
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexander Lazzaro | -- | 4052 | 2023-06-08 18:33:44 | | | |
| 2 | Peter Tang | -121 word(s) | 3931 | 2023-06-09 05:10:45 | | |
Video Upload Options
Hepatocellular carcinoma (HCC) is an aggressive, primary malignant liver tumor. It is estimated that HCC accounts for approximately 85% of all primary liver tumors. HCC is a highly prevalent cancer worldwide and the incidence is growing due not only to alcoholism, hepatitis B and C, but also to steatohepatitis. HCC, like renal cell carcinoma and melanoma, is a cancer largely resistant to chemotherapy but the advent of anti-angiogenic, targeted and immune therapies have improved survival for all of these cancers.
1. Introduction
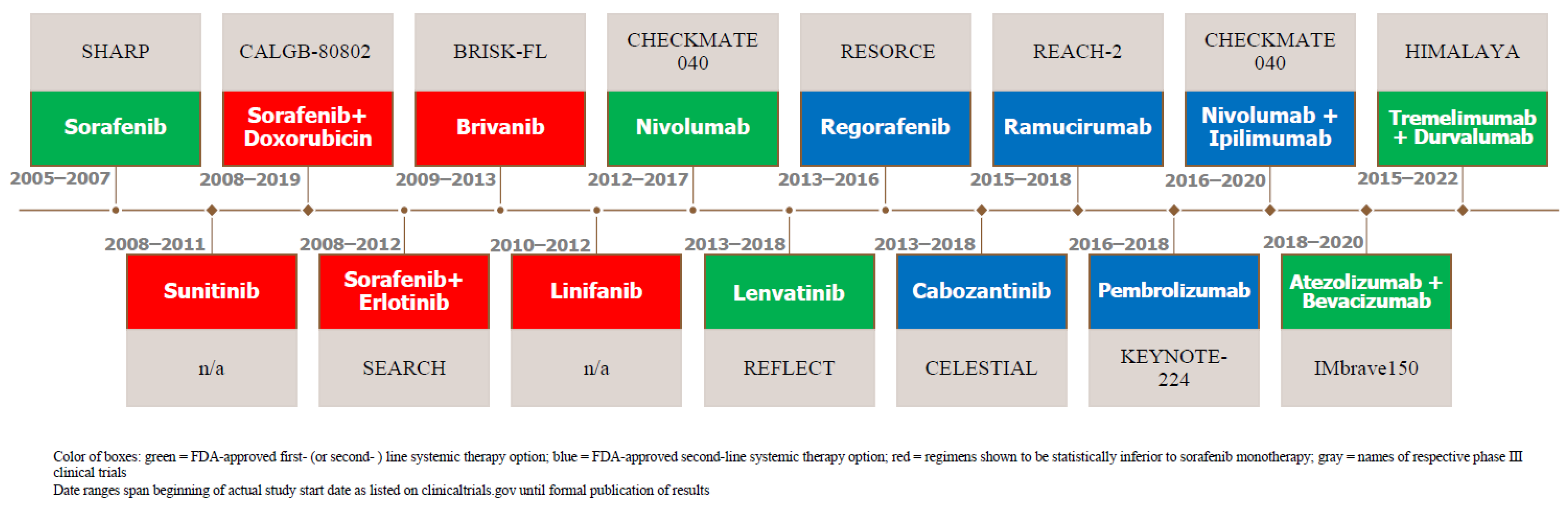
2. Systemic Therapy as First-Line Therapy
2.1. For HCC Patients with Preserved Liver Function and Functional Status
2.2. Sorafenib
2.3. Unsuccessful TKI Challengers of Sorafenib
2.4. Unsuccessful Combination Therapy: Sorafenib and Doxorubicin
2.5. Unsuccessful Combination Therapy: Sorafenib and an EGFR Inhibitor
2.6. Lenvatinib, the First Approved Alternative to Sorafenib
2.7. Bevacizumab and the Emergence of Anti-VEGF Monoclonal Antibodies
2.8. Immune Checkpoint Inhibitors (ICIs): Origin in Treatment for Advanced HCC
2.9. Combination Therapy: Atezolizumab and Bevacizumab
2.10. Combination Therapy: Sintilimab and Bevacizumab
2.11. Combination Therapy: Tremelimumab and Durvalumab
|
Regimen |
Trial Name |
Authors |
Year |
Study Arm |
# of Pts |
Primary Endpoint Results |
|
|---|---|---|---|---|---|---|---|
|
“Preferred Regimens:” |
Atezolizumab (Checkpoint inhibitor: anti-PD-L1 monoclonal Ab) + Bevacizumab (anti-VEGF-A monoclonal Ab) combination therapy |
IMbrave150 |
Finn et al. [44] |
2020 |
Atezolizumab + bevacizumab vs. sorafenib (first-line setting) |
501 |
OS: at 12 months, 67.2% (95% CI, 61.3 to 73.1) with atezolizumab—bevacizumab and 54.6% (95% CI, 45.2 to 64.0) with sorafenib. mPFS: 6.8 months (95% CI, 5.7 to 8.3) and 4.3 months (95% CI, 4.0 to 5.6) in the respective groups (hazard ratio for disease progression or death, 0.59; 95% CI, 0.47 to 0.76; p < 0.001). |
|
STRIDE (Tremelimumab (anti-CTLA-4 monoclonal Ab) + Durvalumab (checkpoint inhibitor: anti-PD-L1 monoclonal Ab)) combination therapy |
HIMALAYA |
Abou-Alfa et al. [47] |
2022 |
STRIDE vs. durvalumab vs. sorafenib (first-line setting) |
1171 |
OS: 16.4 months (14.2–19.6) w/STRIDE (single tremelimumab + regular interval durvalumab) and 13.8 months (12.3–16.1) with sorafenib (HR for death, 0.78; 96% CI, 0.65–0.92; p = 0.0035) |
|
|
“Other Recommended Regimens:” |
Sorafenib (TKI of VEGF-R1–3, PDGF beta and serine-threonine kinase inhibitor of Raf-1 and B-Raf) monotherapy |
SHARP |
