Antimicrobial, Immunological and Biochemical Effects of Florfenicol and Garlic (Allium sativum) on Rabbits Infected with Escherichia coli Serotype O55: H7. Vet. Res. Commun. 2022, 46, 363–376.
- Ren, S.Y.; Geng, Y.; Wang, K.Y.; Zhou, Z.Y.; Liu, X.X.; He, M.; Peng, X.; Wu, C.Y.; Lai, W.M. Streptococcus Agalactiae Infection in Domestic Rabbits, Oryctolagus cuniculus. Transbound. Emerg. Dis. 2014, 61, e92–e95.
- Abd El-Aty, A.M.; Goudah, A.; Abo El-Sooud, K.; El-Zorba, H.Y.; Shimoda, M.; Zhou, H. Pharmacokinetics and Bioavailability of Florfenicol Following Intravenous, Intramuscular and Oral Administration in Rabbits. Vet. Res. Commun. 2004, 28, 515–524.
- Liu, C.; Wang, S.-J.; Zhang, Q.; Shao, Y.-X. Influence of Three Coccidiostats on the Pharmacokinetics of Florfenicol in Rabbits. Exp. Anim. 2015, 64, 73–79.
- Jain, D.; Banerjee, R. Comparison of Ciprofloxacin Hydrochloride-Loaded Protein, Lipid, and Chitosan Nanoparticles for Drug Delivery. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 105–112.
- Perrett, T.; Abutarbush, S.M.; Fuchs, M.T.; Schunicht, O.C.; Pollock, C.M.; Fenton, R.K.; Jim, G.K.; Guichon, P.T.; Booker, C.W. A Comparison of Florfenicol and Tulathromycin for the Treatment of Undifferentiated Fever in Feedlot Calves. Vet. Ther. 2008, 9, 128–140.
- Varma, K.J.; Adams, P.E.; Powers, T.E.; Powers, J.D.; Lamendolat, J.F.; Lamendola, J.D.; Pharmacokinetics, J.F. Pharmacokinetics of Florfenicol in Veal Calves. J. Vet. Pharmacol. Ther. 1986, 9, 412–425.
- Apley, M.D. Clinical Evidence for Individual Animal Therapy for Papillomatous Digital Dermatitis (Hairy Heel Wart) and Infectious Bovine Pododermatitis (Foot Rot). Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 81–95.
- White, D.G.; Hudson, C.; Maurer, J.J.; Ayers, S.; Zhao, S.; Lee, M.D.; Bolton, L.; Foley, T.; Sherwood, J. Characterization of Chloramphenicol and Florfenicol Resistance in Escherichia coli Associated with Bovine Diarrhea. J. Clin. Microbiol. 2000, 38, 4593–4598.
- Liu, J.; Yu, F.; Call, D.R.; Mills, D.A.; Zhang, A.; Zhao, Z. On-Farm Soil Resistome Is Modified after Treating Dairy Calves with the Antibiotic Florfenicol. Sci. Total Environ. 2021, 750, 141694.
- Richards, E.D.; Pereira, R.V.; Davis, J.L.; Rowe, J.D.; Clapham, M.O.; Wetzlich, S.E.; Rupchis, B.A.; Tell, L.A. Comparison of Florfenicol Depletion in Dairy Goat Milk Using Ultra-Performance Liquid Chromatography with Tandem Mass Spectrometry and a Commercial on-Farm Test. Front. Vet. Sci. 2022, 9, 1275.
- Soback, S.; Paape, M.J.; Filep, R.; Varma, K.J. Florfenicol Pharmacokinetics in Lactating Cows after Intravenous, Intramuscular and Intramammay Administration. J. Vet. Pharmacol. Ther. 1995, 18, 413–417.
- Shah, J.M.; Qureshi, T.A.; Arain, M.A.; Bhutto, Z.A.; Saeed, M.; Siyal, F.A. Impact of therapeutic and high doses of florfenicol on kidney and liver functional indicators in goat. Vet. World 2016, 9, 1135–1140.
- Ural, K.; Kirkan, Ş.; Ural, D.A.; Erbas, G.; Gültekin, M.; Parın, U.; Balıkçı, C. Florfenicol Therapy During Naturally Occuring Corynebacterium Pseudotuberculosis Infection in Sheep and Goats in Aydin, Turkey. Anim. Health Prod. Hyg. 2014, 3, 278–283.
- Batey, R.G. Pathogenesis of Caseous Lymphadenitis in Sheep and Goats. Aust. Vet. J. 1986, 63, 272–296.
- Mckellar, Q.A.; Varma, K.J. Pharmacokinetics and Tolerance of Florfenicol in Equidae. Equine Vet. J. 1996, 28, 209–213.
