1. Introduction
Prior to the SHARP trial and approval of sorafenib, systemic therapy had not been used routinely for patients with advanced HCC for a myriad of reasons including but not limited to HCC’s high rate of expression of drug resistance genes and difficulties with chemo-toleration owing to underlying hepatic dysfunction [
8]. Ablative approaches including transarterial chemoembolization or palliative systemic therapy with conventional cytotoxic chemotherapy agents were variably used [
9]. The use of sorafenib was based on a recognition of the important roles of proangiogenic factors, vascular endothelial growth factor (VEGF), the platelet-derived growth factor (PDGF), and the fibroblast growth factor (FGF) in neovascularization, invasiveness, and metastatic potential. Since the demonstration of overall survival (OS) benefits of sorafenib, there has been an explosion of systemic therapy clinical trials for HCC, some of which have failed to meet or improve the survival benefits of sorafenib. However, more recent trials, particularly those incorporating immunotherapy, have shown improved survival compared with sorafenib. A chronological timeline of such HCC systemic therapy clinical trials, including those that resulted in the approval of agents as first- and second-line options as well as relevant ones that failed along the way, is illustrated in
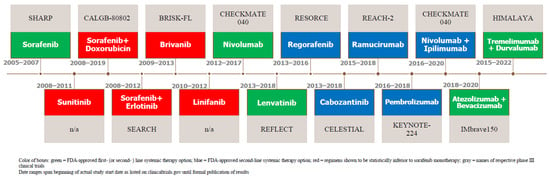
Figure 1.
Figure 1. Timeline of Notable HCC Systemic Therapy Trials.
Currently, systemic therapy is appropriate for those HCC patients who are not amenable to curative or locoregional therapy and have adequate performance status and underlying liver function. This includes HCC patients with extrahepatic spread, with tumors confined to the liver which have progressed after locoregional therapies, extensive vascular invasion, or a large intrahepatic tumor burden unsuitable for locoregional approaches. Lastly, prior to initiating systemic treatment, it is important to note the parameters for viral hepatitis screening as well as the predictive markers of response. In the former, a 2020 provisional clinical opinion from the American Society of Clinical Oncology (ASCO), which endorses universal HBV screening for all patients beginning systemic anti-cancer therapy (cytotoxic chemotherapy, immunotherapy, and molecularly targeted therapy) using HbSAg, anti-HBc, IgG, anti-HBs, and less compelling HCV screening recommendations prior to initiating potentially immunosuppressive chemotherapy, is supported [
10]. In addition to overall survival (OS; the principally used primary endpoint in cancer research), other potential surrogate endpoints, such as response rate and progression-free survival (PFS), are also currently in use [
11]. To validate and provide a common framework for assessing treatment response in clinical trials on HCC, first the RECIST (the Response Evaluation Criteria in Solid Tumors) and then the modified RECIST (mRECIST) guidelines, which mainly measure tumor viability, were established [
11]. In the case of sorafenib, survival extension occurred despite the low objective response rate (ORR) assessed by standard RECIST criteria.
2. Systemic Therapy as First-Line Therapy
2.1. For HCC Patients with Preserved Liver Function and Functional Status
Systemic therapy as a first-line option is appropriate for patients with advanced, unresectable HCC who are unsuitable for locoregional therapy and whose liver function is adequate enough to tolerate therapy (i.e., Child–Pugh class A or B cirrhosis). The recommended approach is dependent firstly upon active ongoing clinical trials testing new therapeutic strategies.
Currently, for healthy patients with an ECOG (Eastern Cooperative Oncology Group) performance status of 0 or 1, no worse than Child–Pugh class A cirrhosis, tumors that have not recurred following liver transplantation, and those following the management of esophageal varices, the suggested first-line therapy options are outlined below and summarized in Table 1. Notably, the selection criteria and stratification factors just described, were established by the benchmark for clinical trial design for HCC, the previously mentioned SHARP study.