Lovet et al. [1] |
2008 |
Sorafenib vs. placebo (first-line setting) |
602 |
OS: 10.7 months with sorafenib and 7.9 months with placebo (HR, 0.69; 95% CI, 0.55 to 0.87; p < 0.001) mPFS: 4.1 months vs. 4.9 months (p = 0.77) |
|
Lenvatinib (TKI of VEGF-R1–3, FGF receptors 1–4, PDGF receptor alpha, RET, KIT) monotherapy |
REFLECT |
Kudo et al. [21] |
2018 |
Lenvatinib vs. sorafenib (first-line setting) |
954 |
OS: 13.6 months (95% CI, 12.1–14.9) for lenvatinib was non-inferior to 12.3 months (10.4–13.9; HR, 0.92; 95% CI, 0.79–1.06) for sorafenib |
|
|
Durvalumab (checkpoint inhibitor: anti-PD-L1 monoclonal Ab) monotherapy |
HIMALAYA |
Abou-Alfa et al. [47] |
2022 |
STRIDE vs. durvalumab vs. sorafenib (first-line setting) |
1171 |
OS: 16.6 months for durvalumab monotherapy vs. 13.8 months for sorafenib monotherapy (HR, 0.86; 95% CI, 0.73–1.03). Judged to be non-inferior. |
|
|
Pembrolizumab (checkpoint inhibitor: anti-PD-1 Monoclonal Ab) monotherapy |
KEYNOTE-224, 2021 update of Cohort 2 |
Verset et al. [39] |
2022 |
Phase II study of pembrolizumab |
51 |
ORR: 16% (95% CI, 7–29) for pembrolizumab monotherapy |
|
|
“Useful in Certain Circumstances:” |
Nivolumab (checkpoint inhibitor: anti-PD-1 monoclonal Ab) monotherapy |
CheckMate 459 |
Yau et al. [40] |
2021 |
Nivolumab vs. sorafenib (first-line setting) |
743 |
OS: 16.4 months (95% CI 13.9–18.4) with nivolumab and 14.7 months (11.9–17.2) with sorafenib (HR, 0.85; 95% CI, 0.72–1.02; p = 0.075) |
2.12. Other Trials Combining TKIs and ICIs
3. Second-Line Systemic Therapies
|
Regimen |
Trial Name |
Authors |
Year |
Study Arm |
# of Pts |
Primary Endpoint Results |
|
|---|---|---|---|---|---|---|---|
|
“Preferred Regimens:” |
Regorafenib (a multikinase inhibitor targeting VEGFR 1–3, FGFR 1–2, angiopoietin-1 receptor (TIE2) and PDFRs alpha and beta) |
RESORCE |
Bruix et al. [51] |
2017 |
Regorafenib vs. placebo (second-line setting) |
573 |
OS: 10.6 months (95% CI 9.1–12.1) for regorafenib versus 7.8 months (6.3–8.8) for placebo (HR of 0.63; 95% CI, 0.50–0.79; one-sided p < 0·0001) |
|
Cabozantinib (a multikinase inhibitor (TKI) of kinases involved in tumor pathogenesis including VEGF, MET and the TAM family (TYRO3, AXL, MER) |
CELESTIAL |
Kelley et al. [52] |
2020 |
Cabozantinib vs. placebo (second-line setting) |
707 |
OS: Cabozantinib improved OS relative to placebo in the overall second-line population who had received only prior sorafenib (median 11.3 vs. 7.2 months; HR, 0.70; 95% CI, 0.55 to 0.88) |
|
|
Ramucirumab (humanized monoclonal antibody directed against VEGF 2) monotherapy |
REACH-2 |
Zhu et al. [53] |
2019 |
Ramucirumab vs. placebo (second-line setting) |
292 |