- Dowling, P.M.; Acvcp, A. Adverse Drug Reactions in Horses. Vet. Clin. N. Am. Equine Pract. 1987, 3, 153–179.
- Wang, Y.; Zhang, L.; Ahmed, S.; Liu, Y.; Li, X. Pharmacokinetic of Florfenicol in Pulmonary Epithelial Lining Fluid of Swine and Effects of Anesthetic Agent on Drug Plasma Disposition Kinetics. Arq. Bras. Med. Vet. Zootec. 2018, 70, 1497–1504.
- De Smet, J.; Boyen, F.; Croubels, S.; Rasschaert, G.; Haesebrouck, F.; De Backer, P.; Devreese, M. Similar Gastro-Intestinal Exposure to Florfenicol after Oral or Intramuscular Administration in Pigs, Leading to Resistance Selection in Commensal Escherichia coli. Front. Pharmacol. 2018, 9, 1265.
- Ling, Z.; Yonghong, L.; Junfeng, L.; Li, Z.; Xianqiang, L. Tilmicosin- and Florfenicol-Loaded Hydrogenated Castor Oil-Solid Lipid Nanoparticles to Pigs: Combined Antibacterial Activities and Pharmacokinetics. J. Vet. Pharmacol. Ther. 2018, 41, 307–313.
- Somogyi, Z.; Mag, P.; Kovács, D.; Kerek, Á.; Szabó, P.; Makrai, L.; Jerzsele, Á. Synovial and Systemic Pharmacokinetics of Florfenicol and PK/PD Integration against Streptococcus suis in Pigs. Pharmaceutics 2022, 14, 109.
- Abu-Basha, E.A.H.; Gehring, R.; Al-Shunnaq, A.F.; Gharaibeh, S.M. Pharmacokinetics and Bioequivalence of Florfenicol Oral Solution Formulations (Flonicol® and Veterin®10%) in Broiler Chickens. J. Bioequivalence Bioavailab. 2012, 4, 1–5.
- Marien, M.; Nauwynck, H.; Duchateau, L.; Martel, A.; Chiers, K.; Devriese, L.; Froyman, R.; Decostere, A. Comparison of the Efficacy of Four Antimicrobial Treatment Schemes against Experimental Ornithobacterium rhinotracheale Infection in Turkey Poults Pre-Infected with Avian Pneumovirus. Avian Pathol. 2006, 35, 230–237.
- Shin, S.J.; Kang, S.G.; Nabin, R.; Kang, M.L.; Yoo, H.S. Evaluation of the Antimicrobial Activity of Florfenicol against Bacteria Isolated from Bovine and Porcine Respiratory Disease. Vet. Microbiol. 2005, 106, 73–77.
- Tavakkoli, H.; Derakhshanfar, A.; Gooshki, S.N. The Effect of Florfenicol Egg-Injection on Embryonated Chicken Egg. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 496–503.
- Fukui, H.; Fujihara, Y.; Kano, T. In Vitro and in Vivo Antibacterial Activities of Florfenicol, a New Fluorinated Analog of Thiamphenicol, Against Fish Pathogens. Fish Pathol. 1987, 22, 201–207.
- Gaunt, P.S.; Gao, D.; Sun, F.; Endris, R. Efficacy of Florfenicol for Control of Mortality Caused by Flavobacterium Columnare Infection in Channel Catfish. J. Aquat. Anim. Health 2010, 22, 115–122.
- Samuelsen, O.B.; Hjeltnes, B.; Glette, J. Communications Efficacy of Orally Administered Florfenicol in the Treatment of Furunculosis in Atlantic Salmon. J. Aquat. Anim. Health 1998, 10, 56–61.
- Schmidt, A.S.; Bruun, M.S.; Dalsgaard, I.; Pedersen, K.; Larsen, J.L. Occurrence of Antimicrobial Resistance in Fish-Pathogenic and Environmental Bacteria Associated with Four Danish Rainbow Trout Farms. Appl. Environ. Microbiol. 2000, 66, 4908–4915.
- Michel, C.; Kerouault, B.; Martin, C. Chloramphenicol and Florfenicol Susceptibility of Fish-Pathogenic Bacteria Isolated in France: Comparison of Minimum Inhibitory Concentration, Using Recommended Provisory Standards for Fish Bacteria. J. Appl. Microbiol. 2003, 95, 1008–1015.
- InglisV, R.R.H. The in Vitro Susceptibility of Aeromonas Salmonicida and Other Fish-Pathogenic Bacteria to 29 Antimicrobial Agents. J. Fish Dis. 1991, 14, 641–650.
More
Information
Subjects:
Veterinary Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
10 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