2.2. Sorafenib
Until 2007, no effective systemic therapy existed that improved survival for patients with advanced HCC [
9]. This changed with preliminary studies suggesting that sorafenib, an oral multikinase inhibitor of serine-threonine kinases Raf-1 and B-Raf and the receptor tyrosine kinase inhibitor (TKI) of VEGFRs-1, 2, and 3 and platelet-derived growth factor receptor beta (PDGFR-beta), may be therapeutically effective for HCC given the kinases’ role in cellular signaling implicated in the molecular pathogenesis of HCC [
12]. In preclinical experiments, sorafenib demonstrated anti-proliferative activity in liver cancer cell lines, reduction in tumor angiogenesis and tumor-cell signaling, and an increase in tumor cell apoptosis in a mouse xenograft model of human HCC [
13]. Eventual experimentation and publication of the multicenter, phase III, double-blind, placebo-controlled SHARP trial examined the primary outcomes of OS and the time to symptomatic progression. Median overall survival (mOS) was 10.7 months in the sorafenib group and 7.9 months in the placebo group (Hazard ratio (HR) in the sorafenib group, 0.69; 95% confidence interval (CI), 0.55 to 0.87;
p < 0.001) and thus efficacy was established for sorafenib over a placebo as a first-line systemic therapy option in unresectable, advanced HCC [
8]. Notably, the overall incidence of treatment-related adverse events was 80% in the sorafenib group and 52% in the placebo group. Most commonly, the adverse events reported were predominantly grade 1 or 2 in severity and GI (diarrhea), constitutional (weight loss), or dermatological (hand-foot-skin reaction, alopecia, etc.) in nature [
8]. Nonetheless, sorafenib was approved by the US FDA in November 2007 for advanced HCC as the first-line standard-of-care treatment. An additional trial confirmed the survival benefit of sorafenib in an Asian-Pacific population [
14].
2.3. Unsuccessful TKI Challengers of Sorafenib
Since the groundbreaking success of sorafenib in the SHARP trial, several further phase III clinical trials (up until 2018) failed to demonstrate equivalence with sorafenib as first-line advanced HCC treatments. This includes brivanib, a multitargeted TKI of VEGFRs and fibroblast growth factor receptors (FGFRs), in the BRISK-FL study which did not meet its primary endpoint of OS non-inferiority versus sorafenib; sunitinib, a multitargeted TKI of PDGF-Rs, VEGFRs, RET, G-CSF-R, FLT-3 and c-KIT, which was significantly inferior to sorafenib with more frequent and severe toxicity; linifanib, a multitargeted TKI of VEGFRs and PDGFRs, which did not meet the predefined superiority and non-inferiority OS primary endpoints and had a worsened safety profile [
15,
16,
17].
2.4. Unsuccessful Combination Therapy: Sorafenib and Doxorubicin
In addition, studies investigated the combination of chemotherapy agents with sorafenib in efforts to demonstrate improved clinical benefits as a first line. Notably, doxorubicin, an anthracycline drug that previously showed promise with feasibility and tolerability in a phase I trial and then a significant improvement in OS in a randomized, double-blind, phase II study when joined with sorafenib, was further explored as a potential pairing with sorafenib [
18,
19]. The hypotheses at the time suggested a possible synergism between the two via the inhibition of the Ras/Raf/MEK/ERK pathway preventing the activation of the multidrug resistance pathway and additive effects via anthracyclines’ modulation of angiogenesis [
20,
21]. Ultimately, however, the CALGB 80802 phase III clinical trial studying sorafenib and doxorubicin compared to sorafenib monotherapy was halted after the accrual of 356 (out of planned 480) patients with a futility boundary crossed at a planned interim analysis [
22]. Overall, the primary endpoint of mOS was 9.3 months (95% CI, 7.3–10.8 months) in the doxorubicin and sorafenib arm and 9.4 months (95% CI, 7.3–12.9 months) in the sorafenib alone arm (HR, 1.05; 95% CI, 0.83–1.31). In addition, hematologic toxicities, especially grade 3 or 4 neutropenia (36.8%) and thrombocytopenia (17.5% vs. 2.4%) occurred significantly more frequently in the combination group [
22]. The discrepancy between the phase II and III trials could in part be explained by the use of doxorubicin in lieu of sorafenib as the control for the CALGB phase II trial [
22,
23].