OS: At a median follow-up of 7.6 months (IQR 4.0–12.5), mOS was 8.5 months (95% CI 7.0–10.6) in the ramucirumab group vs. 7.3 months (5.4–9.1) in the placebo group (HR, 0.710; 95% CI, 0.531–0.949; p = 0.0199) |
|
|
Lenvatinib (TKI of VEGF-R1–3, FGF receptors 1–4, PDGF receptor alpha, RET, and KIT) monotherapy |
REFLECT |
Kudo et al. [21] |
2018 |
Lenvatinib vs. sorafenib (first-line setting) |
954 |
OS: 13.6 months (95% CI, 12.1–14.9) for Lenvatinib was non-inferior to 12.3 months (10.4–13.9; HR, 0.92; 95% CI 0.79–1.06) for sorafenib |
|
|
Sorafenib (TKI of VEGF-R1–3, PDGF beta and serine-threonine kinase inhibitor of Raf-1 and B-Raf) monotherapy |
SHARP |
Lovet et al. [1] |
2008 |
Sorafenib vs. placebo (first-line setting) |
602 |
OS: 10.7 months with sorafenib and 7.9 months with placebo; HR, 0.69; 95% CI, 0.55 to 0.87; p < 0.001 mPFS: 4.1 months vs. 4.9 months; p = 0.77 |
|
|
“Other Recommended Regimens:” |
Nivolumab (checkpoint inhibitor: anti-PD-1 monoclonal antibody) + Ipilimumab (anti-CTLA-4 humanized monoclonal antibody) |
CheckMate 040 |
Yau et al. [54] |
2020 |
Phase I/II, three-arm study of nivolumab and ipilimumab (second-line setting) |
148 |
ORR: 32% (95% CI, 20–47%) in arm A, 27% (95% CI, 15–41%) in arm B, and 29% (95% CI, 17–43%) in arm C, with the respective arms differing in quantity and time for the administration of drugs |
|
Pembrolizumab (checkpoint inhibitor: anti-PD-1 monoclonal antibody monotherapy |
Keynote-240 |
Finn et al. [55] |
2020 |
Pembrolizumab vs. placebo (second-line setting) |
413 |
OS: 13.9 months (95% CI, 11.6 to 16.0 months) for pembrolizumab versus 10.6 months (95% CI, 8.3 to 13.5 months) for placebo (HR, 0.781; 95% CI, 0.611 to 0.998; p = 0.0238). mPFS (with predefined one-sided significance thresholds; p = 0.0174 for final analysis) for pembrolizumab was 3.0 months (95% CI, 2.8 to 4.1 months) versus 2.8 months (95% CI, 1.6 to 3.0 months) at final analysis (HR, 0.718; 95% CI, 0.570 to 0.904; p = 0.0022). |
|
|
“Useful in Certain Circumstances:” |
Nivolumab monotherapy |
CheckMate 040 |
El-Khoueiry et al. [56] |
2017 |
Phase I/II dose escalation and expansion trial assessing safety and efficacy of nivolumab monotherapy |
262 |
ORR: 20% (95% CI 15–26) in patients treated with nivolumab 3 mg/kg in the dose-expansion phase and 15% (95% CI 6–28) in the dose-escalation phase |
|
Dostarlimab-gxly (humanized anti-PD-1 monoclonal antibody) monotherapy |
GARNET |
Andre et al. [57] |
N/a |
Phase I study evaluating safety of dostarlimab |
N/a |
N/a |
|
|
Selpercatinib (highly selective RET kinase inhibitor) monotherapy |
LIBRETTO-001 |
Subbiah et al. [58] |
2022 |
Phase ½ study evaluating safety and efficacy of selpercatinib in RET fusion-positive advanced solid non-lung or thyroid tumors |
N/a |
N/a |
References
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390.