2.5. Unsuccessful Combination Therapy: Sorafenib and an EGFR Inhibitor
Next, in the SEARCH phase III clinical trial, erlotinib, a TKI of EGFR (endothelial growth factor receptor), was combined with sorafenib and compared against sorafenib monotherapy in the first-line systemic therapy setting. It was hypothesized that EGFR inhibition, via erlotinib, would enhance tumor response due to both the implicated roles of the EGFR pathway in the pathogenesis of HCC and that of EGFR activation interfering with HCC response to sorafenib [
24,
25]. A phase I trial of sorafenib and erlotinib in patients with advanced solid tumors revealed good toleration and no pharmacokinetic interactions [
26]. Ultimately, in the SEARCH trial, there was no improvement of OS or PFS in patients with unresectable HCC compared to sorafenib monotherapy [
27].
2.6. Lenvatinib, the First Approved Alternative to Sorafenib
Finally, studies conducted on lenvatinib, an inhibitor of VEGF receptors 1–3, FGF receptors 1–4, PDGF receptors alpha, RET, and KIT revealed promising early results. Its molecular nature was smaller than that of sorafenib with more potent activity against VEGF receptors and the FGF family. In the open-label, phase III, multicenter, non-inferiority, REFLECT clinical trial, OS as a primary endpoint was compared between unresectable HCC patients receiving sorafenib vs. lenvatinib. The median survival time for lenvatinib of 13.6 months (95% CI, 12.1–14.9) was non-inferior to that of sorafenib (12.3 months, 10.4–13.9; HR, 0.92; 95% CI 0.79–1.06) [
28]. Compared to sorafenib, similar rates and types of adverse events were reported with the exception of increased reports of grade 3- or 4-associated hypertension (23 vs. 14 percent) and proteinuria in the lenvatinib group [
28]. In contrast, lenvatinib appeared to have less hand–foot syndrome than sorafenib did. Consequently, US FDA approval was granted in August 2018, thus marking the first alternative to sorafenib (albeit not superior to it) for the first-line treatment of unresectable HCC, with NCCN (National Comprehensive Cancer Network) guidelines suggesting limiting its use to individuals with no worse than Child-Pugh class A cirrhosis.
2.7. Bevacizumab and the Emergence of Anti-VEGF Monoclonal Antibodies
Throughout this time interval, studies were underway on monoclonal antibodies directed against VEGF, in similar hopes of reducing tumor vascularity and growth. Bevacizumab is a humanized monoclonal antibody that binds to VEGF-A [
29]. Various initial experiments and subsequent phase II clinical trials were conducted that investigated bevacizumab first in vitro, then in mouse models and then as either monotherapy, combination therapy with chemotherapy (capecitabine, oxaliplatin, and gemcitabine), or combination therapy with EGFR inhibitors in efforts to demonstrate its efficacy as an alternative first-line systemic therapy option for unresectable HCC [
30,
31,
32,
33,
34,
35,
36,
37]. A systematic review of the efficacy and safety of bevacizumab for the treatment of advanced HCC was carried out, which included 8 trials and 300 patients with bevacizumab given in various regimens, as mentioned above. Ultimately, the drug showed promise as an effective and tolerable agent that compared favorably to sorafenib and warranted comprehensive examination in the phase III setting [
38].
2.8. Immune Checkpoint Inhibitors (ICIs): Origin in Treatment for Advanced HCC
Concomitantly, immune checkpoint inhibitor immunotherapy research was underway to find alternative treatment modalities for cancer research at large, including that for unresectable HCC. The premise is based on the dual understanding that the development of cancers is a multi-step process, that is in short characterized by the accumulation of genetic and epigenetic alterations that drive or reflect tumor progression, in addition to the fact that developing cancer cells become distinguished from their normal counterparts yet are rarely rejected spontaneously, reflecting their ability to maintain an immunosuppressive microenvironment [
39]. Notably, programmed death ligand 1 (PD-L1, i.e., B7-H1) is noted to be expressed on many cancer and immune cells and by binding to programmed death-1 (PD-1) and CD80, negative regulators of T-lymphocyte activation, it suppresses T-cell migration, proliferation, and secretion of cytotoxic mediators, thereby restricting tumor cell killing and blocking the cancer immunity cycle [
40]. Hence, extensive research has been underway to block the binding of PD-L1 to its receptors, enhancing anti-cancer immunity, with hopes of developing efficacious advanced HCC treatment options.
Despite the clear clinical benefits achieved with ICI therapy thus far, it is worth noting their use has been associated with a new spectrum of side effects, related to their mechanism of action, which is distinct from that of other systemic therapies such as cytotoxic chemotherapy [
41]. Notably, their side effects predominantly effect the GI, dermatologic, hepatic, endocrine, and pulmonary systems though any bodily system may be affected. The incidence and onset of immune-related adverse effects has been shown to depend on the type of cancer, the class and dose of ICI used, and specific factors related to the patient [
41]. Because HCC by and large develops within a background of cirrhosis, which on its own leads to systemic manifestations, the potential for harmful risks could not be ignored. However, in general, studies have shown that metrics for immune related adverse events are not significantly higher for HCC treatment than they are for other cancers [
42]. Certain patient populations with specific co-morbidities must be more carefully examined when undergoing ICI therapy, as is evident in those with IBD (inflammatory bowel disease) undergoing disease reactivation at higher rates [
43].
Initial immune checkpoint inhibitor studies on HCC were developed upon the success highlighted in melanoma research seen in the form of pembrolizumab treatment, in the Keynote-001 study, and nivolumab, in the CheckMate 066 study—both monoclonal antibodies directed against PD-1 [
44,
45]. When adapted for advanced first and second-line HCC treatment there were mixed results. In an updated report of the second cohort of patients (51 in total) who had not received any prior systemic therapy for advanced HCC in the Keynote-224 phase II trial, an objective response rate (ORR) of 16% and OS of 17 months with a consistent safety profile was observed in patients receiving pembrolizumab [
46]. In the CheckMate 459 study, a randomized, multicenter, open-label, phase III study investigating nivolumab monotherapy vs. sorafenib monotherapy, a mOS of 16.4 months (95% CI, 13.9–18.4) compared with the 14.7 months (95% CI, 11.9–17.2) for sorafenib (HR, 0.85; 95% CI, 0.72–1.02;
p = 0.075) was demonstrated [
47]. Although nivolumab did not significantly improve OS compared to sorafenib, clinical activity and a favorable safety profile were observed, thus leading to recommendations for its use to patients in whom TKIs and anti-angiogenic drugs are contraindicated or have substantial risks.
In slightly earlier ICI studies, the role of CTLA-4 (cytotoxic T-lymphocyte associated protein-4)-directed monoclonal antibodies was explored following the success of ipilimumab against melanoma in 2011 [
48]. With the understanding that CTLA-4 is an inhibitory co-receptor that interferes with T-cell activation and proliferation, a pilot phase I study of tremelimumab, an anti-CTLA-4 monoclonal antibody, was conducted and demonstrated a good safety profile with a partial response rate of 17.6% amongst 21 enrolled patients [
49]. Thus, further studies were warranted.