- Lopez, P.M.; Villanueva, A.; Llovet, J.M. Systematic review: Evidence-based management of hepatocellular carcinoma—An updated analysis of randomized controlled trials. Aliment. Pharm. Ther. 2006, 23, 1535–1547.
- Hwang, J.P.; Feld, J.J.; Hammond, S.P.; Wang, S.H.; Alston-Johnson, D.E.; Cryer, D.R.; Hershman, D.L.; Loehrer, A.P.; Sabichi, A.L.; Symington, B.E.; et al. Hepatitis B Virus Screening and Management for Patients with Cancer Prior to Therapy: ASCO Provisional Clinical Opinion Update. J. Clin. Oncol. 2020, 38, 3698–3715.
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 52–60.
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res. 2004, 64, 7099–7109.
- Liu, L.; Cao, Y.; Chen, C.; Zhang, X.; McNabola, A.; Wilkie, D.; Wilhelm, S.; Lynch, M.; Carter, C. Sorafenib Blocks the RAF/MEK/ERK Pathway, Inhibits Tumor Angiogenesis, and Induces Tumor Cell Apoptosis in Hepatocellular Carcinoma Model PLC/PRF/5. Cancer Res. 2006, 66, 11851–11858.
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2008, 10, 25–34.
- Johnson, P.J.; Qin, S.; Park, J.-W.; Poon, R.T.; Raoul, J.-L.; Philip, P.A.; Hsu, C.-H.; Hu, T.-H.; Heo, J.; Xu, J.; et al. Brivanib Versus Sorafenib as First-Line Therapy in Patients With Unresectable, Advanced Hepatocellular Carcinoma: Results From the Randomized Phase III BRISK-FL Study. J. Clin. Oncol. 2013, 31, 3517–3524.
- Cheng, A.-L.; Kang, Y.-K.; Lin, D.-Y.; Park, J.-W.; Kudo, M.; Qin, S.; Chung, H.-C.; Song, X.; Xu, J.; Poggi, G.; et al. Sunitinib Versus Sorafenib in Advanced Hepatocellular Cancer: Results of a Randomized Phase III Trial. J. Clin. Oncol. 2013, 31, 4067–4075.
- Cainap, C.; Qin, S.; Huang, W.-T.; Chung, I.J.; Pan, H.; Cheng, Y.; Kudo, M.; Kang, Y.-K.; Chen, P.-J.; Toh, H.-C.; et al. Linifanib Versus Sorafenib in Patients with Advanced Hepatocellular Carcinoma: Results of a Randomized Phase III Trial. J. Clin. Oncol. 2015, 33, 172–179.
- Richly, H.; Henning, B.F.; Kupsch, P.; Passarge, K.; Grubert, M.; Hilger, R.A.; Christensen, O.; Brendel, E.; Schwartz, B.; Ludwig, M.; et al. Results of a Phase I trial of sorafenib (BAY 43-9006) in combination with doxorubicin in patients with refractory solid tumors. Ann. Oncol. 2006, 17, 866–873.
- Abou-Alfa, G.K.; Johnson, P.; Knox, J.J.; Capanu, M.; Davidenko, I.; Lacava, J.; Leung, T.; Gansukh, B.; Saltz, L. Doxorubicin Plus Sorafenib vs. Doxorubicin Alone in Patients with Advanced Hepatocellular Carcinoma. JAMA 2010, 304, 2154–2160.
- McCubrey, J.A.; Steelman, L.S.; Abrams, S.L.; Lee, J.T.; Chang, F.; Bertrand, F.E.; Navolanic, P.M.; Terrian, D.M.; Franklin, R.A.; D’assoro, A.B.; et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzym. Regul. 2006, 46, 249–279.