2.9. Combination Therapy: Atezolizumab and Bevacizumab
Combination studies were conducted to explore the additive effects of ICIs and anti-VEGF therapies. A phase Ib study of atezolizumab, a monoclonal antibody checkpoint inhibitor selectively targeting PD-L1, and bevacizumab was conducted in patients with unresectable HCC. It demonstrated an ORR of 36%, a median progression-free survival (mPFS) advantage of 5.6 vs. 3.4 months, and an acceptable side-effect profile [
50]. In the follow-up, global, open-label, phase III IMbrave150 trial, patients with unresectable HCC who had not previously received systemic treatment were randomized to receive either bevacizumab and atezolizumab or sorafenib until unacceptable toxic effects occurred or there was a loss of clinical benefit. The primary endpoints were OS and PFS. In the intention-to-treat population, OS at 12 months was 67.2% (95% CI, 0.42 to 0.79;
p < 0.001) with atezolizumab–bevacizumab and 54.6% (95% CI, 45.2 to 64.0) with sorafenib. The median PFS was 6.8 months (95% CI, 5.7 to 8.3) and 4.3 months (95% CI, 4.0 to 5.6) in the respective groups (HR for disease progression or death, 0.59; 95% CI, 0.47 to 0.76;
p < 0.001). In conclusion, when compared head-to-head, in patients with unresectable HCC, atezolizumab combined with bevacizumab resulted in better overall and progression-free survival outcomes than did sorafenib, a previously preferred systemic therapy first-line option [
51]. An updated OS analysis following an additional 12 months of follow up was revealed at the 2021 ASCO Gastrointestinal Cancer Symposium and demonstrated a sustained clinical efficacy benefit with atezolizumab + bevacizumab vs. sorafenib with a mean OS of 19.2 months vs. 13.4 months (HR, 0.66; 95% CI, 0.52–0.85;
p < 0.0009) [
52]. Thus, sorafenib was firmly, for the first time, supplanted as the recommended first-line systemic therapy option for advanced HCC, with the exception being patients with untreated varices or those with contraindications for VEGF inhibitors or immunotherapy.
2.10. Combination Therapy: Sintilimab and Bevacizumab
Next, the Chinese ORIENT-32 Trial was published in 2021 with an investigation into the combination of sintilimab, an alternate anti-PD-1 monoclonal antibody, and a bevacizumab biosimilar (IBI305). In this randomized, open-label, phase II-III study, the combination was compared against sorafenib monotherapy in unresectable HCC [
53]. Similarly, this combination regimen showed a significant OS and PFS benefit versus sorafenib monotherapy in the first-line setting for unresectable (exclusively HBV-associated) HCC, with an acceptable safety profile. To date, this regimen remains under regulatory review and does not yet have FDA approval as an alternative systemic HCC therapy option.
2.11. Combination Therapy: Tremelimumab and Durvalumab
Results from the HIMALAYA phase III clinical trial, a study investigating the combination therapy of tremelimumab and durvalumab, an anti-PD-L1 monoclonal antibody, as a first-line systemic therapy for advanced HCC, were first presented at the June 2022 American Society of Clinical Oncology annual meeting. In this study, patients were randomized to arms taking the single high priming dose of tremelimumab and durvalumab, an infusion regimen termed STRIDE (Single Tremelimumab Regular Interval Durvalumab), durvalumab monotherapy, or sorafenib monotherapy. The median OS was 16.43 months (95% CI, 14.16–19.58), 16.56 months (95% CI, 14.06–19.12), and 13.77 months (95% CI, 12.25–16.13), respectively, with an overall survival HR for STRIDE versus sorafenib of 0.78 (96% CI, 0.65–0.93;
p < 0.0035) [
54]. Additionally, of note is that durvalumab monotherapy was demonstrated to be non-inferior to sorafenib. Lastly, no new safety signals were noted. The regimen was approved by the US FDA in October 2022 and recommended as an additional first-line systemic therapy option for advanced HCC.
Table 1. A summary of first-line systemic therapy options for HCC as outlined by NCCN guidelines.
2.12. Other Trials Combining TKIs and ICIs
Around the same timeframe, the phase III COSMIC-312 trial compared the regimen of cabozantinib, a multikinase inhibitor (TKI) of kinases involved in tumor pathogenesis including VEGF, MET and the TAM family (TYRO3, AXL, MER), and the previously mentioned atezolizumab. This regimen was compared against sorafenib in the first-line setting for advanced HCC. The results of the dual predefined primary endpoints of PFS per RECIST 1.1 and OS were mixed. The median PFS was 6.8 months (99% CI, 5.6–8.3) in the combination treatment group versus 4.2 months (2.8–7.0) in the sorafenib group (HR, 0.63; 99% CI, 0.44–0.91;
p = 0.0012). The median OS was 15.4 months (96% CI, 13.7–17.6) in the combination treatment group versus 15.5 months (12.1-not estimable) in the sorafenib group (HR, 0.90; 96% CI, 0.69–1.18;
p = 0.44). Furthermore, serious treatment-related adverse events occurred in 18% of patients in the combination group (with five fatal events) compared to 8% in the sorafenib group (with one fatal event) [
55]. Consequently, there was no formal adoption by the NCCN or a recommendation of this regimen.
A phase Ib study combining lenvatinib and pembrolizumab in patients with unresectable HCC showed promising anti-tumor activity, with a mOS of 22 months, and no new identifiable safety signals [
56]. In the ensuing phase III, global, randomized, double-blind LEAP-002 study, lenvatinib and pembrolizumab was compared to. lenvatinib monotherapy. Unfortunately, the primary endpoints of OS at the final analysis and PFS at an interim analysis I did not meet the pre-specified statistical significance [
57].
3. Second-Line Systemic Therapies
Despite the fact that there are no comparative data with which to define optimal treatment after first-line systemic therapy, there currently exist 10 NCCN recommended regimens, with various degrees of US FDA approval for the treatment of advanced, progressed HCC in various circumstances. Notably, second-line therapy is an option for patients whose HCC progresses while in first-line therapy as well as for those patients whose presenting performance status and liver function are sufficient to tolerate it. The options are presented in Table 2. Importantly, the bulk of these studies were conducted on patients with disease progression on or after sorafenib monotherapy.
| |
Regimen
|
Trial Name
|
Authors
|
Year
|
Study Arm
|
# of Pts
|
Primary Endpoint Results
|
|
“Preferred Regimens:”
|
Regorafenib (a multikinase inhibitor targeting VEGFR 1–3, FGFR 1–2, angiopoietin-1 receptor (TIE2) and PDFRs alpha and beta)
|
RESORCE
|
Bruix et al. [58]
|
2017
|
Regorafenib vs. placebo (second-line setting)
|
573
|
OS: 10.6 months (95% CI 9.1–12.1) for regorafenib versus 7.8 months (6.3–8.8) for placebo (HR of 0.63; 95% CI, 0.50–0.79; one-sided p < 0·0001)
|
| |
Cabozantinib (a multikinase inhibitor (TKI) of kinases involved in tumor pathogenesis including VEGF, MET and the TAM family (TYRO3, AXL, MER)
|
CELESTIAL
|
Kelley et al. [59]
|
2020
|
Cabozantinib vs. placebo (second-line setting)
|
707
|