- Wakabayashi, I.; Groschner, K. Vascular actions of anthracycline antibiotics. Curr. Med. Chem. 2003, 10, 427–436.
- Abou-Alfa, G.K.; Shi, Q.; Knox, J.J.; Kaubisch, A.; Niedzwiecki, D.; Posey, J.; Tan, B.R., Jr.; Kavan, P.; Goel, R.; Lammers, P.E.; et al. Assessment of Treatment with Sorafenib Plus Doxorubicin vs Sorafenib Alone in Patients With Advanced Hepatocellular Carcinoma: Phase 3 CALGB 80802 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1582–1588, Erratum in JAMA Oncol. 2019, 5, 1643.
- El Dika, I.; Capanu, M.; Chou, J.F.; Harding, J.J.; Ly, M.; Hrabovsky, A.D.; Do, R.K.G.; Shia, J.; Millang, B.; Ma, J.; et al. Phase II trial of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma after disease progression on sorafenib. Cancer Med. 2020, 9, 7453–7459.
- Berasain, C.; Nicou, A.; Garcia-Irigoyen, O.; Latasa, M.U.; Urtasun, R.; Elizalde, M.; Salis, F.; Perugorría, M.J.; Prieto, J.; Recio, J.A.; et al. Epidermal Growth Factor Receptor Signaling in Hepatocellular Carcinoma: Inflammatory Activation and a New Intracellular Regulatory Mechanism. Dig. Dis. 2012, 30, 524–531.
- Blivet-Van Eggelpoël, M.-J.; Chettouh, H.; Fartoux, L.; Aoudjehane, L.; Barbu, V.; Rey, C.; Priam, S.; Housset, C.; Rosmorduc, O.; Desbois-Mouthon, C. Epidermal growth factor receptor and HER-3 restrict cell response to sorafenib in hepatocellular carcinoma cells. J. Hepatol. 2012, 57, 108–115.
- Duran, I.; Hotté, S.J.; Hirte, H.; Chen, E.X.; MacLean, M.; Turner, S.; Duan, L.; Pond, G.R.; Lathia, C.; Walsh, S.; et al. Phase I Targeted Combination Trial of Sorafenib and Erlotinib in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2007, 13, 4849–4857.
- Zhu, A.X.; Rosmorduc, O.; Evans, T.J.; Ross, P.J.; Santoro, A.; Carrilho, F.J.; Bruix, J.; Qin, S.; Thuluvath, P.J.; Llovet, J.M.; et al. SEARCH: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Sorafenib Plus Erlotinib in Patients with Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2015, 33, 559–566.
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173.
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335.
- Siegel, A.B.; Cohen, E.I.; Ocean, A.; Lehrer, D.; Goldenberg, A.; Knox, J.J.; Chen, H.; Clark-Garvey, S.; Weinberg, A.; Mandeli, J.; et al. Phase II Trial Evaluating the Clinical and Biologic Effects of Bevacizumab in Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2008, 26, 2992–2998.
- Hsu, C.-H.; Yang, T.-S.; Toh, H.C.; Epstein, R.J.; Hsiao, L.-T.; Chen, P.-J.; Lin, Z.-Z.; Chao, T.-Y.; Cheng, A.-L. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br. J. Cancer 2010, 102, 981–986.
- Sun, W.; Sohal, D.; Haller, D.G.; Mykulowycz, K.; Rosen, M.; Soulen, M.C.; Caparro, M.; Teitelbaum, U.R.; Giantonio, B.; O’Dwyer, P.J.; et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011, 117, 3187–3192.
- Thomas, M.B.; Morris, J.S.; Chadha, R.; Iwasaki, M.; Kaur, H.; Lin, E.; Kaseb, A.; Glover, K.; Davila, M.; Abbruzzese, J. Phase II Trial of the Combination of Bevacizumab and Erlotinib in Patients Who Have Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2009, 27, 843–850, Erratum in J. Clin. Oncol. 2009, 27, 3263.
- Kaseb, A.; Garrett-Mayer, E.; Morris, J.; Xiao, L.; Lin, E.; Onicescu, G.; Hassan, M.; Hassabo, H.; Iwasaki, M.; Deaton, F.; et al. Efficacy of Bevacizumab plus Erlotinib for Advanced Hepatocellular Carcinoma and Predictors of Outcome: Final Results of a Phase II Trial. Oncology 2012, 82, 67–74.