OS: Cabozantinib improved OS relative to placebo in the overall second-line population who had received only prior sorafenib (median 11.3 vs. 7.2 months; HR, 0.70; 95% CI, 0.55 to 0.88)
|
| |
Ramucirumab (humanized monoclonal antibody directed against VEGF 2) monotherapy
|
REACH-2
|
Zhu et al. [60]
|
2019
|
Ramucirumab vs. placebo (second-line setting)
|
292
|
OS: At a median follow-up of 7.6 months (IQR 4.0–12.5), mOS was 8.5 months (95% CI 7.0–10.6) in the ramucirumab group vs. 7.3 months (5.4–9.1) in the placebo group (HR, 0.710; 95% CI, 0.531–0.949; p = 0.0199)
|
| |
Lenvatinib (TKI of VEGF-R1–3, FGF receptors 1–4, PDGF receptor alpha, RET, and KIT) monotherapy
|
REFLECT
|
Kudo et al. [28]
|
2018
|
Lenvatinib vs. sorafenib (first-line setting)
|
954
|
OS: 13.6 months (95% CI, 12.1–14.9) for Lenvatinib was non-inferior to 12.3 months (10.4–13.9; HR, 0.92; 95% CI 0.79–1.06) for sorafenib
|
| |
Sorafenib (TKI of VEGF-R1–3, PDGF beta and serine-threonine kinase inhibitor of Raf-1 and B-Raf) monotherapy
|
SHARP
|
Lovet et al. [8]
|
2008
|
Sorafenib vs. placebo (first-line setting)
|
602
|
OS: 10.7 months with sorafenib and 7.9 months with placebo; HR, 0.69; 95% CI, 0.55 to 0.87; p < 0.001
mPFS: 4.1 months vs. 4.9 months; p = 0.77
|
|
“Other Recommended Regimens:”
|
Nivolumab (checkpoint inhibitor: anti-PD-1 monoclonal antibody) + Ipilimumab (anti-CTLA-4 humanized monoclonal antibody)
|
CheckMate 040
|
Yau et al. [63]
|
2020
|
Phase I/II, three-arm study of nivolumab and ipilimumab (second-line setting)
|
148
|
ORR: 32% (95% CI, 20–47%) in arm A, 27% (95% CI, 15–41%) in arm B, and 29% (95% CI, 17–43%) in arm C, with the respective arms differing in quantity and time for the administration of drugs
|
| |
Pembrolizumab (checkpoint inhibitor: anti-PD-1 monoclonal antibody monotherapy
|
Keynote-240
|
Finn et al. [61]
|
2020
|
Pembrolizumab vs. placebo (second-line setting)
|
413
|
OS: 13.9 months (95% CI, 11.6 to 16.0 months) for pembrolizumab versus 10.6 months (95% CI, 8.3 to 13.5 months) for placebo (HR, 0.781; 95% CI, 0.611 to 0.998; p = 0.0238).
mPFS (with predefined one-sided significance thresholds; p = 0.0174 for final analysis) for pembrolizumab was 3.0 months (95% CI, 2.8 to 4.1 months) versus 2.8 months (95% CI, 1.6 to 3.0 months) at final analysis (HR, 0.718; 95% CI, 0.570 to 0.904; p = 0.0022).
|
|
“Useful in Certain Circumstances:”
|
Nivolumab monotherapy
|
CheckMate 040
|
El-Khoueiry et al. [62]
|
2017
|
Phase I/II dose escalation and expansion trial assessing safety and efficacy of nivolumab monotherapy
|
262
|
ORR: 20% (95% CI 15–26) in patients treated with nivolumab 3 mg/kg in the dose-expansion phase and 15% (95% CI 6–28) in the dose-escalation phase
|
| |
Dostarlimab-gxly (humanized anti-PD-1 monoclonal antibody) monotherapy
|
GARNET
|
Andre et al. [77]
|
N/a
|
Phase I study evaluating safety of dostarlimab
|
N/a
|
N/a
|
| |
Selpercatinib (highly selective RET kinase inhibitor) monotherapy
|
LIBRETTO-001
|
Subbiah et al. [78]
|
2022
|
Phase ½ study evaluating safety and efficacy of selpercatinib in RET fusion-positive advanced solid non-lung or thyroid tumors
|
N/a
|
N/a
|
This entry is adapted from the peer-reviewed paper 10.3390/cancers15092506