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.-L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148.
- Finn, R.S.; Bentley, G.; Britten, C.D.; Amado, R.; Busuttil, R.W. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int. 2009, 29, 284–290.
- Boige, V.; Malka, D.; Bourredjem, A.; Dromain, C.; Baey, C.; Jacques, N.; Pignon, J.-P.; Vimond, N.; Bouvet-Forteau, N.; De Baere, T.; et al. Efficacy, Safety, and Biomarkers of Single-Agent Bevacizumab Therapy in Patients with Advanced Hepatocellular Carcinoma. Oncologist 2012, 17, 1063–1072.
- Fang, P.; Hu, J.-H.; Cheng, Z.-G.; Liu, Z.-F.; Wang, J.-L.; Jiao, S.-C. Efficacy and Safety of Bevacizumab for the Treatment of Advanced Hepatocellular Carcinoma: A Systematic Review of Phase II Trials. PLoS ONE 2012, 7, e49717.
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10.
- Park, J.-J.; Omiya, R.; Matsumura, Y.; Sakoda, Y.; Kuramasu, A.; Augustine, M.M.; Yao, S.; Tsushima, F.; Narazaki, H.; Anand, S.; et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 2010, 116, 1291–1298.
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126, Erratum in J. Clin. Oncol. 2022, 40, 315.
- Sangro, B.; Chan, S.L.; Meyer, T.; Reig, M.; El-Khoueiry, A.; Galle, P.R. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 2020, 72, 320–341.
- Abu-Sbeih, H.; Faleck, D.M.; Ricciuti, B.; Mendelsohn, R.B.; Naqash, A.R.; Cohen, J.V.; Sellers, M.C.; Balaji, A.; Ben-Betzalel, G.; Hajir, I.; et al. Immune Checkpoint Inhibitor Therapy in Patients with Preexisting Inflammatory Bowel Disease. J. Clin. Oncol. 2020, 38, 576–583.
- Kang, S.P.; Gergich, K.; Lubiniecki, G.M.; de Alwis, D.P.; Chen, C.; Tice, M.A.B.; Rubin, E.H. Pembrolizumab KEYNOTE-001: An adaptive study leading to accelerated approval for two indications and a companion diagnostic. Ann. Oncol. 2017, 28, 1388–1398.
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330.
- Verset, G.; Borbath, I.; Karwal, M.; Verslype, C.; Van Vlierberghe, H.; Kardosh, A.; Zagonel, V.; Stal, P.; Sarker, D.; Palmer, D.H.; et al. Pembrolizumab Monotherapy for Previously Untreated Advanced Hepatocellular Carcinoma: Data from the Open-Label, Phase II KEYNOTE-224 Trial. Clin. Cancer Res. 2022, 28, 2547–2554.
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2021, 23, 77–90.
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723, Erratum in N. Engl. J. Med. 2010, 363, 1290.
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88.
- Lee, M.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Randomised efficacy and safety results for atezolizumab (Atezo) + bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Ann. Oncol. 2019, 30, v875.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Finn, S.Q.R.S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.V.; Merle, P.; Kaseb, A.O.; et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39 (Suppl. S33), 267.
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990, Erratum in Lancet Oncol. 2021, 22, e347.
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Dao, T.V.; De Toni, E.N.; et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 2022, 40, 379.
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008.
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970.
- Finn, R.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.-Y.; Ren, Z.; et al. LBA34 Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2022, 33, S1401.
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66, Erratum in Lancet 2017, 389, 36.
- Kelley, R.K.; Ryoo, B.-Y.; Merle, P.; Park, J.-W.; Bolondi, L.; Chan, S.L.; Lim, H.Y.; Baron, A.D.; Parnis, F.; Knox, J.; et al. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: A subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open 2020, 5, e000714.
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296.
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564, Erratum in JAMA Oncol. 2021, 7, 140.
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202.
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502.
- Andre, T.; Berton, D.; Curigliano, G.; Ellard, S.; Pérez, J.M.T.; Arkenau, H.-T.; Abdeddaim, C.; Moreno, V.; Guo, W.; Im, E.; et al. Safety and efficacy of anti–PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: Results from GARNET study. J. Clin. Oncol. 2021, 39, 9.
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022, 23, 1261–1273.




